Archive : Article / Volume 1, Issue 2
Case Report | DOI: https://doi.org/10.58489/2836-2187/007
A Review on Succession of Bioremediation Including Microbial Interventions for Reducing Heavy Metal Ions Contamination of Natural Environment
1Department of Biotechnology, SR Institute of Management & Technology, Bakshi ka Talab, Sitapur Road (NH 24), Lucknow 226201,U.P., India;
2Department of Biotechnology Era University, Lucknow-226003, U.P., India;
3Regional Food Research & Analysis Centre (RFRAC), Directorate of Horticulture & Food Processing, Udyan Bhavan Campus, 2- Sapru Marg, Lucknow-226001, U.P., India
Correspondng Author: Sanjay Mishra
Citation: J. James, Mohd. Ahmad, Uday K. Gupta, P.Jha, S. Maurya, P. Gupta, R. Pandey, Amit M. Tiwari, S.K. Chauhan and Sanjay Mishra, (2022). A Review on Succession of Bioremediation Including Microbial Interventions for Reducing Heavy Metal Ions Contamination of Natural Environment. Journal of Microbes and Research. 1(2). DOI: 10.58489/2836-2187/007
Copyright: © 2022, Sanjay Mishra, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-10-10, Received Date: 2022-10-10, Published Date: 2022-11-30
Abstract Keywords: Bioremediation, biosorption, contaminants, environment, heavy metals, microorganisms, organic matter, soil, water.
Abstract
As a consequence of urbanization and industrialization, it has been a global impact on water and soil profile. These activities like industry of heavy equipment works, traffic and waste increase the conductivity, TDS (Total Dissolved Solids), pH and redox potential in the water bodies like river, canals and ponds. Also,heavy metals have an adverseeffect on soil fertility. This overview covers the enthralling succession of âBioremediationâ at physiological, biochemical and molecular level. An enhancement of heavy metal in water bodies as well in the soil in close vicinity to industriesmakes it toxic and for controlling it, bioremediation is brought into function including a series of processes, which with the assistance of metal resistance microorganisms, concentrate followed by accumulation of metal like Zinc, Cadmium, Arsenic,etc. As there is still requirement for more studiesto develop bioremediation technologies in order to find more biological solutions for bioremediation of heavy metal contamination from different environmental systems, the collection of data concomitant with significant hypotheses together with this overview provides new insights into developing a platform to explore certain novel bioremediation experimental model that could berapid, precise and cost effective.
Introduction
In fact, heavy metal effluence is a serious concern because of hazardous impacts at even nano concentrations. Heavy metals are non-biodegradable, bioaccumulate in tissues and are biomagnified along with the trophic levels [1-3]. The nature of heavy metals releasedfrom the industrial waste depends on natureof industrial effluentand certain other
factors such as the innate chemical profile of the soil, climate, nature, and composition of the soil and other anthropogenic activities in the specificregion [2,4]. Subsequentreleases and the entry of heavy metals into thefood chain depend on their concentration and uptake by thelocal flora and fauna [2,5]. The genetic and epigenetic effects of these elements are associated with an increased risk of differentcancer types [1-4,6].Epigenetic mechanisms play an equally important if not a more prominent role than genetic events in carcinogenesis. These effects occur most frequently during the early stages of tumor development.Epigenetic measures includereversible modification of histone proteins and CpG islands of gene promoters that affect not only gene expression of germ and somatic cells, but also cause indirect gene-sequence changes [7,8].
In ranking the carcinogens, heavy metals have been classified by the International Agency for Researchon Cancer (IARC)and Environmental Protection Agency (EPA) as the first group, except for selenium that has been listed within group 3 (not carcinogenic to humans) of the IARC classification [9]. The impact of heavy metals on the qualityof water and soil due to urbanization and industrialization concomitant with Bioremediation applying certain potential devices has been overviewed under following headings:
Modes of Exposure to Toxicants
Animals includinghuman usually get exposed to the toxicants through: (a) respiratory (for gaseous and particulate matters); (b) the skin (chemicals able to cross skin barrier); (c) digestive tract (for food contaminants). Afterentering the body, the metal deposited in nasopharyngeal, tracheobronchial, or pulmonary compartments may be transported through the mucociliary action to the gastrointestinal tract. Macrophages phagocytethe wandering metals. Food is a principal source of essential and toxic elements. Some elements like mercury (Hg) are biologically magnified at higher trophic level. The dietary contribution for toxic metal intake has been extensively studied [10]. If an individual is deficient in minerals and trace elements its body will absorb heavy metals on their place. Every cell membrane breaks down and rebuilds every two weeks but does not releasethe heavy metals if essential fats are not properly ingestedor if poor quality fats are ingested. The liver that performs detoxification 100% of the time cannot perform this important task without a complete profileof essential nutrients.
