Archive : Article / Volume 1, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2994-2624/002
A Review of The Essence of Stability Constants in The Thermodynamic Assessments of Chemical Compounds
1.Department of Chemistry, Federal University of Technology, Owerri, 1526, Nigeria.
2.Department of Chemistry, Chukwuemeka Odumegwu Ojukwu University, Uli Campus Anambra, 431124, Nigeria.
Onyeocha Veronica Ogechi
Onyeocha V.O., Onuchukwu A.I., (2022). A Review of The Essence of Stability Constants in The Thermodynamic Assessments of Chemical Compounds. International Journal of Anesthesiology and Practice. 1(1). 10.58489/2994-2624/002
© 2022 Onyeocha Veronica Ogechi, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 08-11-2022
- Accepted Date: 30-11-2022
- Published Date: 23-12-2022
Chemical compounds, Stability of compounds, Stability constant, Thermodynamic assessment, Gibbâs free energy, Chemical equilibrium.
Abstract
Background: This treatise discusses the following topics:
- Chemical compounds
- Factors affecting stability of chemical compounds
- Stability constants
- The significance of stability constants vis-a-vis equilibrium constant, Keq
The different forms of stability constant are reviewed and the thermodynamic assessment of stability constant is explained.
Method: The approach involved the review from reputable authors and writers, of the topics that are associated with stability constant and the generalization is remarked following the thermodynamic assessment of the stability constant of chemical compounds.
Findings:
The thermodynamic analysis of stability constant of chemical compounds and of reactions shows that reactions proceed in the direction of decreasing Gibbâs free energy, G, where the standard reaction Gibbâs free energy,
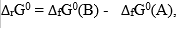
defines the formation of the pure substance B, from the pure substance A, at its standard state.
Introduction
Chemistry is the study of matter and its changes into many products under various conditions. Matter is any substance that has mass and takes up space. An element is a basic unadulterated simple item that cannot be decomposed further and from which all forms of compounds are built. However, the smallest particle of an element that exists is called an atom. An element is a substance composed of only one kind of atom. Thus, matter is made up of various combinations of the simple forms of elements called the chemical substance. In a wider scale, the chemical combination of different elements constitutes a compound. The substances around us are combinations of elements i.e., compounds rather than single elements [1]. A compound is a substance that contains more than one element in a definite ratio. Water is a compound that contains two elements of hydrogen and an element of oxygen. The common table salt (i.e., Sodium Chloride) is a compound too. It contains one sodium atom for each chlorine atom. Some compounds show specific combination of atoms. These compounds are classified as either organic or inorganic [2]. For ease of carriage in science, with respect to Chemistry, combinations of either same elements, as in Oxygen or different elements, such as in water, both are referred to as the molecule, i.e., a molecule of Oxygen and a molecule of Water respectively. Thus, a molecule may be either a compound of same elements (Oxygen) or a compound of different elements (Water). Note: Students are often confused by this simple usage of these terms for convenience. On the other hand, an ion is either a changed element or a charged molecule - this charge can be positive or negative that are called a cation and an anion respectively. Compounds are classified as molecular if they consist of molecules and as ionic if they consist of ions [2]. A compound can be transformed into a different substance by chemical reactions which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. [Chemical compound - Wikipedia]
Chemical compounds
There are four major types of compounds - these compounds are distinguished by how the constituent atoms are bonded together. They are:
- molecular compounds, held together by covalent bonds
- ionic compounds, held together by ionic bonds
- intermetallic compounds, held together by metallic bonds
- coordination complexes, held together by coordinate covalent bonds
