Archive : Article / Volume 2, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-2187/009
A narrative review of alterations in PANDAS syndrome: Where do we draw the line?
1. Department of Medical Laboratory Sciences, Varastegan Institute for Medical Sciences, Mashhad, Iran
3. Department of Microbiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
4. Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Iran
5.Antimicrobial Resistance Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran
6.Department of Microbiology and virology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Sepideh Hasanzadeh
Shaghayegh Zahmatkesh Moghadam, Behnam Azari, Davoud Mansouri, Ali Mehri, Kiarash Ghazvini, Sepideh Hasanzadeh, A narrative review of alterations in PANDAS syndrome: Where do we draw the line? Journal of Microbes and Research 2(2). DOI: 10.58489/2836-2187/009
© 2023 Sepideh Hasanzadeh, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 01-01-2023
- Accepted Date: 12-01-2023
- Published Date: 18-01-2023
PANDAS/PANS; Obsessive-Compulsive Disorder; Tic disorder, Group A Streptococcus; Gut Microbiota
Abstract
PANDAS or Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection is a concerning disorder in childhood. Co-existence of the OCD and tic disorders after the infection of group A β-hemolytic streptococcus may cause PANDAS. PANDAS supported molecular mimicry as the cause of disorder. Characteristic changes that may be related to the alterations in the nervous system are demonstrated to be the first line of diagnosis. In PANDAS, some parts of the brain may undergo changes like dysfunction in basal ganglia and inflammation in striatum, etc. In this disorder not only do the symptoms tangle the nervous system, but also alterations in gut microbiota which correlate with the brain in psychiatric diseases are feasible. In addition, the studies on genetic aspects of PANDAS showed that changes can lead to the exacerbation in PANDAS. In this paper, we provide data related to signs and symptoms of PANDAS to clarify the importance of this disorder in childhood and adulthood as a side effect of streptococcal infection.
Highlights
- Presence of Autoantibodies against group A beta-hemolytic streptococcal antigen
- The acute onset of PANDAS in childhood and adolescence
- The impact of PANDAS on behavior, the nervous system, the gut microbiota, etc
- Effects of genetics on the severity of signs and symptoms of PANDAS
Introduction
The term Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection or PANDAS is a contentious topic that many details of it are still unclear [1, 2]. It is defined as part of a larger group called PANS or Pediatric Acute-onset Neuropsychiatric Syndrome [2]. The first description of this rare mental disorder was presented by Swedo et al. in 1998, based on 50 children with a sudden onset of obsessive-compulsive disorder (OCD) and tics [3]. Epidemiologically, PANDAS is more common in men [2.6:1] and usually occurs in childhood, but a minor percentage of cases show that PANDAS can happen after puberty [4, 5]. In recent years, scientists have suggested a connection between group A β-hemolytic streptococcus (GABHS) infections and subsequent development of OCD and tic disorders in children, the actual difference between PANS and PANDAS [1]. Consequently, there are some non-streptococcal infections such as Bornavirus or Mycoplasma pneumoniae that reveal the symptoms similar to those in PANDAS [6-8].
The hypothesis about the pathophysiology of PANDAS is molecular mimicry which involves antibodies produced against streptococcal antigens that can cross-react with brain proteins, particularly in the basal ganglia [3]. These antineuronal antibodies include anti-pyruvate kinase, anti-dopamine receptor, anti-Tubulin, and anti-lysoganglioside GM1, plus the receptors that antibodies cross-react with, including N-acetyl-β-D-glucosamine, lysoganglioside, and group A carbohydrate epitopes, and receptors of Dopamine (D1 and D2L) [2, 4, 5].
Neuroimmune interactions play a significant role in the pathophysiology of a variety of neuropsychiatric illnesses [1]. For instance, Both OCD and Tourette syndrome occur with alterations in the Cortico-basal ganglia circuitry [1, 9]. Although PANDAS is similar to a mental disorder called Sydenham chorea (SC), there are differences in the age of conflict and Systemic involvement [2].