Chemical elements present in the form of free ions are readilyionized and eventually get absorbed totallyby the body. Transition metals readily form stable covalentcomplexes and generallyintermingle as parts of macromolecules (proteins, enzymes, hormones,etc.) according to their chemical uniqueness including oxidation state [1,2]. The behavior of metal ion release into biofluid is regulated by the electrochemical rule. Released metal ions do not all the time combine with biomolecules to come out toxicity as active ion immediately combine with a water molecule or an anion in close proximity to the ion to form an oxide, hydroxide, or inorganic salt. Consequently, there is only a small chancethat the ion will merge with biomolecules to cause cytotoxicity, allergy, and other biological effects [11]. Health damage caused by toxic metals may be less (irritation) or acute (mutagenic, teratogenic and carcinogenic). These reactive elements of food fabricate complexes with fiber, reveal low solubility within the intestinal lumen and are weakly absorbed(Table 1). Absorption of these mineralsis augmented at low concentration of fiber, and in the absence of phytates and oxalates in the diet [12,13]. Micronutrients can interact with toxic metalsin the body at severalpoints: (a) absorption; (b) transport; (c) binding to target proteins; (d) metabolism; (e) sequestration; (f) excretionof toxic
Table 1: Food Sourcesof Toxic Metals
| Metal | Food Source |
| Pb | Egg, cocoapowder, rice, wheat,potato, calcium supplement, smoked food, wine,beer, milk, carrot, raisins |
| As | Green papaya,rice, tomato, carrot, seafood, Indian mustard, bovine and chickenmeat, wine, milk |
| Hg | Egg, mushroom, seafood, fish oil |
| Cd | Egg, fish, mushroom, garlic, spinach, wheat,rice, oat, corn,soyabean, peanuts, mushroom |
Source: Reference: [1,13] metals; and (g) finally in secondary symptoms of toxicity such as oxidative stress. The role of oxidative stress in the destruction of immune cells has been elucidated [14-17].Therefore, a diet poor in micronutrients can lead to enhancement in the toxicity. The prevalence and mortalitydue to multifactorial polygenic diseases;hypertension, coronary artery disease (CAD), diabetes and cancer vary depending upon enetic susceptibility as well as environmental pollutants generated as a consequence of numerous chemicals, metal ions and metalloids. Speedy changes in diet and lifestyle may manipulate heritability ofthe variant phenotypes that are dependenton the nutraceutical or functional food supplementation for their expression [18]. It is possible to recognize the interaction of specific nutraceuticals, with the genetic code possessed by all nucleated cells. There is evidence that South Asians have an increasedsusceptibility to CAD, diabetesmellitus, central obesity and insulin resistance at younger age, which may be due to interaction of gene and nutraceutical (especially micronutrients) environment. These populations appear to have enherited predisposition and may have interaction of internal nutritional status and environmental factors, mainly metal ions. Higher intake of refined starches and sugar increases generation of super oxide anion in the leucocytes and mononuclear cells, and free fatty acids (FFA), as well as higher amount and activity of nuclear factor-kB (NF-kB), a transcriptional factor regulating the activity of at least 125genes, most of which are pro-inflammatory [18]. Glucose intake also causes an increasein two other pro- inflammatory transcription factors; activating protein-1 (AP- 1) and early growth response protein-1 (Egr-1), the first regulating the transcription of matrix metallo-proteinases and the second modulating the transcription of tissue factor and plasminogen activator inhibitor-1. Refined food, mixed meal inducesactivation of NF-kB associated with free radicals’ generation by mononuclear cells. The superoxide anion is an activator of at least two major pro inflammatory transcription factors, NF-kB and AP-1. Increased intake oflinoleic acid, saturatedfat, trans fat and refinedstarches and sugars can increase the generation of free radicals and activate the NF-kB, leadingto rapid expression of proinflammatory genes. It is possible that nutraceuticals; antioxidants, micronutrients, minerals, vitamins, coenzymeQ10 and w-3 fatty acids may inhibit the generation of super oxideand suppress NF-kB as well as AP-1, and Egr-1 leading to suppression of phenotypic expressions. It is known that genes are important in determining enzymes, receptors, cofactors, structural components involvedin regulation of blood pressure,the metabolism of lipids, lipoproteins and inflammatory and coagulation factors that are involvedin determining individual risk for vasculardiseases and diabetes.It seems that these phenotypic expressions may be silenced by targeting simple sequence differences known as single nucleotide polymorphisms (SNP) by nutraceuticals and slowly absorbed wild foods or functional foods enriched with certain protective macro- or micro-nutrients as well as nutraceuticals [18].
In biological fluids and tissues,the majority of metals and metalloids are not present as free cations. In blood they are generally bound to red cells or to plasma proteins. Lead and cadmium are almost totally bound to red blood cells. The chemicalelements bound to plasma proteins constitute the fraction available for transport into and out of the tissues. Albumin, a plasma protein, has an enormous capacityto bind several metals.
Endurable Daily Intake Approach
In view of avoidingundesirable health hazardsconsequent of "excessive" intake of toxicants(including toxic metals),international and nationalscientific organisms such as FAO/WHO, FDA, European Union, etc have used the safety factor approach for establishing acceptable or tolerableintakes of substances that exhibit threshold toxicity [19,20]. The acceptable daily intake (ADI) or Endurabledaily intake (EDI) or provisional endurable weekly intakes(PEWI) are used to describe"safe" levels of intake for several toxicants including toxic metals [21]. For chemicals that give rise to such toxic effects, a tolerable daily intake (EDI), i.e., an estimateof the amount of a substance in food, expressed on a body weight basis (mg.kg-1 or mg.kg-1 of body weight)that can be ingested over a lifetime without appreciable health risk. Exposureover and above the TDI value for short periods has not been reported to reveal deleterious effects on human health [19-21].However, acute effectsmay occur if the TDI is substantially exceeded even for short periodsof time. Besides,contaminants possessing very long half-lives can be accumulated in the body and chronic effectsare most often observed when critical concentrations are reached in target tissues.The comprehensive accountof health hazards rendered principally by aluminium (Al), arsenic (As),cadmium (Cd), lead (Pb), mercury (Hg), Selenium (Se) and Lithium(Li) is represented as follows:
- Aluminium: The compoundsof this elementhave a wide range of applications in different industries, including cosmetics, and food additives[22]. Aluminum-induced carcinogenesis is related to its abilityto bind to the estrogenreceptor and mimic estrogen functions, therefore its named metallo-estrogen [22]. Metallo-estrogen triggers expression in genes that contain estrogen responsive-element (ERE) on their promoters. In mammary gland cells, this gives rise to an increasein the number of divisionsof breast cells,thus increasing replication errors in cancer-related genes [23]. It has been shown that if antiperspirants containing aluminum applied on the skin around the underarm and breast areas are not effectively washed, some aluminum salts remains in the area. This gives rise to continuous exposure,and enhances the risk of breast cancer [24].
- Arsenic: Arsenic is mostly known as an epigenetic carcinogen metalloid when in the form of an inorganic compound. Trivalent arsenite (As+3) has more carcinogenic properties than the pentavalent arsenate (As+5) [25, 26]. Trivalent arseniccan bind with high affinityto thiol groupsof proteins and reduced glutathione (GSH) [27]. Long time uptake of drinking-water containing low levels of arsenite, induces carcinogenesis in skin, lung, bladder, and kidney tissues,resulting from alteration in multiple signalingpathways [28].
- Cadmium: Certain compounds of cadmium (Cd) are highly toxic to humans. Cadmium is employed in several industrial processessuch as: (a) protective coatings(electroplating) for metals like iron; (b) preparation of Cd-Ni batteries, control rods and shields within nuclear reactors and television phosphors. Cadmium is a cumulative toxicant and carcinogenic that affects kidneys, generates various toxic effects in the body, disturbs bone metabolism and deformsreproductive tract as well as endocrine system[1,29]. There are several morphopathological changes in thekidneys due to long-term exposureto cadmium. Increasing intakes of zinc can reduce the renal toxicity of cadmium. An exposure to cadmium increasescalcium excretion thus causes skeletal demineralization, probably leading to increases in bone fragility and risk of fractures [30]. Cadmium and its compounds are currently classified by IARC as a Group 1 carcinogen for humans. Cadmiumexposure, during human pregnancy, leads to reduced birth weightsand premature birth[31].
