Archive : Article / Volume 2, Issue 2
- Research Article | DOI:
- https://doi.org/10.58489/2836-2322/021
Analysis Of Bioactive Compounds in Ethanolic Extract of Xylopia Aethiopica Leaves Using Gas Chromatography and Mass Spectrometry (Gc-Ms) Technique
1,2Department of Animal Nutrition and Biochemistry, Sumitra Research Institute, Gujarat, India
3,4,5Department of Animal Science, University of Abuja, Nigeria
Alagbe J.O
Alagbe J.O, (2023). Analysis Of Bioactive Compounds in Ethanolic Extract of Xylopia Aethiopica Leaves Using Gas Chromatography and Mass Spectrometry (Gc-Ms) Technique Pharmacy and Drug Development. 2(2); DOI: 10.58489/2836-2322/021
© 2023 Alagbe J.O, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 23-03-2023
- Accepted Date: 14-04-2023
- Published Date: 11-08-2023
Xylopia aethiopica, anti-microbial, phytochemicals, gas chromatography, mass spectrometry, free radicals.
Abstract
This study investigated the bioactive compounds in ethanolic extract of Xylopia aethiopica leaves using gas chromatography and mass spectrometry (GC-MS) technique. The phytoconstituents present in Xylopia aethiopica leaves were: flavonoids (951.82 mg/g), tannins (282.70 mg/g), alkaloids (188.47 mg/g), phenols (603.25 mg/g), saponins (11.47 mg/g), steroids (91.20 mg/g), oxalates (190.32 mg/g) and glycosides (190.32 mg/g). A total number of 30 bioactive compounds were identified based on their peak areas. The major compounds greater than 1 % were; 13-docosenamide (21.09 %), terpineol (10.07 %), 1,6-cyclodecadiene (9.37 %), copaene (2.88 %), caryophyllene (8.15 %), β-ocimene (6.05 %), β-myrcene (5.09 %), copaene (2.38 %), 2 â methoxy-4-vinylphenol (1.72 %), β-elemenone (1.31 %), 3,4-dimethylphenyl heptyl ether (1.26 %), ethyl oleate (1.07 %) and γ-elemene (1.27 %) while those less than 1 % (Ë 1 %) were; 2- methylenebornane (0.66 %), 2-methoxy-2-prophenyl (0.72 %), hexadec-7-enal (0.23 %), hexadecanoic acid (0.02 %), didodecyl benzene 1,2 dicarboxylate (0.09 %), methyl stearate (0.16 %), 9,12-octadecadienoic acid (0.08 %), hexadeca-7,10 â dienal (0.47 %), 1,1,5 âtrimethyl -1,2-dihydronaphthalene (0.01 %), propane, 1,1 â oxybis -3- chloro (0.08 %), 1-trimethylsilypent-1-en-4-yne (0.03 %), bicyclo[13.1.0] hexade can-2-one (0.02 %), methyl octadeca-9-yn-11-trans-enoate (0.51 %), cis-linaloxide (0.22 %), tetradecanoic acid, 10,13 âdimethyl ester (0.18 %), didodecyl benzene 1,2 âdicarboxylate (0.47 %) and 2-cyclopentene -1-one, 2 â hyroxy (0.09 %). However, all the compounds have a wide range of pharmacological activities including- antimicrobial, antioxidant, anti-malarial, antifungal, anti-arrhythmic, anti-viral, hepato-protective, anti-proliferative, anti-depressant, antipyretic and antihelminthic.
Introduction
The demand of herbal medicines has increased globally due to the growing recognition with common consideration that plant-based medicines are safe, non-toxic, environmentally friendly, easily available and affordable (Nikul, 2020; Alagbe et al., 2023). The efficacy of herbs can be linked to the presence of phytochemicals or bioactive compounds which performs therapeutic effects (antioxidants, anti-microbial, hepat-protective, immune-modulatory, hypolipidemic, anti-tumor, antifungal, antiviral, anti-proliferative, antipyretic, anti-depressant, anti-fibrotic, antihelminthic, anti-androgenic and analgesics) in human being and animals (Singh et al., 2022; Oluwafemi et al., 2019; Agubosi et al., 2022). Phytochemicals are generally regarded as chemicals of plant origin used by plants for growth, defense against competitors, predators and pathogens (Akintayo and Alagbe, 2020; Shittu and Alagbe, 2020). According to Adewale et al. (2021), there are over 300,000 species of herbal plants with pharmaceutical properties. Among the probable and underutilized herbal plant is Xylopia aethiopica.