Non-stoichiometric crystals form a marginal case that could be given special considerations. [Chemical compound - Wikipedia] The chemical formula of a compound is a statement of its composition in terms of the chemical symbols of the elements present. The molecular compound shows how many atoms of each type of element present in the molecule [1]. A covalent bond described as a molecular bond involves the sharing of electrons between two atoms. It occurs between atoms of elements that fall close to each other on the Periodic Table of elements. Elements that fall close to each other on the Periodic Table have both similar electronegativities and affinity for electrons. Since neither element has a stronger affinity to donate or gain electrons, it causes the elements to share electrons, thereby both elements have a more stable octet. Chemical compounds are unique because they established chemical structures that are held together in a defined spatial arrangement by chemical bonds. Chemical compounds could be molecular compounds held together by covalent bond, salts held together by ionic bonds, intermetallic compounds held together by metallic bonds, or the subset of chemical complexes that are held together by coordinate covalent bonds. The chemical formula of a compound is a statement of its composition in terms of the chemical symbols of the elements present [1]. [Chemical compound - Wikipedia]
Factor affecting stability of chemical compounds
In Chemistry, chemical stability could be described by the thermodynamic stability of the chemical system. Thermodynamic stability occurs when a system is in its lowest energy state or in chemical equilibrium with its environment. Chemical systems undergo changes in the phase of matter or a set of chemical reactions. Generally, a chemical substance is stable if it is not reactive in the environment or during normal use and retains its useful properties on the timescale of its expected usefulness. The usefulness is retained in the presence of air, moisture or heat, and under the expected conditions of application. A material is said to be unstable if it corrodes, decomposes, polymerizes, burns or explodes under the conditions of anticipated use or normal environmental conditions. [Chemical stability - Wikipedia] Chemical stability is important in the comprehensive assessment of pharmaceutical properties, activity, and selectivity during drug discovery. As recorded in [Chemical stability - Wikipedia], some of the questions that are considered in the formation of stable compounds are: “will an active ingredient be stable under the influence of heat? Again, is the product stable in water? How can the viscosity of the compound be influenced? What kind of container should the preparation be dispensed in? What kind of storage condition should be needed? What should this compound look like? Stability could be defined as the extent to which a product retains the same properties and characteristics that it had when it was produced throughout its shelf life. [THE PCCA BLOG | Factors That Affect the Stability of Compounded M (pccarx.com)] The general stability of a compound is made up of five different types, namely: chemical, physical, microbiological, therapeutic and toxicological stabilities. Chemical stability means that each active pharmaceutical ingredient (API) maintains its chemical integrity and potency. Physical stability means that properties like appearance, solubility, ability to be suspended and particle size are maintained. Microbiological stability means that microbial growth is avoided. Therapeutic stability ensures that the therapeutic effect does not change. Toxicological stability means that there is no significant increase in toxicity. These five stability issues are considered during release inspections.
Stability constants
A stability constant, often referred to as either formation constant or binding constant, is the thermodynamic equilibrium constant for the formation of a complex from its reactants in solution. It is a measure of the strength of the interaction between the reactants to form the complex product(s). [stability constant - Search (bing.com)] Stability constant may refer to: [Stability constant - Wikipedia]
- Equilibrium constant
- Acid dissociation constant
- Stability constants of complexes
There are also thermodynamic and kinetic stability constants. Thermodynamic stability constant describes the transformation energy from reactants to products as described by change of the Gibb’s free energy (ΔG) for the reaction at equilibrium. This paper limits its scope on the thermodynamic stability constant. Also, in Coordination Chemistry, stability constant is considered either as formation constant or binding constant. This is same as an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. The stability constant gives the information needed to calculate the concentrations of the complexes in solution. The applications of equilibrium constant are observed in Chemistry, Biology, Medicine, such as in pharmaceutical products, etc. [Stability constants of complexes - Wikipedia]
Thermodynamic analysis of equilibrium constant
Thermodynamic stability describes the energetics associated with equilibrium constant for any reactions at equilibrium. [Stability Constants | Definition, Examples, Diagrams (toppr.com)] The thermodynamic analysis of equilibrium constant is as illustrated below.