- Lead: Lead (Pb) is used in storage batteries, cable coverings, plumbing, ammunition, manufacture of tetraethyl Pb, sound absorbers, radiationshields around X-ray equipment and nuclear reactors, paints, while the oxide is used in producing fine "crystal glass" and "flint glass" with a high refractive index for achromatic lenses, solder and insecticides. Lead enters the human body in many ways. It can be inhaled in dust from lead paints,or waste gases from leaded gasoline. It is found in trace amounts in various foods, notablyfish, as a consequence of inefficient and non- hygienic food processing. Plants can absorb Pb from soils and from a PbEt4 traffic-induced air pollution (90 % of totalPb emissions into the atmosphere). Pb can contaminate water and consequently enter the aquatic food chains [32]. Nevertheless, lead (Pb) toxicity has been a thrust area for environmental scientists because of its toxic effect on plants, animals, and humans [33]. An enhancement in several Pb related industrial activities and use of Pb containing products such as agrochemicals, oil and paint, mining, etc. can result in Pb contamination in the environment and thus,can enter the food chain [33]. Among different Pb- remediation approaches, certain advanced approaches such as microbialassisted phytoremediation have been highlighted. It could possibly minimize the Pb load from the resources in a sustainable manner and would be a viable option to ensure a safe food production system [33]. Being one of the most toxic heavy metals,Pb ingestion throughthe food chain has proven to be a potential health hazard for both plants and humans [34]. Research work on Pb toxicity and its effects on plants, soil, and human health have been comprehensively updated [34].
- Selenium: Dietary selenium (Se) supplementation with differentorigin and chemicalforms is generallyuse for overcoming selenium deficiency and maintaining high productive and reproductive performance of farm animals.Excess amount of selenium is found as pro-oxidant and can be toxic for all animal species and man depending on the dose and duration of intake. The precise mechanism of selenium toxicityis still obscure;however, there are a number of proposalslike oxidative stress mechanism, supportedby in vitro as well as in vivo outcome [35].
- Mercury: Mercury (Hg) and its compounds are highly toxic, especially methylmercury - a potent neurotoxin. It has caused a significant number of human fatalities in several accidents around the world. Due to its wide dispersion through the atmosphere, Hg is considered a global pollutant, being deposited even in remote pristine aquatic systems, where it is biomagnified through the food chain. Hg and its compounds are highly toxic, have wide dispersion through the atmosphere. It is biomagnified through the food chain. Hg use in dental amalgams, thermometers, barometers, and the development of large-scale industrial processes (e.g., chlor-alkali plants and PVC production) and release into the environment. Hg occurs in nature in mineral, cinnabar,metacinnabar and hyper cinnabar [36]. Diet can be the major source of inorganic and organomercurials particularly seafood, while dental amalgams have been noticed to be the foremost exposure source to elemental mercury [36]. Mercury is organomercurial in the form of methylmercury,which have toxicological uniqueness. Mercurial fungicides treated wheat seeds cause poisoning and death of 5,000 to 50,000 people [19]. Tokuomi et al. [37] were the first to describe the symptoms of methylmercury poisoning. Thus,the symptoms were named the Hunter-Russell syndrome. Elemental Hg can be oxidized to Hg2+ that accumulates preferentially in the kidneys. The increased excretion of low molecular-weight proteins confirmed at low-level exposure, and related to damage to the renal tubes. It is a potent neurotoxin to human due to their ability to cross the blood- brain barrier. It is absorbedin the gastrointestinal tract, immediately entering the blood stream, very often, affecting the developing nervous system of the foetus [19].
- Lithium: Lithium (Li) is transferred in the food chain from soils via flora and fauna to human beings. Till date, it is not measured as an essentialelement for animal and human. The postulated normative lithium necessities amount to < 100>
- Nickel: Water-insoluble nickel compounds including nickel sulfides, disulfides, and oxides readily enter the cell and are very potent carcinogens [40]. In contrast, water-soluble nickel compoundsincluding acetate, chloride,nitrate, and sulfatedo not enter the cells as readilyas water- insoluble nickel compounds [41]. The increase in the usage of nickel compounds and the spread of nickel due to itsdissolution from nickel ore-bearing rocks are the main causes of nickel presence in the environment. The primary source of nickelin drinking-water is the leachingof metals in water network [42]. However, food is the major source of nickel exposure in the non-smoking, non-occupationally exposed population, but nickel absorption from water, was significantly higher than absorption of nickel from beverages like tea, coffee, or orange juice and milk [42]. Ni2+ induces carcinogenesis through several processes including DNA hypermethylation (H3K9 mono- and dimethylation), DNMT inhibition, DNA mutation, ROS generation, inhibiting histone H2A, H2B, H3 and H4 acetylation, converting the tumor suppressor genes to the heterochromatin, and substantialincreases of the ubiquitination of H2A and H2B [43]. Therefore, nickel plays an important role in the suppression or silencing of genes.
- Chromium: Trivalent chromiumis an epigenetic carcinogen factor since it can form stable compounds withmacromolecules such as DNA and cysteine residueof proteins and glutathione [44]. The trivalentform of chromium cannot pass the cell membrane; however, the hexavalent salts are able to enter the cell and are converted to the trivalent form [45]. Thus, dependingon the situation, reducing agents can affect carcinogenic properties of chromium and inside the cell, chromium(VI) can be converted to a carcinogen. During Cr (VI) reduction, many compounds such as oxygen radicals,DNA inter-strand cross links (ICLs), and single-strand breaks (SSBs) may be formed [45].ICLs act as physical barriersto DNA replication and transcription events, thus inducing apoptosis [46]. The chromium carcinogenicity, particularly in lung epithelial cells and fibroblasts, is imposed through hypermethylation of a putative promoter, namely,CYP1A1 promoter [46]. Chromium recruit’shistone deacetylase 1 (HDAC1) and DNMT1, especially to CYP1A1 promoter,and this assemblyrecruits BP1 and inhibits CYP1A1 gene expression [46]. CYP1A1 issignificant in the metabolism of carcinogens such as polycyclic aromatichydrocarbons (PAHs) and heterocyclic amines that are widely distributed widely in our environment through automobile exhausts, cigarette smoke,charcoal-broiled cooking, and industrial waste.In contrast to other cytochrome P450 enzymessuch as epoxidehydrolase and dihydrodiol dehydrogenase that are involved in PAH- and Benzo (α)pyrene- induced carcinogenesis, CYP1A1 inhibits PAH carcinogenesis. Thus, inhibition of CYP1A1 by chromium leads to the production of a PAH [45]. PAHs have an important role in the activation of cytosolic ligand-activated transcription factor named aromatic hydrocarbon receptor (AhR) [47]. After formation, the PAH-AhR complex is transferred into the nucleus.In the nucleus, PAH is detached from the complex and AhR binds to its nuclearpartner, Arnt. This new complex acts as a transcription factor and interacts with DRE of CYP1A1 gene, leading to the activation of CYP1A1 gene expression, thus causing bioactivation of exogenous procarcinogens of both hepatocellular and lung carcinomas [48]. It is interesting that PAH through binding to transcription factor AhR, activates CYP1a1 gene expression, and CYP1A1 inhibitsPAH carcinogenesis, but in the presence of Cr, the promoter of CYP1a1 is inactivated and PAH can act as carcinogens.Benzo(α) pyrene is also a member of polycyclic aromatic hydrocarbon (PAHs) family that is metabolically transformed from its pro-carcinogenic status to the carcinogenic metabolite [(BP-7,8-dihydrodiol-9,10- epoxide (BPDE)], that can bind covalently to DNA and form BPDE–DNA adducts and reactive oxygen species (ROS) [49]. BPDE activates apoptosis through p53 –independent and – dependentmanner [48]. P53 dependent Cr-induced apoptosis takes place by increasing p53 phosphorylation at serine 392, as well as up-regulation of pro-apoptotic gene bcl-XS, and caspase-7, and down-regulation of several anti-apoptoticgenes from Bcl2-family (bcl-W and bcl-XL), and bax. These apoptotic events result in the destruction of the mitochondria and release of cytochrome c [50, 51]. Moreover, Cr induces the ATM proteinproduction, which phosphorylates and activates Chk2 protein. The phosphorylated Chk2 in turn phosphorylates and activates p53. The phosphorylated p53 does not bind to MDM2 protein [52]. Cr exposure at very high concentrations activatesall subclasses of MAPK throughphosphorylation; therefore, Cr acts as a MAPK kinase and increases survival/proliferation in a dose- dependent manner.This function is associated with its abilityin ROS generation [53].