Xylopia aethiopica (African pepper) belongs to Annonaceae family is an aromatic, evergreen tree native to low land rain forest in the savannah zones of Africa and most parts of Asia (Orwa et al., 2009; Burkill, 1985). The tree can grow up to 15 – 30 meters high and about 60 – 70 cm in diameter. The fruits have a small twisted bean shaped pods and are characterized by deep brown colour (Soladoye et al., 2012). Xylopia aethiopica leaves and seeds have been reported to contain several phytochemicals such as; tannins, alkaloids, saponins, flavonoids, anthraquinones, phlobatannins and glycosides making them exhibit a wide range of biological effects (Tapsell et al., 2006; Tan et al., 2010).
Various parts of Xylopia aethiopica plant extracts (seeds, leaves, flowers, fruits, stem bark and roots) are being employed traditionally for the treatment of gastrointestinal infections, diarrhea, cough, skin infections, respiratory diseases, tooth ache, sexually transmitted infections, cough, malaria, diabetes, uterine fibroids, hemorrhoids, asthma, rheumatism and female sterility (Feste et al., 2016). A decoction of Xylopia aethiopica root and stem bark can be used to treat tooth ache due to the presence of minerals (copper, zinc, calcium, phosphorus and potassium) (Obodo et al., 2013) and it has antimicrobial effects on several pathogenic bacteria including; Bacillus spp, Staphylococcus aureus, Escherichia coli, Salmonella typhi and Klebsiella spp (Konan et al., 2009). Xylopia aethiopica leaves have also been reported to be loaded with vitamins A, B2 (folic acid), B12 (cobalamins), ascorbic acid (vitamin C) and tocopherol (vitamin E) which performs various biochemical functions in the body (Kiran and Devi, 2007).
Drug research makes the use of ethno botany to search for pharmacologically active substance in nature and has in this way discovered hundreds of useful compounds (Sushila, 2017). Phyto-medicinal reports for each of the medicinal plants including information on physiological effects, efficacy and references needed to be developed (Nikul, 2020). Therefore, this experiment was designed to examine the bioactive compounds of
Xylopia aethiopica
using gas chromatography technique.
Materials and methods
Experimental site
The study was carried out at the Department of Animal Nutrition and Biochemistry, Sumitra Research Institute, Gujarat, India located between the coordinate 23o 13’N 72o41’E with a coastline of 1600 Km (Bose Ashish, 1991).
Collection, authentication and processing of Xylopia aethiopica leaf extract
Fresh leaves of Xylopia aethiopica were collected within the premises of Sumitra Research Institute, Gujarat India and authenticated by a certified taxonomist. It was washed with distilled water and shade dried for 14 days. Dried leaves of Xylopia aethiopica were grinded into powder form with the aid of an electric blender. 200 grams of Xylopia aethiopica powder was imbibed with 1000 mL of 90 % ethanol for 2 days with occasional stirring. Finally, the ethanolic extract of Xylopia aethiopica was obtained by sieving the sample using Whatman’s No.1 filter paper, stored in a sterile air tight container and stored in a cool dry place before transporting it to the laboratory for further analysis.
Quantitative determination of phytochemical components
Total flavonoids, tannins and phenols were estimated using Aluminium chloride and Folin – Ciocalteau method described by Otles and Yacin (2012). Saponins and alkaloids were quantified using colourimetric and gravimetric technique described by Madhu et al. (2016). Glycosides, steroids and phytates were analyzed using anion exchange methods described by Adeniyi et al. (2009).