As of 2017, The diagnostic criteria for PANDAS comprise five sections [4, 5]:
- Presence of OCD and/or tic disorders (chronic motor or vocal tic disorder)
- The onset of OCD and tics in childhood (between the age of 3 years old and period of puberty)
- Association with group A β-hemolytic streptococcus (GABHS) infection
- Co-existing with neurological abnormalities such as attention deficit hyperactivity disorder (ADHD), choreiform movements, etc.
- Sudden onset and episodic course of symptoms
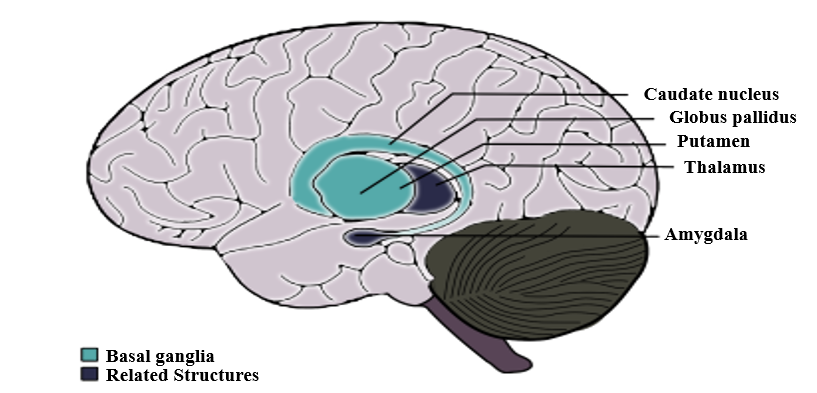
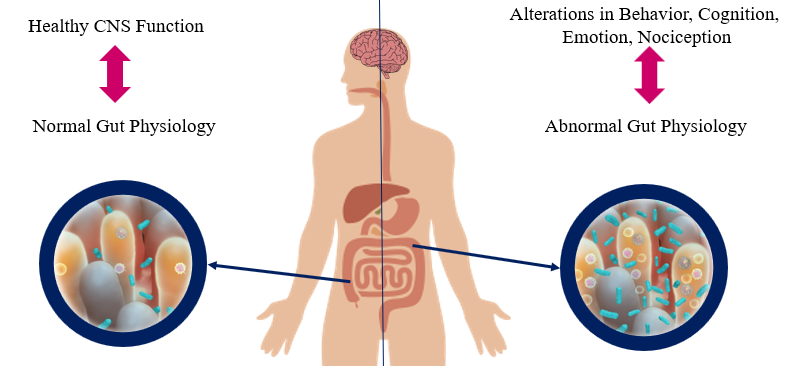
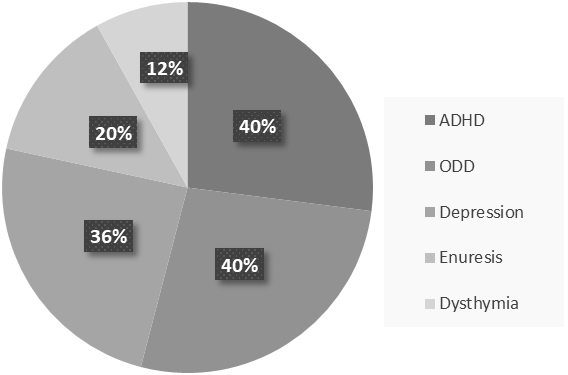
In addition, the state of Streptococcus pyogenes infection is analyzed and measured by the antibody titers of ASO and/or anti-DNase B in the blood sample and a positive throat culture of GABHS [3-5]. However, as a result of 2019, the increased number in specific antibodies of Streptococcus pyogenes is more accurate than a positive culture and titer evaluations because these are somehow misleading ways to a carrier state [10]. Serological methods consider the possibility of the carrier state [3]. One of the more convenient methods to diagnose PANDAS is Cunningham Panel. Based on auto-antibody levels, it has shown an overall of 90 There is evidence that monoclonal antibodies attend to signal neuronal cells and stimulate them to release dopamine and activate calcium-calmodulin dependent protein (CaM) kinase II in basal ganglia [4, 36]. This activation is associated with the progression of the choreic movements [4]. The OCD and tics show abnormalities in cortico-basal ganglia circuitry [37]. The autoantibodies against M18 strain of Streptococcus pyogenes bind to the receptors in basal ganglia [38]. And also, the presence of anti-basal ganglia antibodies in affected individuals’ serum is a marker with high sensitivity and specificity [39]. Epidemiologically, two-third of PANDAS patients show these antibodies [40]. On one hand, the corpus striatum and basal ganglia are enlarged due to an increased number of antineuronal antibody titers [41]. On the other hand, it is clear that the gray matter of basal ganglia had a greater volume, although the white matter shows a reduced amount (based on Cabrera et al.) [42]. Nevertheless, the size of related structures such as caudate, putamen, and globus pallidus increased while the size of thalamus and cerebrum stayed the same (Figure 1) [43]. Surprisingly, the severity of symptoms doesn’t correlate with the enlargement of basal ganglia [4]. The variations within basal ganglia volumes are recognizable whereas there is no evidence about the changes in basal ganglia [4]. As Giedd et al. reported and by PET imaging, the enlargement in the striatum in PANDAS and SC patients is observable [37, 43]. There is a close interaction between the dysregulation within striatal neurons and PANDAS behavioral symptoms and it plays a significant role in tic disorders [37, 44]. More recently, the striatum may be inflamed in PANDAS and Tourette syndrome [37]. However, the inflammation is higher through the bilateral caudate and lentiform nucleus (1, 4). The pathophysiology of tic disorder and Tourette syndrome is associated with cholinergic interneurons [45]. Furthermore, the cholinergic interneurons express dopamine receptors, which is a building-up situation for autoantibodies to bind in patients [4, 37]. The CD68+/Iba1+ activated microglia in the olfactory bulb increases due to frequent internasal GABHS infections [46]. However, it has shown that microglia are beneficial to present the GABHS antigens to immune cells such as Th17 [1, 47]. As mentioned before, microglia are necessary