Bioremediation Approach for Heavy Metal Pollution
- Soil: Bioremediation has been considered as one of the safer, cleaner, cost- effective and eco- friendly technology for makingheavy metals’ polluted sites free of contamination [54].The term bioremediation has been introduced to describe the process of using biological agent to remove toxic waste from environment. The process of bioremediation uses various agents such as bacteria, fungi,algae and higher plants as major tools in treating heavy metals present in the environment [3, 54-56]. Bioremediation, both in situ and ex-situ have also enjoyed strong scientific growth, in part due to the increased use of natural attenuation, since most natural attenuation is due to biodegradation [55]. Bioremediation has also considered as a solution for emerging contaminant problems. Microbes are very helpfulto remediate the contaminated environment [54, 55]. Soil heavy metal pollution is difficult to control due to its strong toxicity, wide distribution, and easy transformation [56]. The remediation of heavy metal contaminated soil is a long-term and arduous process.Besides, remediation technology has been constantly developing, from a single remediation to hybrid technology revealing a single effect to a combination of advantages, respectively. The restoration is shifting from high-cost technologies with obvious side effectsto low-cost, green and environmentally-friendly technologies, and new and more efficientrestoration materials are continuously synthesized from ordinary materials. Among all remediation technologies, compared with physical and chemical remediation methods, microbial remediation can be carried out in situ, which has been both cost-effective and eco-friendly. Besides,microbial remediation is an improvement of natural processes and thus generally does not produce secondary pollution, with a wide range of application prospects. But meanwhile, there are disadvantages such as long-timeconsumption, specificity in most cases, and small remediation scope.Therefore, when selectingremediation technology, environmental, time, and economicfactors should be considered comprehensively to choose the most suitable remediation plan [3, 54-58].
- Water: Mercury removalfrom synthetic wastewater using a bioreactor has been systematically documented [59]. The wastewater bioremediation is reliant on various factors e.g., pH [60]. The pH affects their bioavailability by affectingthe solution chemistrythrough processes like complexation, hydrolysis, redox as well as precipitation [61]. Microbial biomasssurface area and pretreatment processes (modifying the surfacearea) tend to influence the bioremediation process[62]. Microbial biomassmay be required to be immobilized in matrices like alginate and silica gel to developa suitable commercial bio sorbent with appropriate strengthand porosity [62]. Encapsulation commences physicochemical stability as well as heat resistance. Encapsulated Agrobacterium sp. in alginatewith nanoparticles of Fe has revealed excellentadsorption ability for continuously five cycles [63]. Poor selectivity and hurdles in recycling biomassare some of the limitations of the process. Bioremediation has furtherbeen mediated throughmicrobial biofilms with high resistance and tolerance for metal ions. Rhodotorula sp. has been reported to have a removal efficiency of up to 95%.
- Air: The air pollution of toxic heavy metals has been considered one of the most significant environmental issues which have accelerated noticeably due to altering industrial activities [63].Various most common techniques, strategies, and biological approaches of heavy metal bioremediation have been practiced so far [64]. Besides, certain specific roles of microorganisms in the bioremediation of heavy metals in polluted air have been well elucidated [65; Figure I.] Advanced methods of heavy metal remediation include physicochemical and biological methods; the latter can be further classified into in situ and ex situ bioremediation. The in-situ process includesbioventing, biosparging, biostimulation, bioaugmentation, and phytoremediation [65]. Ex situ bioremediation includes land farming, composting and biopiles. Bioremediation uses naturally occurringmicroorganisms such as Pseudomonas, Sphingomonas and Rhodococcus, Alcaligenes. In general, bioremediation is of very less effort,less labor intensive, cheap, eco- friendly,sustainable as well as comparatively easy to implement. Most of the disadvantages of bioremediation narrate to the slowness and time-consumption; additionally,the products of biodegradation sometimes become more toxic than the original compound.The performance evaluation of bioremediation might be difficult as it has no acceptable endpoint. There is still requirement for more studies to develop bioremediation technologies in order to find more biological solutions for bioremediation of heavy metal contamination from different environmental systems including air [65].