Analysis of bioactive compounds of Xylopia aethiopica leaves using GC-MS technique
Analysis of bioactive compounds in ethanolic extract of
Xylopia aethiopica
leaves were analyzed using Skyray GC-MS 6800 (USA). The GC specifications are; inlet temperature (Max. 450
oC
), pressure range (0 ˷ 100 psi), pressure control mode (electronic pressure control), split mode (split/splitless, max. split ratio: 1000:1), column oven working temperature (+4
oC
˷ 450
oC
), heating rate (up to 120
oC
/min), temperature programming (7 stages/8 platforms) and auto sampler (optional) and MS specifications: EI source ionization energy (5eV – 250 eV), mass range (1.5 – 1000 amu), resolution (unit resolution), ion source temperature (100 - 350
oC
), filament emission current (0 - 350 µ A), GC-MS interface temperature (Max. 450
oC
), stability (± 0.10 amu/48 hours), sensitivity (full scan. 1 pg OFN at m/z 272 with S/N ˃ 30:1), scan rate (up to 1000 amu/s), vacuum (Turbo molecular pumps: 67 L/s) and detector (high energy dynode electron multiplier).
Results and discussion
Phyto-constituents of Xylopia aethiopica leaf extract
Phytochemical constituents of Xylopia aethiopica leaf extract is presented in Table 1. The values of flavonoids, tannins, phenols, alkaloids, glycosides, oxalates, steroids and saponins were 951.82 mg/g, 282.70 mg/g, 603.25 mg/g, 188.47 mg/g, 190.32 mg/g, 23.74 mg/g, 91.20 mg/g and 11.47 mg/g respectively. Flavonoids had the highest concentration (951.82 mg/g) while saponins had the lowest concentration (11.47 mg/g). Plants are complex matrices producing a range of secondary metabolites with different functional groups and polarities (Oluwafemi et al., 2020). Flavonoids are group of compounds with antioxidant activities against free radicals, cellular signaling, inflammation allergies and platelet aggregation (Akintayo and Alagbe, 2000; Agubosi et al., 2021). Alkaloids have a wide range of pharmacological activities including; antimalarial, antiarrhythmic and analgesics (Okwu, 2004). Plants rich in alkaloids have bitter taste thus preventing consumption from insects and chordates (Sexena et al., 2013; Stary, 1998). Tannins are complex mixtures of organic compounds used as astrigents as they precipitate tissue protein (Saxena et al., 2013). They can also be used for the treatment of diarrhea (Sczkowski et al., 1988). The presence of phenols in Xylopia aethiopica leaf extracts supports its use as anti-inflammatory and antioxidant thus preventing the incidence of coronary disease (Poumarad et al., 2006). Plants containing glycosides and steroids can be used as flavouring agents and cardiac drugs (Saker and Nahar, 2007). In addition, steroids possess medicinal properties such as; anti-carcinogenic, antispasmodic and fertility boosting activity (Feste et al., 2016). Overload of oxalate in a body can cause kidney stones and heart diseases (Kuete, 2014). The results on phyto-constituents of Xylopia aethiopica leaf extract is in agreement with the findings of Aguoru et al. (2016).