for brain development and its regular action by synaptic pruning [1]. An animal experiment showed that synaptic pruning will increase in PANDAS [47]. Fractalkine/fractalkine receptor signaling pathways may disrupt in PANDAS and result in neural and behavioral changes [48]. Gut microbiome includes anaerobic bacteria, fungi, parasites, and viruses that are significant as a part of every mammalian immune system and play a critical role in adult development and homeostasis [49, 50]. If the homeostasis of Gastrointestinal altered, the development and function of the nervous system would considerably be affected (Figure 2) [50, 51]. For instance, the brain-derived neurotrophic factor (BDNF) levels can be affected by gut microbiota and bacterial dysbiosis may produce systemic inflammation [49, 52]. The axis develops during the intrauterine period [4]. The antimicrobial treatment during pregnancy, vaccination, exposure to chemical or other microorganisms, types of childbirth, etc. may affect this axis [53, 54]. Indeed, some disorders prove the close interaction such as Parkinson's disease, Alzheimer's disease, schizophrenia, multiple sclerosis, anxiety, autism, anorexia nervosa, ADHD, alcohol dependence, bipolar disorders, and migraine pain [4, 49]. The bacterial commensals that produce γ-aminobutyric acid in the gastrointestinal tract may induce neuropsychiatric symptoms, too [4]. It is concluded that the changes in gut microbiota can lead us to enhance PANDAS development. In his studies about the interactions between human microbiota, gut, and brain, Quagliariello et al. found that the amount of some bacterial families may change due to PANDAS (Table 2) (55). Table 2. Summary of the discrepancy between gut microbiota Age Higher level Lower level Total absence 4-8 years old Bacteroidaceae*, Rikenellaceae, and Odoribacteriaceae Firmicutes Actinobacteria Saccharibacteria Some of the firmicutes include Turicibacteraceae, Tissierellaceae, Gemellaceae, and Carnobacteriaceae Corynebacteriaceae and Lachnospiracea Above 9 years old Peptostreptococcaceae and Erysipelotrichaceae Rikenellaceae and Barnesiellacea _ The group of 4-8 years old patients presented the increased level of Bacteroidaceae, Rikenellaceae, and Odoribacteriaceae [4]. Contrariwise, the level of Firmicutes and Actinobacteria were lower than expected in this group of patients [4]. Also, TM7 phylum or Saccharibacteria, and some of the Firmicutes families such as Turicibacteraceae, Tissierellaceae, Gemellaceae, Carnobacteriaceae Corynebacteriaceae, and Lachnospiracea were absent during the incidence of PANDAS [4]. Given the fact that the age of conflict is a crucial factor in the discrepancy between gut microbiota levels, the patients above 9 years old represent different results from the other group [4]. An increased level of Peptostreptococcaceae and Erysipelotrichaceae and lower levels of Rikenellaceae and Barnesiellacea were dramatically observable in these patients [4]. Furthermore, it is essential to mention that Bacteroidaceae presented as the most inclusive family of gut microbiota in PANDAS patients [4]. Genetic correlation with PANDAS There are some genetic susceptibilities to psychiatric diseases like OCD, anxiety, attention deficit hyperactivity disorder (ADHD), pervasive developmental disorder (PDD), etc. [21]. According to what Murphy et al. mentioned, the rate of maternal autoimmune diseases increased in a group of patients with either OCD, tic disorders, or both [56]. The expression of the Trk-like family member 1 (SLITRK1) gene is associated with the cholinergic interneurons in the adult striatum [27]. On the other side, GABHS is highly