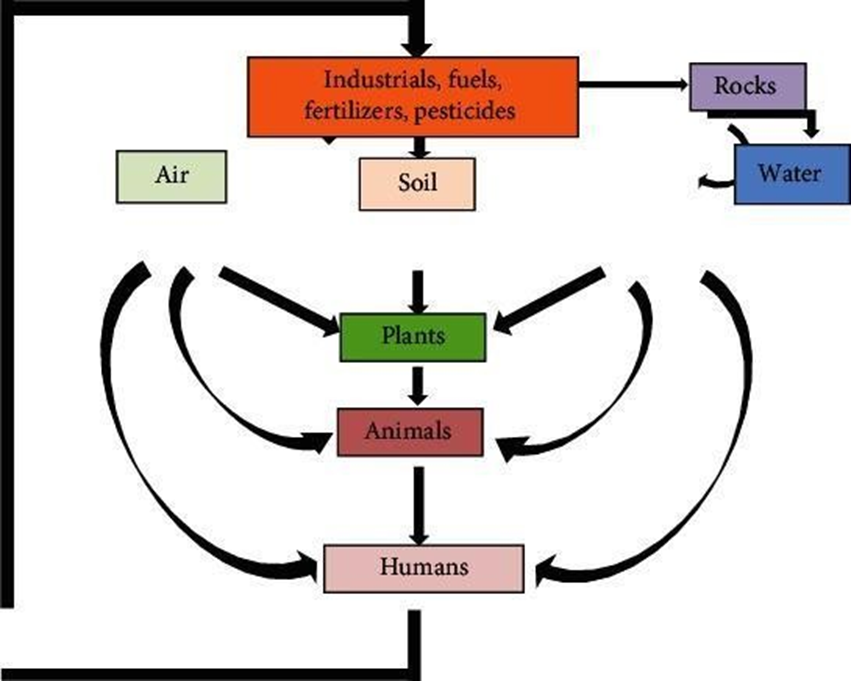
Microbial Mechanism Implicated in Heavy Metal Bioremediation
Recently,a range of processes of heavy metal bioremediation, such as biosorption, bioleaching, biomineralization, biotransformation, and intracellular accumulation, as well as the application of genetically modifiedmicrobes and immobilized microbial cells for heavy metal bioremediation, have been well overviewed [66]. For the elimination of heavy metal ions from the polluted sites,bioremediation methods are in practice[67]. Generally, these techniques engrossthe absorption/adsorption of toxic metal ions, reducingassociated side effects[68]. In additionto various natural resources like wood bark/dust, coconut husk/shells, agro wastes, seaweeds,seeds, discarded coffee beans, and aquatic plants,microorganisms are beingused frequently to reduce the number of metal ions from the place of their source,in which microbes(algae, fungi, bacteria,yeasts, etc.) play a pivotal role [69]. Microbes transform the heavy metals’ionic state ultimately influencing the solubility, bioavailability and movementin the soil as well as in the aquaticambiance [70]. Mobilization or immobilization of heavy metals assists microbialremediation that is next followedby oxidation-reduction, chelation, modification of the metallic complex,and biomethylation [67]. The enzymatic catalysis by microbes solubilizes the metals with higher oxidation state to lower oxidation state, e.g., Thiobacillus ferrooxidans and Serratia, are commonly applied[71, 72]. Metal transformation, immobilization, chelation, or solubilization is supported by the fabrication of exopolysaccharides by plant growth promoting bacterium(PGPB), like oxidases,reductases, siderophores, and organic acids, thus, promotingphytoremediation of heavy metal ions. PGPB reduces the pH of the soil by producing organic acids, ultimately aiding in the removal of heavy metal ions. Metal resistant siderophore-producing bacteriafound in the vicinity of rhizosphere deliver nutrients to the plants, namely, iron,possibly reducing the negative consequences of metal pollution [73, 74]. Siderophore (metal-chelating agents with low molecular masses, ranging 200–2000 Da, produced by microorganisms and plants,especially under Fe-limiting conditions) is also accountable for the formation of stable complexeswith radionuclides and metals concerning environment like Cd, Ga, Al, Cu, Zn, In, and Pb [75, 76]. The synergistic effects of bioaugmentation and phytoremediation leading to rhizoremediation may triumph over the difficulties that arise while both processes are employed distinctly. Furthermore, the remediation of heavy metals with the aid of higher plants has also been reported [76, 77]. It has also been observedthat planting Salix in Cd-polluted soil improved the diversity of beneficial microbes, such as thebacteria genera Arthrobacter and Bacillus [77]. Plant- associated microorganisms play an important role in controlling heavy metal uptake and accumulation in aerial parts. The microbial community and its interaction with Cd accumulation by willow were assessedto explore the association of phytoextraction efficiency and rhizosphericmicrobial populations [77]. Therefore, the rhizosphere microbial compositions of three willow genotypes grown in two Cd polluted sites were investigated, focusing on their interactions with phytoremediation potential. Principal coordinate analysisrevealed a significant effect of genotypeon the rhizosphere microbial communities [77]. Distinct beneficial microorganisms, such as plant growth promoting bacteria (PGPB) and mycorrhizal fungi, were assembled in the rhizosphere of different willow genotypes. Linear mixed models showed that the relative abundance of PGPB was positively associated (p < 0.01) with Cd accumulation, since these microbes significantly increased willow growth. The higher abundance of arbuscular mycorrhizal fungi in the rhizosphere of Salix × aureo-pendula CL 'J1011' at the Kejing site, showed a negative correlation with the Cd content, but a positive correlation with biomass [77]. On the other hand, mycorrhizal fungi, were more abundant in the rhizosphere of S. × jiangsuensis CL. 'J2345' and positively interrelated with the Cd content in willow tissues. This study provides new insightsinto the distinctive microbial communities in rhizosphere of different willow genotypes,which may probably be consistent with the phytoremediation prospective [77].
Advantages and Disadvantages of Bioremediation
Bioremediation is a straightforward procedure used by several researchers in the waste treatment processfor contaminated environments such as soil and water. The microbial organisms that degrade the contaminant augmentin numbers and release harmlessproducts. The residuesfor the treatmentare generally harmlessproducts, namely, carbondioxide, water and cell biomass.Bioremediation is of basically very less effort, less laborious, as well as cost effective compared to other methods that are in practice for the removal of hazardous waste.Besides, bioremediation is ecofriendly, sustainable, reasonably easy to implement, and useful for the total destruction of a wide range of contaminants [78]. Many hazardouscompounds can be altered into harmless products. Furthermore, bioremediation can be implemented on the site of contamination itself without causing a principal disruption of standard activities.There is no need of transporting huge numbers of waste off- site, there is no latent human health risk, and the environment will continue uncontaminated. The majority ofthe disadvantages of bioremediation narrateto it requiring a longer time to be accomplished as compared with other options, namely,excavation and removingpollutants from the site. Moreover, there is a complexity of bioremediation in treating inorganiccontaminants and in ascertaining whethercontaminants have been perfectly destroyed or not. In addition, there is a sluggishness of highly chlorinated materials biodegradation and generation of extra toxic or carcinogenic by-products [79]. Lastly, the products of biodegradation sometimesbecome more toxic than the original compound. Its biological processes are also highly specific with efficient site factors including the presence of microbial populations, growth conditions, and quantity of nutrients concomitant with pollutants [65, 80-82].