Table 1: Phyto-constituents of Xylopia aethiopica leaf extract
| Constituents | Composition (Mg/g) |
| Flavonoids | 951.82 |
| Tannins | 282.70 |
| Phenols | 603.25 |
| Alkaloids | 188.47 |
| Glycosides | 190.32 |
| Oxalates | 23.74 |
| Steroids | 91.20 |
| Saponins | 11.47 |
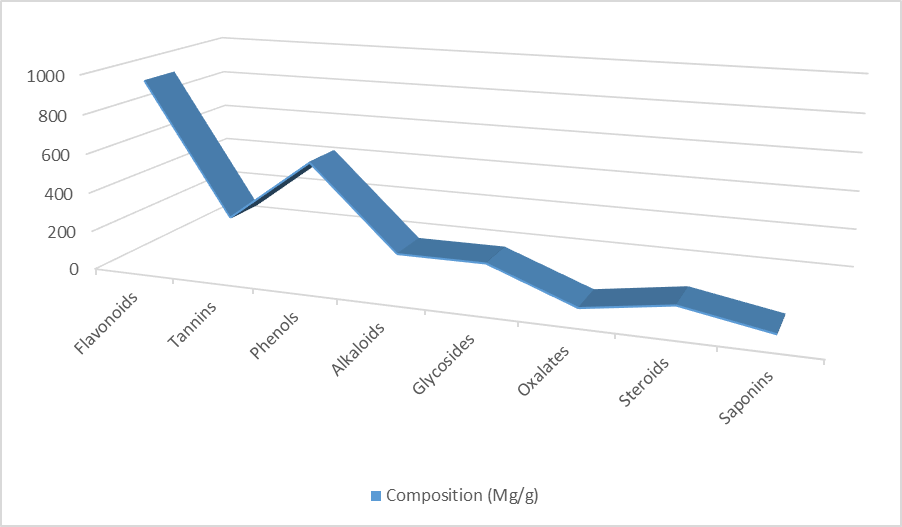
Figure1: Phyto-constituents of Xylopia aethiopica leaf extract
Bioactive compounds of Xylopia aethiopica leaf extracts by Gas chromatography and mass spectrometry technique
The bioactive compounds of
Xylopia aethiopica
leaf extracts by GC-MS is presented in Table 2. Thirty compounds were identified based on their peak areas and retention time. The major compounds greater than 1 % were; 13-docosenamide (21.09 %), terpineol (10.07 %), 1,6-cyclodecadiene (9.37 %), copaene (2.88 %), caryophyllene (8.15 %), β-ocimene (6.05 %), β-myrcene (5.09 %), copaene (2.38 %), 2 – methoxy-4-vinylphenol (1.72 %), β-elemenone (1.31 %), 3,4-dimethylphenyl heptyl ether (1.26 %), ethyl oleate (1.07 %) and γ-elemene (1.27 %) while the minor compounds less than 1 % (˂ 1 %) were; 2- methylenebornane (0.66 %), 2-methoxy-2-prophenyl (0.72 %), hexadec-7-enal (0.23 %), hexadecanoic acid (0.02 %), didodecyl benzene 1,2 dicarboxylate (0.09 %), methyl stearate (0.16 %), 9,12-octadecadienoic acid (0.08 %), hexadeca-7,10 – dienal (0.47 %), 1,1,5 –trimethyl -1,2-dihydronaphthalene (0.01 %), propane, 1,1 – oxybis -3- chloro (0.08 %), 1-trimethylsilypent-1-en-4-yne (0.03 %), bicyclo[13.1.0] hexade can-2-one (0.02 %), methyl octadeca-9-yn-11-trans-enoate (0.51 %), cis-linaloxide (0.22 %), tetradecanoic acid, 10,13 –dimethyl ester (0.18 %), didodecyl benzene 1,2 –dicarboxylate (0.47 %) and 2-cyclopentene -1-one, 2 – hyroxy (0.09 %). Ethyl oleate, γ-elemene and β-ocimene was reported to be found in
Luffa aegyptiaca leaves (Alagbe et al., 2023) and Strychnos innocua root bark. Hexadecanoic acid was found in Delonix regia root and leaves (Alagbe et al., 2020). Hexade can-2-one, 1,1, 5 –trimethyl -1,2-dihydronaphthalene and methyl octadeca-9-yn-11-trans-enoate have been reported to effectively treat female infertility, gastro-intestinal disease and skin infections (Paula et al., 2008; Singh et al., 2010; Adams et al., 2020). Caryophyllene, copaene and methyl stearate have been detected in Prosopis africana oil, Baccharis spp, Strychnos spinosa, Zollingeriana indigofera stem bark (Agubosi et al
., 2021; Hoet et al., 2007). They have a wide range of therapeutic properties including; anti-inflammatory, anti-carcinogenic, cytotoxic and antioxidant (Hongxiang et al., 2005).
Conclusion
It was concluded that Xylopia aethiopica leaf extract have several phyto-constituents which have a wide range of pharmacological or therapeutic functions making them useful in the treatment of gastro-intestinal disease, skin infection, cough, malaria, sexually transmitted infections, hemorrhoids, infertility, diabetes and uterine fibroids among others.