susceptible to bind to Mannose-binding lectin (MBL), which is a critical part of immune responses [4]. Surprisingly, any changes like variants in exon 1 or polymorphism of codon 54 of MBL2 increases the probability of PANDAS [57]. Changes in TNF-α like, TNF-α-308 AA polymorphism, is associated with the exacerbation of PANDAS symptoms [26]. Since the incidence and severity of autoimmune disorders like PANDAS vary among identical siblings, we would have concluded that environmental factors mostly affect this discordance [25]. Moreover, prenatal and postnatal difficulties and early exposure to GABHS may affect the development of the immune system at the epigenetic level. Methylation of the DNA or modifications in the way histones package the DNA are examples of epigenetic changes [25]. Exacerbation in remission period PANDAS treatment varies including antibiotic therapy (acute antibiotic therapy and prophylaxis) such as Penicillin V, Azithromycin, Amoxicillin-clavulanate, Cephalosporins, psychoactive drugs such as selective serotonin reuptake inhibitors (SSRIs) like fluoxetine, Escitalopram, Lorazepam, Nonsteroidal anti-inflammatory drugs (NSAIDs), Corticosteroid, Tonsillectomy, Intravenous immunoglobulin therapy (IVIG), Plasmapheresis, Vitamin D, and behavioral-cognitive therapy [4, 58, 59]. The remission period of PANDAS may take 3.3 years on average (based on Leon et al.) [13]. Unlike PANDAS, SC completes a remission in less than a year [60]. This exacerbation includes ADHD, oppositional defiant disorder (ODD), depression, enuresis, and dysthymia (Figure 3) [61]. After the remission of OCD and/or tic disorders, it is probable that children with PANDAS show signs of inattention, impulsiveness, and emotional lability, too [4]. Affected children also rarely encounter constant anxiety and fear as well as OCD, whereas other symptoms have disappeared [4]. Even though PANDAS was first introduced in 1998 by Swedo et al., it is still tough to distinguish its differences between a typical OCD or tic disorder [4]. PANDAS can be counted as a subgroup of PANS, characterized by restricted food intake after infection [2]. PANDAS has a high similarity to Sydenham chorea, which is another side effect of GABHS infection [62]. Environmental factors, coexisting diseases such as OCD, and genetic predispositions may be associated with this disease [63]. Experts define PANDAS by its characteristic symptoms ranging from typical anxiety to acute onset of OCD. The size of some parts of the brain like basal ganglia, striatum, caudate, and putamen is altered in PANDAS by binding autoantibodies in the brain. As seen in some cases, PANDAS may affect the gut microbiota, which in turn can exacerbate the onset of disease. Besides the signs and symptoms, the antibody titers of ASO or anti-DNase B in the blood sample and a positive throat culture of GABHS are helpful in the diagnosis of PANDAS [3-5]. Another method used these days is Cunningham Panel, which shows autoantibodies produced in PANDAS [9]. Although the variety of PANDAS treatments may take 3.3 years on average to cure children, it has proven that children may experience psychiatric symptoms in the remission period [48]. PANDAS has been the topic for many articles, but despite available studies, some aspects of it are still unclear. Conflicts of Interest None to declare. Ethical Approval Patient relative's consent was obtained for publication. Basal ganglia
Striatum and Striatal Interneurons
Microglia
Alterations in Gut Microbiota
Conclusion
References
- Frick L, Pittenger C. (2016) Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. Journal of immunology research.;2016.