Conclusion
Bioremediation techniqueis still a useful,natural, and environmentally friendlyprocess in which the polluted environment is biologically biodegraded. Microorganisms play a pivotalrole in the removal of heavy metalspollutants. The heavy metals (e.g.,mercury, silver, lead, cadmium, and arsenic) exert toxic effects on living cells. Examples of degradative aerobic bacteriaare Pseudomonas, Alcaligenes, Sphingomonas, and Rhodococcus. Certain technical strategies i.e., microbe- based as well as hybrid have been discussed, which are presently being applied to mitigate heavy metal contamination in soils and other contaminated surroundings like air. Because of the contribution in the regulation of biogeochemical cycles,influencing climate, soil structure, and fertility, the environmental microbiome is notion to play a pivotal role. Besides, fungus microorganisms can efficiently degrade numerous toxic environmental pollutants. Nevertheless, phytoremediation represents an emerging technology all the way through that plants can be employed to eradicate pollution from soil, water, and air. Bioremediation is of very less effort,less laborious, cost effective, eco-friendly, sustainable, and comparatively easy to implement. The majority of the disadvantages of bioremediation narrate to the slowness and time-consumption; moreover, the products of biodegradation occasionally becomeextra toxic than the originalcompound. Bioremediation may be restricted by irregularity and uncertainty of totality. Though,sustainable policies have been developed and revised frequently, the performance evaluation of bioremediation might be complicated as there is no up to standard endpoint.As there is still requirement for more studies to developbioremediation technologies in order to find more biological solutionsfor bioremediation of heavy metal contamination from diverse environmental systems, the collection of data concomitant with significant hypotheses together with this overviewprovides new insightsinto developing a platform to explore certain novel bioremediation experimental model that could be rapid, preciseand cost effective. Nevertheless, awareness of the negative effects, as well as awarenessof how to reduce heavymetals pollution in the environment (soil, air and water)should be expanded.
Declaration
This review article is an extended and updated versionof the paper [Mudgal,V., Madaan,N., Mudgal, A., Singh, R.B., Mishra, S. (2010). Effect of
Toxic metals on human health.The Open Nutraceuticals Journal3: 94-99.
Conflict of Interests
Authorsdeclare no conflictof interests.
Acknowledgement
This article is the consequent of joint ventureamongst Department of Biotechnology, SR Institute of Management & Technology, Bakshi Ka Talab, Lucknow- 226201, U.P., India; Department of Biotechnology, Era University, Lucknow226003, U.P., India; and Regional Food Research & Analysis Centre (RFRAC), Lucknow-226001, U.P., India. Authors are grateful to Mr. PawanSingh Chauhan, Chairman, SR Institute of Management & Technology, Bakshi Ka Talab,Lucknow- 226201, U.P., India for his generoussupport and throughout inspiration for accomplishment of this study. Besides, authorsare thankful to members of Board of Directors, SR Institute of Management & Technology, Bakshi Ka Talab, Lucknow- 226201, U.P., India for providing necessary facilities and time-to-time encouragement for exploring the R&D in the area of Biotechnology.
References
- Mudgal, V., Madaan, N., Mudgal, A., Singh, R.B., Mishra, S. (2010). Effect of toxic metals on human health. The Open Nutraceuticals Journal 3: 94-99.
- Mishra, S., Dwivedi, S.P. and Singh, R.B. (2010). A review on epigenetic effect of heavy metal carcinogens on human health. The Open Nutraceuticals Journal 3: 188-193.
- Kaphi, M. and Sachdeva, S. (2019). Bioremediation options for heavy metal pollution. Journal of Health & Pollution 9 (24): 191203-191222.
- Tchounwou, P.B., Yedjou, C.G., Patlolla, A.K. and Sutton, D.J. (2012). Heavy metaltoxicity and the environment. Exp Suppl. 101:133-64.
- Leivadara, S.V., Nikolaou, A.D. and Lekkas, T.D. (2008). Determination of organic compounds in bottled waters. Food Chem. 108: 277-86.
- Bower, J.J., Leonard, S.S. and Shi, X. (2005). Conference overview: Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 279: 3-15.
- Jones, P.A. and Baylin, S.B. (2002). The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3: 415-428.
- Vaziri, G.A., Mohammadi, A. and Heidari, M. (2007). In: Molecular Genetics of Cancer, Samer, Tehran, Iran; ISBN: 978-964-91351-0-6.
- Mohammadi, A., Vaziri, G. A., Shakibaie, M.R. (2008). Mutations in tumor suppressor TP53 gene in formalin- fixed, paraffin embedded tissues of squamous cell carcinoma (SCC) of lung cancer. Am. J. Biochem. Biotechnol. 4(1): 1-6.
- Santos, E.E., Lauria, D.C., Porto da Silveira, C.L. (2004). Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Total Environ. 327: 69-79.
- Hanawa, T. (2004). Metal ion release from metal implants. Mater Sci Eng C 24(6-8): 745-752.
- Hazell, T. (1985). Minerals in food: dietary sources, chemical forms, inter[1]actions, bioavailability. World Rev Nutr Diet 46: 1-123.
- Mishra, S., Chauhan, S.K., Nayak, P. (2021). Physiological, biochemical, biotechnological and food technological applications of Mushroom: An overview. IOSR Journal of Biotechnology and Biochemistry (IOSR-JBB) 7 (1): 39-46.
- Gil, L., Martinez, G., Gonzalez, I., et al. (2003). Contribution of characteriza[1]tion of oxidative stress in HIV/AIDS patients. Pharmacol Res. 47: 217-224.
- Gil, L., Martinez, G., Tarinas, A., et al. (2005). Effect of increase of dietary micronutrient intake on oxidative stress indicators in HIV/AIDS patients. Int. J. Vitam. Nutr. Res. 75: 19-27.
- Sharma, S., Kalim, S., Srivastava, M.K., Nanda, S., Mishra, S. (2009). Oxidative stress and coxasackie virus infection as mediators of beta cell damage: a review. Sci. Res. Essays 4: 42-58.
- Mishra, S., Dwivedi, S.., Dwivedi, N., Singh, R.B. (2009). Immune response and possible causes of CD4+ -T cell depletion in human immunodeficiency virus HIV-1 infection. Open Nutra. J. 2: 46-51.
- Mishra, S., Singh, R.B., Dwivedi, S.P., et al. (2009). Effects of nutraceuticals on genetic expression. Open Nutra. J. 2: 70-80.
- Silva, A.L.O., Barrocas, P.R.G., Jacob, S.C., Moreira, J.C. (2005). Dietary intake and health effects of selected toxic elements. Braz. J. Pl. Physiol. 17 (1): 79-93.
- Dolan, L.C., Matulka, R.A., Burdock, G.A. (2010). Naturally occurring food toxins. Toxins 2: 2289-2332. doi:10.3390/toxins2092289
- Kroes, R., Kozianowski, G. (2002). Threshold of toxicological concern in food safety assessment. Toxicol Lett. 127: 43-46.
- Darbre, P.D. (2005). Aluminium, antiperspirants and breast cancer. J. Inorgan. Biochem. 99: 1912-1919.
- Sun, X., Fontaine, J.M., Bartl, I., Behnam, B., Welsh, M.J., Benndorf, R. (2007). Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor-positive breast cancer cells. Cell Stress Chaperones 2: 307-319.
- Stellman, S.D., Djordjevic MV, Britton, J.A., et al. (2000). Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York. Cancer Epidemiol Biomarks Prev. 9: 1241-49.