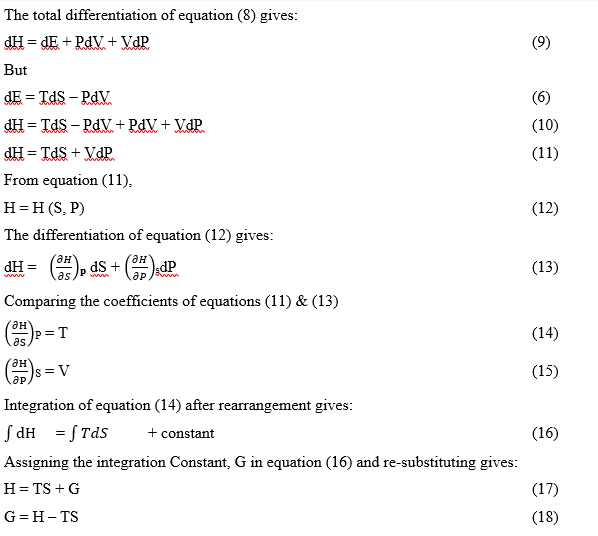
G is the Gibb’s free energy.
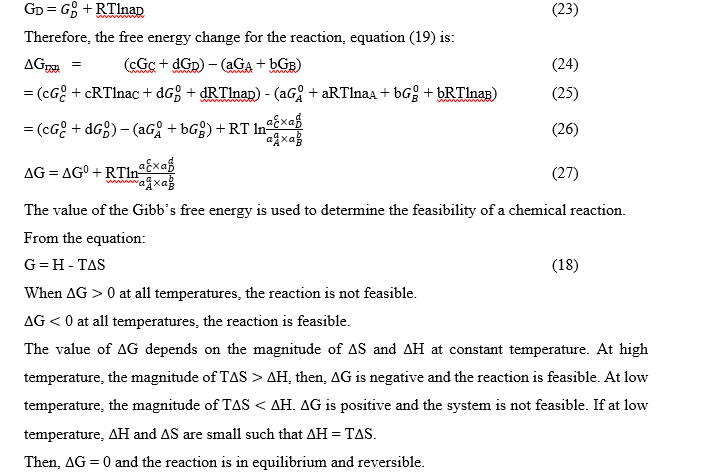
Gibb’s free energy change of a reaction

Brief History [as adapted from Atkins P, Jones L: Chemistry Molecules, Matter and Change. New York: Sumanas, Inc., and W. H. Freeman and Company; Third Edition1997]
The Elements: Thinkers and philosophers have considered the structure of matter since ancient times. The ancient Greeks developed the concept of elements as fundamental substances from which all forms of matter are built. Four types of matter were identified, namely earth, air, fire, and water. These four elements were believed to produce all other substances when combined in the right proportions. This concept of element is consistent with the current belief. There are more than one hundred chemical elements which in various combinations make up all the matter on earth. The ancient Greeks analyzed what would happen if matter is continuously cut into smaller pieces. Would there be a point at which the pieces would no longer have the same properties as the whole? Matter is not continuous. It consists of tiny particles. The smallest particle of an element that exists is called an atom. The first convincing argument for atoms was made in 1807 by the English school teacher and chemist John Dalton. Each element has a name and a unique chemical symbol.
The next question that was considered was where the elements come from? All the elements in the universe except hydrogen and most of the helium were made in the stars. Seconds after the universe came into being with the Big Bang, the only element present were hydrogen and helium. After millions of years, as the universe cooled, the atoms of hydrogen and helium collected together in large clouds under the influence of gravity. These clouds gradually became hotter and hotter as they contracted and in due course they burst into incandescence as stars. Within the stars, intense heat caused atoms of hydrogen to smash together, merge, and become atoms of other elements. When two protons and one or two neutrons merge together, the outcome is an atom of helium (Z = 2, A = 3 or 4). When a third proton and more neutrons join, the atom of lithium (Z = 3, A = 6 or 7) is formed etc. This merging released more heat which generated starlight.