- Gamucci A, Uccella S, Sciarretta L, D'Apruzzo M, Calevo MG, Mancardi MM, et al. PANDAS and PANS: clinical, (2019)neuropsychological, and biological characterization of a monocentric series of patients and proposal for a diagnostic protocol. Journal of child and adolescent psychopharmacology.;29(4):305-12.
- Nielsen MØ, Köhler-Forsberg O, Hjorthøj C, Benros ME, Nordentoft M, (2019)Orlovska-Waast S. Streptococcal infections and exacerbations in PANDAS: a systematic review and meta-analysis. The Pediatric infectious disease journal.;38(2):189-94.
- Baj J, Sitarz E, Forma A, Wróblewska K, Karakuła-Juchnowicz H. (2020)Alterations in the Nervous System and Gut Microbiota after β-Hemolytic Streptococcus Group A Infection—Characteristics and Diagnostic Criteria of PANDAS Recognition. International Journal of Molecular Sciences.;21(4):1476.
- Kırık S, Güngör O, Kırık Y. (2019)Importance of Streptococci Infections in Childhood Neuropsychiatric Disorders. Şişli Etfal Hastanesi tıp Bülteni.;53(4):441.
- Dietrich D, Zhang Y, Bode L, Münte T, Hauser U, Schmorl P, et al. (2005)Brain potential amplitude varies as a function of Borna disease virus-specific immune complexes in obsessive–compulsive disorder. Molecular psychiatry.;10(6):519-20.
- Matsuo M, Tsuchiya K, Hamasaki Y, Singer HS. (2004)Restless legs syndrome: association with streptococcal or mycoplasma infection. Pediatric neurology.;31(2):119-21.
- Müller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele-Horn M. (2004)Mycoplasma pneumoniae infection and Tourette's syndrome. Psychiatry research.;129(2):119-25.
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. (2010)Neurobiological substrates of Tourette's disorder. Journal of child and adolescent psychopharmacology.;20(4):237-47.
- Johnson DR, Kurlan R, Leckman J, Kaplan EL. (2010)The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clinical infectious diseases.;50(4):481-90.
- Shimasaki C, Frye RE, Trifiletti R, Cooperstock M, Kaplan G, Melamed I, et al. (2020)Evaluation of the Cunningham Panel™ in pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS): Changes in antineuronal antibody titers parallel changes in patient symptoms. Journal of Neuroimmunology.;339:577138.
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. (1998)Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. American Journal of Psychiatry.;155(2):264-71.
- Leon J, Hommer R, Grant P, Farmer C, D’Souza P, Kessler R, et al. (2018)Longitudinal outcomes of children with pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS). European child & adolescent psychiatry.;27(5):637-43.
- Macerollo A, Martino D. (2013)Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an evolving concept. Tremor and other hyperkinetic movements.;3.