- Patterson, T.J., Ngo, M., Aronov, P.A., Reznikova, T.V., Green, P.G., Rice, R.H. (2003). Biological activity of inorganic arsenic and antimony reflects oxidation state in cultured human keratinocytes. Chem Res Toxicol. 16: 1624-1631.
- Alkahtani, S. (2009). Antioxidation and hypomethylation effects on genotoxicity and programmed cell death induced in mice somatic cells by arsenic trioxide. J. Biol. Sci. 9 (7): 721-729.
- Suzuki, K.T., Katagiri, A., Sakuma, Y., Ogra, Y., Ohmichi, M. (2004). Distributions and chemical forms of arsenic after intravenous administration of dimethylarsinic and monomethylarsonic acids to rats. Toxicol. Appl. Pharmacol. 198: 336-344.
- Jensen, T.., Wozniak, R.J., Eblin, K.E., Wnek, S.M., Gandolfi, A.J., Futscher, B.W. (2009). Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transforma[1]tion. Toxicol Applied Pharmacol. 235(1): 39-46.
- Genchi, G., Sinicropi, M.S., Lauria, G., Carocci, A., Catalano, A. (2020). The effects of cadmium toxicity. Intl. J. Env. Res. Public Health 17: 3782-3805. doi:10.3390/ijerph17113782
- Wu, X., Jin, T., Wang, Z., Ye, T., Kong, Q., Nordberg, G. (2001). Urinary calcium as a biomarker of renal dysfunction in a general population exposed to cadmium. J. Occup. Environ. Med. 43: 898-904.
- Henson, M.C., Chedrese, P.J. (2004). Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 229: 383-92.
- Kaste, J.M., Friedland, A.J., Sturup, S. (2003). Using stable and radioactive isotopes to trace atmospherically deposited Pb in montane forest soils. Environ. Sci. Technol. 37: 3560-3567.
- Kumar, A., Cabral-Pinto, M.M.S., Chaturvedi, A.K., et al. (2020). Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 17(7): 2179-2211. doi: 10.3390/ijerph17072179.
- Cabral-Pinto M.M.S., Inácio M., Neves O., et al. (2020). Human health risk assessment due to agricultural activities and crop consumption in the surroundings of an industrial area. Expo. Health 12: 629-640.
- Mézes, M., Balogh, K. (2006). Selenium supplimentation in animal and man - positive effects and negative consequences. International Symposium on Trace Element in the Food Chain, pp. 9-15.
- Gliozzo, E. (2021). Pigments- Mercury-based red (cinnabar-vermilion) and white (calomel) and their degradation products. Archaeological and Anthropological Sciences 13: 210-262.
- Tokuomi, H., Kinoshita, Y., Teramoto, J., Imanishi, K. (1977). Hunter-Russel Syndrome. Nippon Rinsho 35(1): 518-519.
- Shen, J., Li, X., Shi, X. et al. (2020). The toxicity of lithium to human cardiomyocytes. Environ. Sci. Eur. 32: 59-70. doi:10.1186/s12302-020-00333-6
- Ermidou-Pollet, S., Pollet, S. (2006). Neuroprotective effects of lithium. International Symposium on Trace Element in the Food Chain, pp. 357-361.
- Dunnick, J.K., Elwell, M.R., Radovsky, A.E., et al. (1995). Comparative carcinogenic effects of nickel subsulfide, nickel oxide, or nickel sulfate hexahydrate chronic exposures in the lung. Cancer Res. 55: 5251-5256.
- Abbracchio, M.P., Heck, J.D., Costa, M. (1982). The phagocytosis and transforming activity of crystalline metal sulfide particles are re[1]lated to their negative surface charge. Carcinogenesis 3: 175- 180.
- WHO. (2005). Nickel in Drinking-Water. Background document for development of WHO guidelines for drinking-water quality.
- Ke, Q., Ellen, T.P., Costa, M. (2008). Nickel compounds induce histone ubiq[1]uitination by inhibiting histone deubiquitinating enzyme activity. Toxicol. Applied. Pharmacol. 228: 190-199.
- Wei, Y.D., Tepperman, K., Huang, M.Y., Sartor, M.A., Puga, A. (2004). Chromium inhibits transcription from polycyclic aromatic hydrocarbon[1]inducible promoters by blocking the release of histone deacetylase and preventing the binding of p300 to chromatin. J. Biol. Chem. 279: 4110-4119.
- Schnekenburger, M., Peng, L., Puga, A. (2007). HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor[1]mediated trans-activation. Biochim. Biophys. Acta. 1769: 569- 578.
- Wu, J.P., Chang, L.P., Yao, H.T., et al. (2008). Involvement of oxidative stress and activation of aryl hydrocarbon receptor in elevation of CYP1A1 expression and activity in lung cells and tissues: An in vitro and in vivo study. Toxicol. Sci. 107: 385-393.
- Nebert, D.W., Roe, A.L., Dieter, M.Z., Solis, W.A., Yang, Y., Dalton, T.P. (2000). Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control and apoptosis. Biochem. Pharmacol. 59: 65-85.
- Li, R., Shugart, Y.Y., Zhou, W., et al. (2009). Common genetic variations of the cytochrome P450 1A1 gene and risk of hepatocellular carci[1]noma in a Chinese population. Eur. J. Cancer. 45: 1239-1247.
- Drukteinis, J.S., Medrano, T., Ablordeppey, E.A., Kitzman, J.M., Shiverick, K.T. (2005). Benzo[α]pyrene, but Not 2,3,7,8-TCDD, induces G2/M cell cycle arrest, p21CIP1 and p53 phosphorylation in human choriocarcinoma JEG-3 cells: A distinct signaling pathway. Placenta 26: S87-S95.
- Carlisle, D.L., Pritchard, D.E., Singh, J., et al. (2000). Apoptosis and P53 induc[1]tion in human lung fibroblasts exposed to chromium (VI): effect of ascorbate and tocopherol. Toxicol. Sci. 55(1): 60-68.
- Ceryak, S., Zingariello, C., O’Brien, T., Patierno, S.R. (2004). Induction of pro[1]apoptotic and cell cycle-inhbiting gene in chromium (VI)-treated human lung fibroblasts: lack of effect of ERK. Mol. Cell. Biochem. 255: 139-149.
- Ha, L., Ceryak, S., Patierno, S.R. (2003). Chromium (VI) activates ATM: requirement of ATM for both apoptosis and recovery from terminal growth arrest. J. Biol. Chem. 278: 17885-17894.
- Gao, N., Jiang, B.H., Leonar, S.S., et al. (2002). p38 signaling-mediated hy[1]poxia-inducible factor 1 and vascular endothelial growth factor induction by Cr (VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 277: 45041-45048.