Many million years after a star was formed and it began to cool, its outer layer might collapse, like a falling roof into its exhausted core. This mighty starquake produced such great shock waves that the star shrugged off its outer layers and sent them into space in a huge explosion called a supernova. Six of such explosions were detected in the galaxy in the past 1000years. The most recent explosion was in 1987. The shock of explosion raised the temperature in the star, making it even brighter than before. The Crab nebula, produced by the supernova of 1054, was visible in broad daylight for three weeks. At a high temperature, the heavy atoms collided violently enough to merge and become heavier atoms. The heavy elements found on Earth, including uranium and gold, were made in this way. The Debris of exploding stars collected together under the influence of gravity and gave rise to a new generation of stars. However, not all the debris collected in a single central body, some collected into smaller bodies that go into orbit around the star. These bodies are the planets and one of them is the earth. All matter on earth was formed in this way in long-dead stars. All the elements other than hydrogen and most of helium, from which everything is made, were formed inside a star. The human flesh is stardust. According to current ideas, hydrogen and helium were formed in the Big Bang. The heavier elements were made inside stars and then scattered throughout space [1].
Chemical Equilibrium
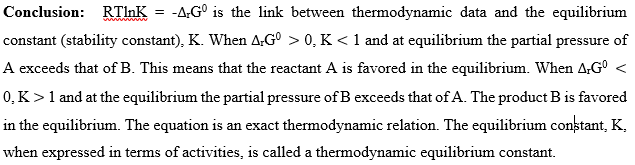
Chemical reactions like the formation and disintegration of compounds move towards a dynamic equilibrium [4]. Thermodynamics gives a guide on the condition and direction of chemical reactions, to achieve economic yield [4]. The direction of spontaneous change at constant temperature and pressure is towards lower values of the Gibbs energy G. The spontaneity of a reaction is determined by calculating the Gibb’s energy [G] of the mixture at various compositions. The minimum in [G] occurs at the composition corresponding to equilibrium. Consider the hypothetical reaction:
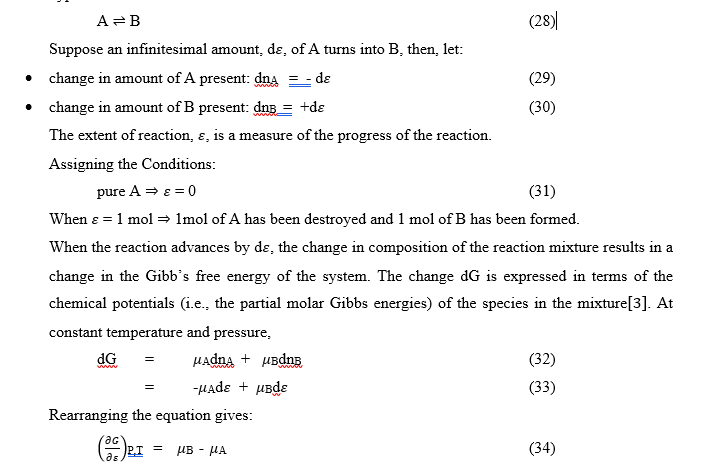
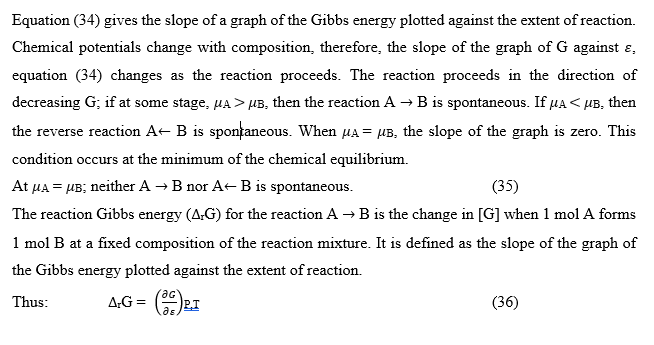
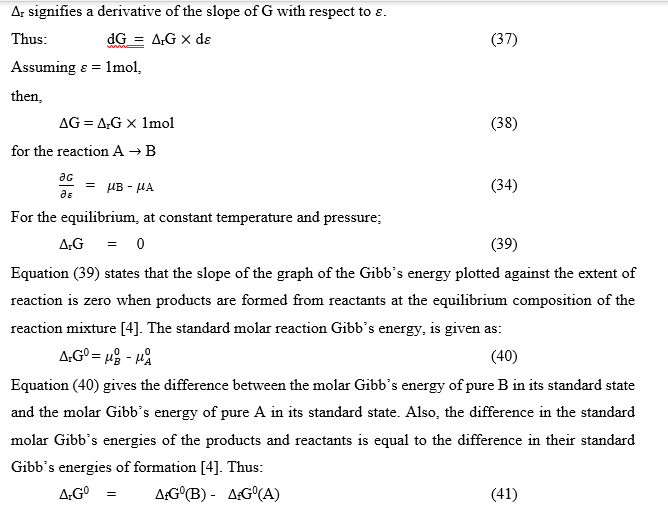

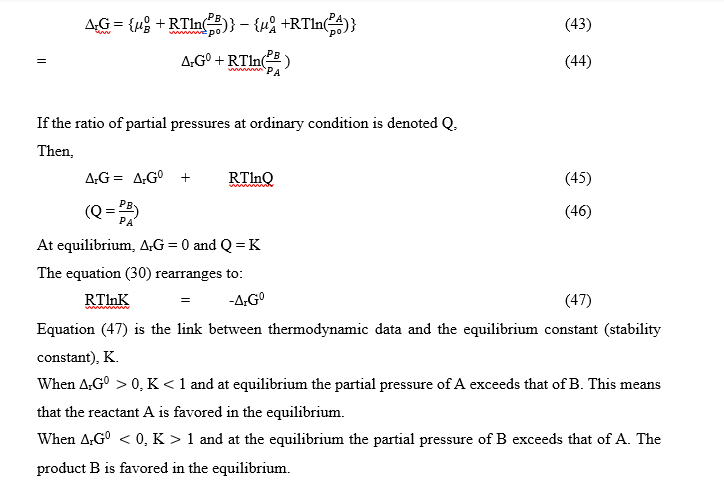
The general case of a reaction
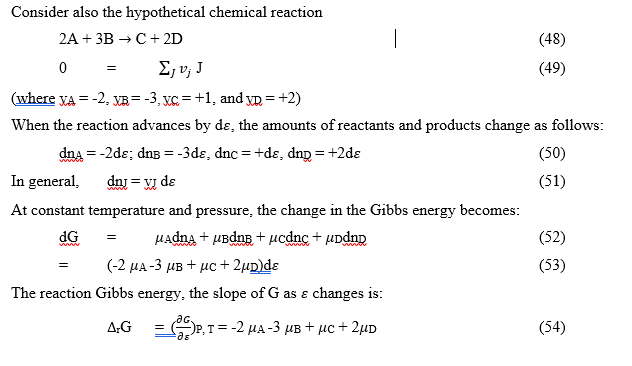
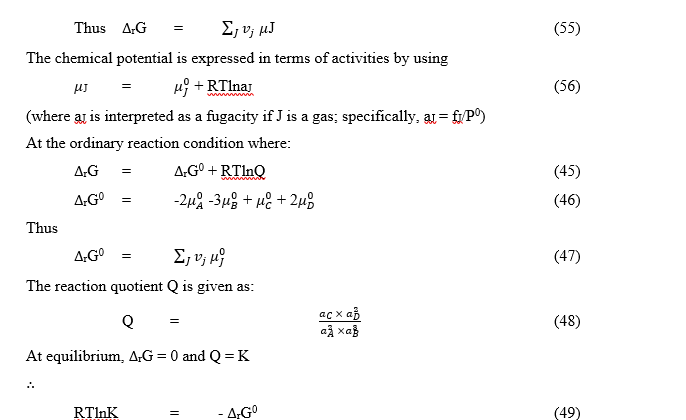
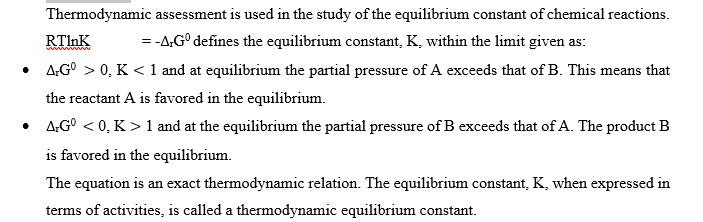
The equation is an exact thermodynamic relation. The equilibrium constant, K, expressed in equation (49), in terms of activities, is called a thermodynamic equilibrium constant.