Table 2: Bioactive compounds of Xylopia aethiopica leaf extract
Bioactive compounds | M.W(g/mol) | Peak area (%) | Retention time (min) |
2- Methylenebornane | 150 | 0.66 | 2.71 |
2 – Methoxy-4-vinylphenol | 136 | 1.72 | 3.92 |
13-Docosenamide | 121 | 21.09 | 4.02 |
Terpineol | 173 | 10.07 | 4.18 |
Copaene | 156 | 2.88 | 4.26 |
1,6-Cyclodecadiene | 112 | 9.37 | 4.44 |
Humulene | 160 | 2.38 | 4.63 |
β-Myrcene | 188 | 5.09 | 4.86 |
β-Ocimene | 167 | 6.05 | 5.11 |
Caryophyllene | 152 | 8.15 | 5.23 |
2-methoxy-2-prophenyl | 216 | 0.72 | 5.47 |
γ-Elemene | 204 | 1.27 | 6.39 |
β-Elemenone | 218 | 1.31 | 6.75 |
Hexadec-7-enal | 238 | 0.23 | 6.97 |
Hexadecanoic acid | 284 | 0.02 | 7.06 |
Didodecyl benzene 1,2 dicarboxylate | 504 | 0.09 | 7.19 |
Methyl stearate | 298 | 0.16 | 7.42 |
9,12-Octadecadienoic acid | 280 | 0.08 | 7.86 |
Hexadeca-7,10 – dienal | 236 | 0.47 | 7.94 |
3,4-dimethylphenyl heptyl ether | 204 | 1.26 | 8.22 |
1,1,5 –Trimethyl -1,2-dihydronaphthalene | 270 | 0.01 | 9.08 |
Propane, 1,1 –Oxybis -3- chloro | 292 | 0.08 | 9.27 |
1-Trimethylsilypent-1-en-4-yne | 280 | 0.03 | 9.63 |
Ethyl Oleate | 310 | 1.07 | 10.04 |
Bicyclo[13.1.0] hexade can-2-one | 236 | 0.02 | 10.32 |
Methyl octadeca-9-yn-11-trans-enoate | 292 | 0.51 | 10.78 |
Cis-Linaloxide | 131 | 0.22 | 18.02 |
Tetradecanoic acid, 10,13 –dimethyl ester | 270 | 0.18 | 18.46 |
Didodecyl benzene 1,2 –dicarboxylate | 504 | 0.47 | 21.75 |
2-Cyclopentene -1-one, 2 – hyroxy | 98 | 0.09 | 28.93 |
M.W: Molecular weight
References
- Muritala, Daniel Shittu., Alagbe, J.O., Ojebiyi, O.O., Ojediran, T.K and Rafiu, T.A. (2022). Growth performance and haematological and serum biochemical parameters of broiler chickens given varied concentrations of Polyalthia longifolia leaf extract in place of conventional antibiotics. Animal Science and Genetics 18(2): 57-71.
- Nikal (2020). Role of medicinal plants and aromatic plants in National economy and export potentials. Pharmacognosy Subject in Diploma in Pharmacy.
- Adel-Rahman and Adel-Ghaffar (2020). Neutraceuticals – Lecture 5 – Phytochemicals.
- Sushila, S. (2017). Medicinal plants seminar presented at Department of Chemistry Deshbandhu College, University of Delhi, India
- Paula, D., Anna, P., Magdalena, N., Natalia T (2018) The use of azelaic acid in selected dermatological disorders. Med Rodz 21(4):307–314
- Alagbe, J.O., Bamigboye, S., Nwosu, G.C., Agbonika, D.A and Kadiri Mercy Cincinsoko. (2023). Characterization of bioactive compounds in Luffa aegyptiaca leaf ethanolic extracts using gas chromatography and mass spectrometry (GC-MS). Drug Discovery, 2023; 17:e10dd1011.
- Adeniyi, S.A., Orjiekwe, C.L, Ehiagbonare, J.E. (2009). Determination of alkaloids and oxalates in some selected food samples in Nigeria. African Journal Biotechnology, 8(1): 110-112.
- Madhu M, Sailaja V, Satyadev TNVSS. (2016). Quantitative phytochemical analysis of selected medicinal plant species by using various organic solvents. Journal of Pharmacogsy Phytochemical, 5(2): 25-29.
- Hoet, S., Pieters, L., Muccioli, G.G., Habib-Jiwan, J., Opperdoes, F.R, Quetin- Leclercq, J. (2007) Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. Journal of National Production, 70:1360–1363
- Otles, S and Yalcin, B. (2012). Phenolic compounds analysis of root, stalk, and leaves of Nettle. Science World Journal. 2012: 564367. DOI: 10.1100/2012/564367.
- Agubosi, O.C.P., Alexander, James and Alagbe, J.O. (2022). Influence of dietary inclusion of Sunflower (Helianthus annus) oil on growth performance and oxidative status of broiler chicks. Central Asian Journal of Medical and Natural Sciences 2(7): 187-195.