- Soderholm AT, Barnett TC, Sweet MJ, Walker MJ. (2018)Group A streptococcal pharyngitis: Immune responses involved in bacterial clearance and GAS‐associated immunopathologies. Journal of Leukocyte Biology.;103(2):193-213.
- Arnold JC, Nizet V. Pharyngitis. (2012)Principles and Practice of Pediatric Infectious Diseases.:199.
- Shaikh N, Swaminathan N, Hooper EG. (2012)Accuracy and precision of the signs and symptoms of streptococcal pharyngitis in children: a systematic review. The Journal of pediatrics.;160(3):487-93. e3.
- Karthikeyan G, Guilherme L. (2018)Acute rheumatic fever. The Lancet.;392(10142):161-74.
- Webb RH, Grant C, Harnden A. (2015)Acute rheumatic fever. Bmj.;351:h3443.
- Punukollu M, Mushet N, Linney M, Hennessy C, Morton M. (2016)Neuropsychiatric manifestations of Sydenham's chorea: a systematic review. Developmental Medicine & Child Neurology.;58(1):16-28.
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. (2015)Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. Journal of Child and Adolescent Psychopharmacology.;25(1):3-13.
- Carelli R, Pallanti S. (2014)Streptococcal infections of skin and PANDAS. Dermatologic Therapy.;27(1):28-30.
- Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Therapeut. (2012);2(2):113.
- Zibordi F, Zorzi G, Carecchio M, Nardocci N. CANS: Childhood acute neuropsychiatric syndromes. European Journal of Paediatric Neurology. (2018);22(2):316-20.
- Lewin AB, Storch EA, Murphy TK. (2011)Pediatric autoimmune neuropsychiatric disorders associated with Streptococcus in identical siblings. Journal of Child and Adolescent Psychopharmacology.;21(2):177-82.
- Luleyap HU, Onatoglu D, Yilmaz MB, Alptekin D, Tahiroglu AY, Cetiner S, et al. (2013)Association between pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections disease and tumor necrosis factor-α gene− 308 g/a,− 850 c/t polymorphisms in 4-12-year-old children in Adana/Turkey. Indian journal of human genetics.;19(2):196.
- O'Roak B, Morgan T, Fishman D, Saus E, Alonso P, Gratacos M, et al. Additional support for the association of SLITRK1 var321 and Tourette syndrome. Molecular psychiatry. (2010);15(5):447-50.
- Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. Journal of child and adolescent psychopharmacology. (2010);20(4):317-31.
- Calaprice D, Tona J, Parker-Athill EC, Murphy TK. (2017)A survey of pediatric acute-onset neuropsychiatric syndrome characteristics and course. Journal of Child and Adolescent Psychopharmacology.;27(7):607-18.
- Murphy TK, Gerardi DM, Parker-Athill EC. The PANDAS Controversy: why (and how) is it still unsettled? Current Developmental Disorders Reports. (2014);1(4):236-44.
- Lanciego JL, Luquin N, Obeso JA. (2012)Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine.;2(12):a009621.
- Báez-Mendoza R, Schultz W. (2013)The role of the striatum in social behavior. Frontiers in Neuroscience.;7:233.
- Wake H, Fields RD. (2011)Physiological function of microglia. Neuron glia biology.;7(1):1.
- Du L, Zhang Y, Chen Y, Zhu J, Yang Y, Zhang H-L. (2017)Role of microglia in neurological disorders and their potentials as a therapeutic target. Molecular Neurobiology.;54(10):7567-84.
- Tremblay M-È, Stevens B, Sierra A, Wake H, Bessis A, (2011)Nimmerjahn A. The role of microglia in the healthy brain. Journal of Neuroscience.;31(45):16064-9.
- da Rocha FF, Correa H, Teixeira AL. (2008) Obsessive–compulsive disorder and immunology: A review. Progress in Neuro-Psychopharmacology and Biological Psychiatry.;32(5):1139-46.