The response of equilibria to conditions
There are namely: the response of equilibria to pressure and the response of equilibria to temperature.
Response of equilibria to pressure:

- Pressure is applied by injecting an inert gas into the reaction system. If the gases are perfect, this addition leaves all the partial pressures gases unchanged. The addition of an inert gas leaves the molar concentration of the original gases unchanged. The gases continue to occupy the same volume. Pressurization by the addition of an inert gas has no effect on the equilibrium composition of the system.
- The pressure of the system may be increased by physical composition, by confining the gases to a smaller volume. Following this, the values of the partial pressures are changed. The molar concentrations are modified because the volume the gases occupy is reduced.
Consider the perfect gas equilibrium;

Response of equilibria to temperature
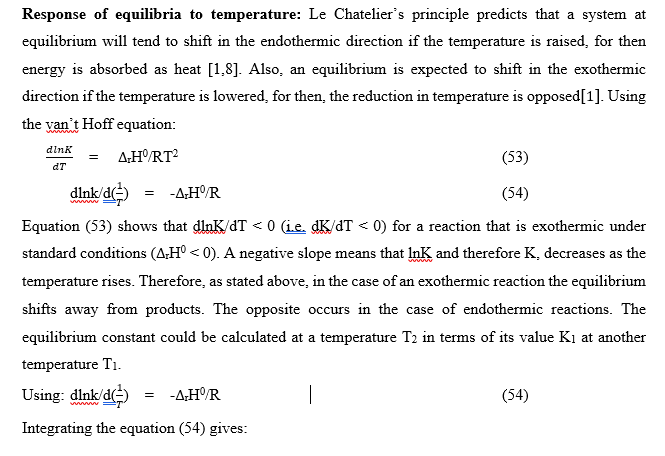

Stability constant
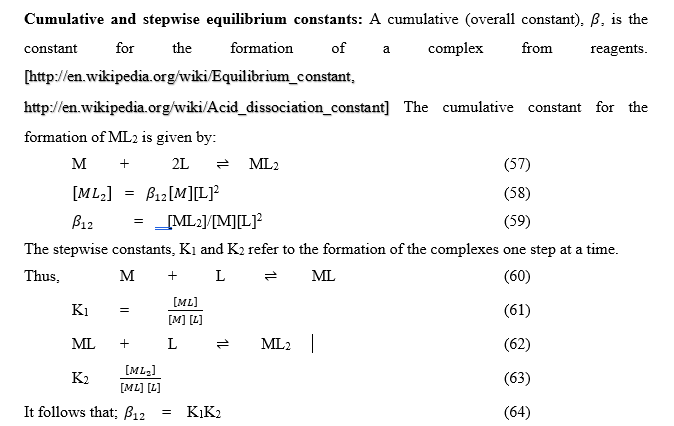
A cumulative constant is expressed as the product of stepwise constant. The two kinds of complex are:
- Compounds formed by the interaction of a metal ion with a ligand.
- Supramolecular complexes: Examples are host-guest complexes and complexes of anions. [http://en.wikipedia.org/wiki/Acid_dissociation_constant] Supramolecular complexes are held together by hydrogen bonding, hydrophobia forces, van der Waals forces, interactions, and electrostatic effects or non-covalent bonding.