- Akshata Jain, A.N., Udayashankara, T. H., Lokesh, K. S. (2014). Review on bioremediation of heavy metals with microbial isolates and amendments on soil residue. International Journal of Science and Research (IJSR) 3 (8): 118-123.
- Madaan, N., Mudgal, V., Mishra, S., A.K. Srivastava, A.K., Singh, R.B. (2011). Studies on biochemical role of accumulation of heavy metals in Safflower. The Open Nutraceuticals Journal 4: 199-204.
- Wood, J.L., Wuxing Liu, W., Tang, C., and Franks, A.E. (2016). Microorganisms in heavy metal bioremediation: strategies for applying microbial-community engineering to remediate soils. AIMS Bioengineering, 3(2): 211-229.
- Jin, T., Shic, C., Wang, P., Liuc, J., Zhan, L. (2021). A review of bioremediation techniques for heavy metals pollution in soil. IOP Conf. Series: Earth and Environmental Science: 687: 012012. doi:10.1088/1755-1315/687/1/012012
- Tarfeen, N., Nisa, K.I., Hamid, B., Bashir, Z., Yatoo, A.M., Dar, M.A., Mohiddin, F.A., Amin, Z., Ahmad, R.A., Sayyed, R.Z. (2022). Microbial remediation: A promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: A review. Processes 10: 1358-1384.
- Volesky, B., Holan, Z.R. (2019). Biosorption of heavy metals. Biotechnol Prog 11(3): 235-250. Available from: https://doi.org/10.1021/bp00033a001 S.
- Hassan, S.H., Awad, Y.M., Kabir, M.H., Oh, S.E., Joo, J.H. (2010). Bacterial biosorption of heavy metals. In: Biotechnology Cracking New Pastures. New Delhi, India: MD Publications Pvt. Ltd.; pp. 79-110.
- Esposito, A., Pagnanelli, F., Lodi, A., Solisio, C., Veglio, F. (2019). Biosorption of heavy metals by Sphaerotilus natans: an equilibrium study at different pH and biomass concentrations. Hydrometall 60(2): 129-41. Available from: https://doi.org/10.1016/S0304-386X(00)00195-X.
- Gupta, R., Ahuja, P., Khan, S., Saxena, R.K., Mohapatra, M. (2000). Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr Sci.78(8): 967-973.
- Tiwari, S., Hasan, A., Pandey, M. (2019). A novel biosorbent comprising encapsulated Agrobacterium fabrum (SLAJ731) and iron oxide nanoparticles for removal of crude oil co-contaminant, lead Pb (II). J. Environ. Chem. Eng. 5(1): 442-452. Available from: https://doi. org/10.1016/j.jece.2016.12.017.
- Coelho, L. M., Rezende, H. C., Coelho, L. M., de Sousa, P.A.R., Melo, D.F.O., Coelho, N.M.M. (2015). Bioremediation of polluted waters using microorganisms. In Advances in Bioremediation of Wastewater and Polluted Soil, N. Shiomi (Ed.), InTech, Shanghai, China.
- Sayqal, A., Ahmed, O.B. (2021). Advances in heavy metal bioremediation: An overview. Appl. Bionics Biomech. 2021: 1609149.
- Pande, V, Pandey, S.C., Sati, D., Bhatt, P., Saman, M. (2022) Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 13:Article 824084. doi: 10.3389/fmicb.2022.824084.
- Pratush, A., Kumar, A., Hu, Z. (2018). Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int. Microbiol. 21: 97–106.
- Njoku, K. L., Asunmo, M. O., Ude, E. O., Adesuyi, A. A., Oyelami, A. O. (2020). The molecular study of microbial and functional diversity of resistant microbes in heavy metal contaminated soil. Environ. Technol. Innov. 17 (2):100606. doi: 10.1016/j.eti.2020.100606.
- Mudila, H., Prasher, P., Kumar, M., Kapoor, H., Kumar, A., Zaidi, M. G. H., et al. (2019). An insight into cadmium poisoning and its removal from aqueous sources by graphene adsorbents. Int. J. Environ. Health Res. 29: 1–21. doi: 10.1080/09603123.2018.1506568.
- Ayangbenro, A.S.; Babalola, O.O. (2017). A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 14: 94. https://doi.org/10.3390/ijerph14010094.
- Carlot, M., Giacomini, A., Casella, S. (2002). Aspects of plant-microbe interactions in heavy metal polluted soil. Acta Biotechnol. 22: 13–20. doi: 10. 1002/1521-3846(200205)22:1/23.0.co;2-9.
- Glick, B. R. (2003). Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 21: 383–393. doi: 10.1016/s0734-9750(03) 00055-7.
- Dimkpa, C. O., Svatoš, A., Dabrowska, P., Schmidt, A., Boland, W., Kothe, E. (2008). Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74: 19–25.
- Sinha, S., Mukherjee, S. K. (2008). Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr. Microbiol. 56: 55–60. doi: 10.1007/s00284-007-9038-z.
- Neubauer, U., Furrer, G., Kayser, A., Schulin, R. (2000). Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int. J. Phytoremediation 2: 353–368. doi: 10.1080/ 15226510008500044.
- Rajkumar, M., Ae, N., Prasad, M. N. V., Freitas, H. (2010). Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 28: 142–149. doi: 10.1016/j.tibtech.2009.12.002.
- Wang, G., Zhang, Q., Du, W., Ai, F., Yin, Y., Ji, R., et al. (2021). Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total Environ. 769:145224. doi: 10.1016/j.scitotenv.2021.145224.
- Zeyaullah, M., Atif, M., Islam, B. et al. (2009). Bioremediation: a tool for environmental cleaning. African Journal of Microbiology Research 3 (6): 310–314.
- Dhokpande, S.R., Kaware, J.P. (2013). Biological methods for heavy metal removal- a review. International Journal of Engineering Science and Innovative Technology 2 (5): 304–309.
- James J. (2015). Isolation of cadmium reducing microorganisms and their use in bioremediation of soil/water. M.Tech. Dissertation, Amity University, Lucknow, Uttar Pradesh, India; pp. 1-57.
- Vidali, M. (2001). Bioremediation: An overview. Pure and Applied Chemistry 73 (7): 1163–1172.
- Singh, A.P., Mishra, S. (2013). Studies on antibiotic production by soil microflora and their biochemical characterization from different industrial waste polluted soil samples in (Uttar Pradesh and Uttarakhand) India. IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) 7 (4): 32- 43.
Quick linksss
- Abstract
- Introduction
- Modes of Exposure to Toxicants
- Endurable Daily Intake Approach
- Bioremediation Approach for Heavy Metal Pollution
- Microbial Mechanism Implicated in Heavy Metal Bioremediation
- Advantages and Disadvantages of Bioremediation
- Conclusion
- Declaration
- Conflict of Interests
- Acknowledgement
- References