- Sarker SD, Nahar L. (2007). Chemistry for Pharmacy Students General, Organic and Natural Product Chemistry. Indian Journal of Physiology and Pharmacology, 2007; 283-359.
- Agubosi, O.C.P., Soliu, M.B and Alagbe, J.O. (2022). Effect of dietary inclusion levels of Moringa oleifera oil on the growth performance and nutrient retention of broiler starter chicks. Central Asian Journal of Theoretical and Applied Sciences 3(3): 30-39.
- Alagbe John Olujimi, Ramalan Sadiq Muhammad., Shittu Muritala Daniel and Olagoke Olayemi Christiana (2022). Effect of Trichilia monadelpha stem bark extract on the fatty acid composition of rabbit’s thigh meat. Journal of Environmental Issues and Climate Change 1(1): 63-71.
- Saxena M, Saxena J, Nema R, Singh D, Gupta, A. (2013). Phytochemistry of Medicinal Plants. Journal of Pharmacognosy and Phytochemistry Center for Microbiology and Bio-Technology Research and Training, Bhopal, India, 8192 (1): 168-182
- Alagbe, J.O. (2023). Bioactive compounds in ethanolic extract of Strychnos innocua root using gas chromatography and mass spectrometry (GC-MS). Drug Discovery, 2023; 17: e4dd1005.
- Alagbe, J.O., Adeoye, Adekemi and Oluwatobi, O.A. (2020). Proximate and mineral analysis of Delonix regia leaves and roots. International Journal on Integrated Education. 3(10): 144-149.
- Singh Sharma., Alagbe Olujimi John., Liu Xing., Sharma Ram and Kumar Amita (2022). Comparative analysis of ethanolic Juniperus thurifera leaf, stem bark and root extract using gas chromatography and mass spectroemetry. International Journal of Agriculture and Animal Production, 2(6): 18-27.
- Adams, D., Midel, M.J., Dastgir, J., Flora, C, Molinari, R.J, Heerinckx, F., Endemann, S., Atwal P, Milner, P and Shchpinor, M.S. (2020) Treatment of infantile neuroaxonal dystrophy with RT001: a di-deuterated ethyl ester of linoleic acid: report of two cases. JIMD Rep 54(1):54–60
- Alagbe, J.O., Adedeji, M.O., Habiba, Z., Nwosu, Gloria and Wyedia Dabara Comfort (2021). Physico-chemical properties of Indigofera zollingineriana seed oil. Asian Journal of Advances in Medical Science 3(4): 306-308.
- Singh, G., Kapoor, I.P.S., Singh, P, Carola, S. (2010) Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chemistry and Toxicology, 48(4):1026–1031.
- Agubosi, O.C.P., Oluwafemi, R.A., and Alagbe, J.O. (2021). Preliminary study on GC-MS analysis of Prosopis africana seed (African mesquite) oil. Journal of Ethics and Diversity in International Communication 1(4): 18-20.
- Hongxiang, S., Cuirong, S and Yuanjian, P. (2005) Cytotoxic activity and constituents of the volatile oil from the roots of Patrinia scabra Bunge. Chemical Biodiversity, 2(10):1351–1357
- Alagbe, J.O., Shittu, M.D and Ushie, F.T. (2021). GC-MS analysis of methanolic stem bark extract of Zollingeriana indigofera. Asian Journal of Advances in Research 11(4): 144-146.
- Musa, B., Alagbe, J.O., Adegbite Motunrade Betty, Omokore, E.A. (2020). Growth performance, caeca microbial population and immune response of broiler chicks fed aqueous extract of Balanites aegyptiaca and Alchornea cordifolia stem bark mixture. United Journal for Research and Technology, 2(2):13-21.
- Oluwafemi, R.A., Isiaka Olawale and Alagbe, J.O. (2020). Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition- A review. Research in: Agricultural and Veterinary Sciences. 4(1): 5-11.
- Alagbe, J.O (2019). Proximate, mineral and phytochemical analysis of Piliostigma thonningii stems bark and roots. International Journal of Biological, Physical and Chemical Studies, 1(1): 1-7.
- Sczkowski CP, Kalinowska M, Wojciechowski Z. (1998). The 3-Oglucosylation of steroidal saponins and alkaloids in eggplant (Solanum melongena); evidence for two separate glycosyl transferences, Phytochemistry, 48: 1151-1159.