The formation of a complex between a metal ion M, and a ligand, L, is a substitution reaction. [http://goldbook.iupac.org/St06785.html] In aqueous solutions, metal ions are present as aqua-ions. The reaction for the formation of the first complex is written as:
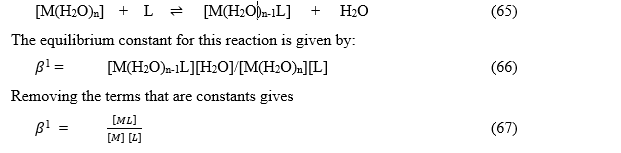
Competition Method
Stepwise constant is used in the determination of stability values outside the normal range for a given method. Examples are EDTA complexes of many metals which are outside the range for the potentiometric method. The stability constants for these complexes were determined by competition with a weaker ligand. [http://en.wikipedia.org/wiki/Stability_constants_of_complexes]
Association and dissociation constants
In Organic Chemistry and Biochemistry, pKa values are used for acid dissociation equilibria. [http://en.wikipedia.org/wiki/Acid_dissociation_constant]
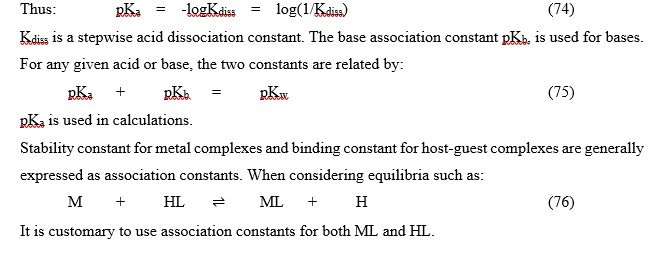
[http://en.wikipedia.org/wiki/Stability_constants_of_complexes] Also, in generalized computer programs dealing with equilibrium constants, it is a general practice to use cumulative constants rather than stepwise constants, and to omit ionic charges from equilibrium expressions. [http://en.wikipedia.org/wiki/Stability_constants_of_complexes] If nitrilotriacetic acid, N(CH2CO2H)3 is designated as H3L and forms complexes ML and MHL with a metal ion M, the following expressions would apply for the dissociation constants. Thus;
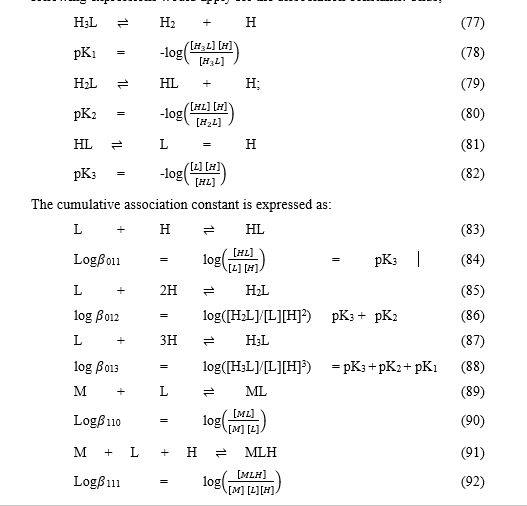
The subscripts define the stoichiometry of each equilibrium product.