- Frick LR, Rapanelli M, Jindachomthong K, Grant P, Leckman JF, Swedo S, et al. (2018)Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain, behavior, and immunity.;69:304-11.
- Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, et al. (2012)Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology.;37(9):2076-87.
- Church A, Dale R, Giovannoni G. (2004)Anti-basal ganglia antibodies: a possible diagnostic utility in idiopathic movement disorders? Archives of disease in childhood.;89(7):611-4.
- Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, et al. (2004)Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatric neurology.;30(2):107-10.
- Giedd J, Rapoport J, Kruesi M, Parker C, Schapiro M, Allen A, et al. (1995)Sydenham's chorea: magnetic resonance imaging of the basal ganglia. Neurology.;45(12):2199-202.
- Cabrera B, Romero-Rebollar C, Jiménez-Ángeles L, Genis-Mendoza AD, Flores J, Lanzagorta N, et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS spectrums. (2019);24(5):533-43.
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. American Journal of Psychiatry. (2000);157(2):281-3.
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, et al. Centrality of striatal cholinergic transmission in basal ganglia function. Frontiers in neuroanatomy. (2011);5:6.
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. Journal of Comparative Neurology. (2010);518(3):277-91.
- Dileepan T, Smith ED, Knowland D, Hsu M, Platt M, Bittner-Eddy P, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. The Journal of clinical investigation. (2016);126(1):303-17.
- Tay TL, Béchade C, D’Andrea I, St-Pierre M-K, Henry MS, Roumier A, et al. (2018)Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Frontiers in molecular neuroscience.;10:421.
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. (2014)Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience.;17(3):400-6.
- Galland L. (2014)The gut microbiome and the brain. Journal of medicinal food.;17(12):1261-72.
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. (2017)Microbiota-gut-brain axis and the central nervous system. Oncotarget.;8(32):53829.
- Gawlik-Kotelnicka O, Polguj M. (2018)Can microbiology affect psychiatry? A link between gut microbiota and psychiatric disorders. Psychiatr Pol.;52(6):1023-39.
- Sherwin E, Sandhu KV, Dinan TG, Cryan JF. (2016) May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS drugs.;30(11):1019-41.
- Jašarević E, Howard CD, Misic AM, Beiting DP, Bale TL. (2017)Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Scientific Reports.;7:44182.
- Milliken S, Allen RM, Lamont RF. (2019)The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert opinion on drug safety.;18(3):173-85.
- Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR, et al. (2018)Gut microbiota profiling and gut–brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Frontiers in microbiology.;9:675.
- Murphy T, Storch E, Turner A, Reid J, Tan J, Lewin A. (2010)Maternal history of autoimmune disease in children presenting with tics and/or obsessive–compulsive disorder. Journal of neuroimmunology.;229(1-2):243-7.
- Çelik GG, Taş DA, Tahiroglu AY, Erken E, Seydaoğlu G, Ray PC, et al. (2019)Mannose-Binding Lectin 2 Gene Polymorphism in PANDAS Patients. Archives of Neuropsychiatry.;56(2):99.
- Sigra S, Hesselmark E, Bejerot S. (2018)Treatment of PANDAS and PANS: a systematic review. Neuroscience & Biobehavioral Reviews.;86:51-65.
- Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ, et al. (2019)PANDAS/PANS in childhood: Controversies and evidence. Paediatrics & child health.;24(2):85-91.
- Murphy TK, Goodman WK, Ayoub EM, Voeller KK. (2000)On defining Sydenham’s chorea: where do we draw the line? Biological psychiatry.;47(10):851-7.
- Williams KA, Swedo SE. (2015)Post-infectious autoimmune disorders: Sydenham’s chorea, PANDAS and beyond. Brain research.;1617:144-54.
- Oosterveer DM, Overweg-Plandsoen WC, Roos RA. (2010)Sydenham's chorea: a practical overview of the current literature. Pediatric neurology.;43(1):1-6.
- Boileau B. (2011)A review of obsessive-compulsive disorder in children and adolescents. Dialogues in clinical neuroscience.;13(4):401.