Micro-constants
When two or more sites in an asymmetrical molecule are involved in an equilibrium reaction, there are more than one possible equilibrium constants. For example, the molecule L-dopa has two non-equivalent hydroxyl groups which may be deprotonated. [http://en.wikipedia.org/wiki/Stability_constants_of_complexes] Denoting L-Dopa as LH2, different species may be formed. The first protonation constants are:

Hydrolysis constant
In aqueous solution the concentrations of the hydroxide ion is related to the concentration of the hydrogen ion by:

Conditional constants
They are also known as apparent constants. They are concentration quotients which are not true equilibrium constants but are derived from them. An example is where pH is fixed at a particular value (buffered). In the case of iron (III) interacting with EDTA, a conditional constant is defined by:
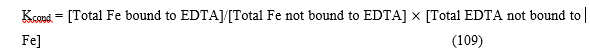
This conditional constant will change with pH. It has a maximum at a certain pH where the ligand sequesters the metal most effectively
Acid dissociation constant
An acid dissociation constant, Ka also known as acid constant or acid ionization constant is a quantitative measure of the strength of an acid in solution. [5,6] It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions. [http://www.acadsoft.co.uk/] The equilibrium is written as:

Gas Phase Equilibria
For equilibria in a gas phase, fugacity, f, is used in place of activity. Fugacity has the dimension of pressure. It is divided by a standard pressure, usually 1 bar, in order to produce a dimensionless quantity, f/pstd. An equilibrium constant is expressed in terms of the dimensionless quantity. [5,6] Example of the equilibrium reaction analysis is given as:
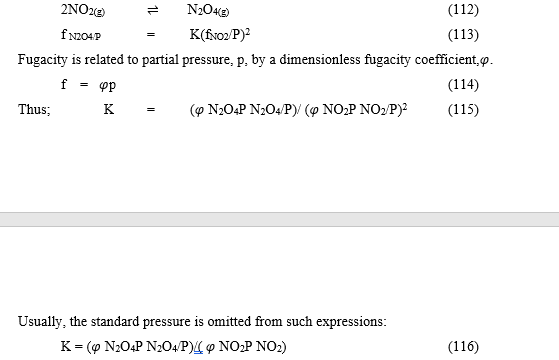
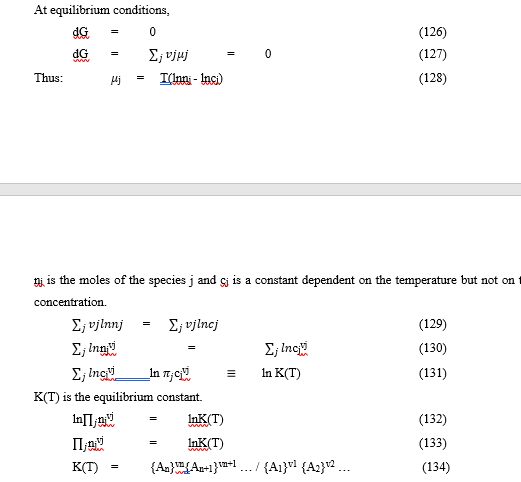
Derivation of Stability Constant from Gibb’s Free energy (A case of a large system)

Conclusion
References
- Atkins P, Jones L: (1997), Chemistry Molecules, Matter and Change. New York: Sumanas, Inc., and W. H. Freeman and Company; Third Edition; pp 1-18, 182-194, 473-495, 587-616.
- Morrison RT, Boyd RN: (2005), Organic Chemistry. Sixth Edition; 375.
- Onuchukwu AI: (2016), Chemical Thermodynamics for Science Students. Owerri: FUTO Press; 4th ed.; 175-205, 214-256.
- Atkins P: (1993), Physical Chemistry. New York: W. H. Freeman and Company; Fifth ed; 213-231.
- Davis RE, Frey R, Sarquis M, et al.: Modern Chemistry. New York: Harcourt Education Company; Teacher Edition. pp 589-628.
- Atkins P: (1993), Physical Chemistry. New York: W. H. Freeman and Company; Fifth ed; 21-48.
- Philips JS, Strozak VS, Wistrom C: (2005), Chemistry Concepts and Applications. New York: Glencoe/McGraw-Hill; 4-44
- Dingrando L, Tallman KG, Hainen N, et al.: (2005), Chemistry Matter and Change. New York: The McGraw-Hill Companies, Inc.; 558–589.
Quick links
- Abstract
- Introduction
- Chemical compounds
- Factor affecting stability of chemical compounds
- Stability constants
- Chemical Equilibrium
- The general case of a reaction
- The response of equilibria to conditions
- Response of equilibria to pressure:
- Response of equilibria to temperature
- Stability constant
- Competition Method
- Association and dissociation constants
- Micro-constants
- Hydrolysis constant
- Conditional constants
- Acid dissociation constant
- Gas Phase Equilibria
- Derivation of Stability Constant from Gibbâs Free energy (A case of a large system)
- Conclusion
- References