- Stary R.R. (1998). The plant cell wall as a source of dietary fiber: chemistry and structure. American Journal of Clinical Nutrition, 39: 320–337
- Alagbe, J.O. (2019). Growth performance and haemato-biochemical parameters of broilers fed different levels of Parkia biglobosa leaf extracts. Academic Journal of Life Sciences. 5(12): 107 – 115.
- Okwu, D.E. (2004). Phytochemical and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain Agric. Environment, 2004; 6:30-37.
- Alagbe, J.O. (2017). Nutrient evaluation of sweet orange (Citrus sinensis) fruit peel as a replacement for maize in the diets of weaner grass cutters. Scholarly Journal of Agricultural Science. 6(8):277-282.
- Oluwafemi, R.A., Agubosi, O.C.P and Alagbe, J.O. (2021). Proximate, minerals, vitamins and amino acid composition of Prosopis africana (African mesquite) seed oil. Asian Journal of Advances in Research 11(1): 21-27.
- Okwu, D.E and Josiah, C. (2006). Evaluation of the chemical composition of two Nigerian medicinal plants. African Journal of Biotechnology, 4: 357-361.
- Oluwafemi, R.A., Lawal Aisha Omolade., Adelowo, Samad Adetope and Alagbe, J.O. (2021). Effects of dietary inclusion of ginger (Zingiber officinale) and garlic (Allium sativum) oil on carcass characteristics and sensory evaluation of broiler chicken. Texas Journal of Multidisciplinary Studies, 2(11): 180-188.
- Adewale, A.O., Alagbe, J.O., Adeoye, Adekemi. O. (2021). Dietary Supplementation of Rauvolfia Vomitoria Root Extract as A Phytogenic Feed Additive in Growing Rabbit Diets: Haematology and serum biochemical indices. International Journal of Orange Technologies, 3(3): 1-12.
- Shittu, M.D and Alagbe, J.O. (2020). Phyto-nutritional profiles of broom weed (Sida acuta) leaf extract. International Journal of Integrated Education. 3(11): 119-124
- Akintayo Balogun Omolere. M and Alagbe, J.O (2020). Probiotics and medicinal plants in poultry nutrition: A review. United International Journal for Research and Technology, 2(1): 7-13.
- Alagbe, J.O. (2022). Prosopis africana (African mesquite) oil as an alternative to antibiotic feed additives on broiler chickens diets: performance and nutrient retention. Discovery 58(314): 134 -142.
- Alagbe, J.O and Ushie, F.T. (2022). Growth performance of broiler chicks fed diets containing different levels of aqueous Citrus aurantium stem bark extracts. Discovery 58(319): 735-741.
- Oyeleke, S.B, Dauda, B.E.N and Boye, O.A. (2008). Antibacterial activity of Ficus capensis. African Journal of Biotechnology, 7(10):1414-1417.
- Alagbe, J.O (2020). Chemical evaluation of proximate, vitamin and amino acid profile of leaf, stem bark and roots of Indigofera tinctoria. International Journal on Integrated Education. 3(10): 150-157.
- Poumarad, S.J., Hosseinimehr, S.J amd Shababimajd, N. (2006). Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. African Journal of Biotechnology, 5(2): 1142-1145.
- Alagbe, J.O., Ajagbe, A.D., Attama Jeremiah, Philemon, K.C and Bello, Kamoru, A (2020). Albizia lebbeck stem bark aqueous extract as alternative to antibiotic feed additives in broiler chicks diets: Haematology, Serum indices and oxidative status. International Journal of Biological, Physical and Chemical Studies, 2(1): 8-15.
- Bose Ashish (1991). Demographic diversity of India census state and district level data. B.R. Publication.
- Alagbe, J.O (2020). Caecal Microbial Population of Growing Grass Cutters (Thyronoyms Swinderianus) Fed Phyllantus Amarus and Pilogstigma Thonngii Leaf Meal Mixture as Partial Replacement for Soya Bean Meal. Concept of Dairy and Veterinary Sciences. 3(5): 350 – 355.
- Tan, A.C., Konczak, I., Sze, D.M. and I. Ramzan. (2010) Towards the discovery of novel phytochemicals for disease prevention from native Australian plants: an ethnobotanical approach. Asian Pacific Journal Clinical Nutrition, 19(3): 330–334.
- Akintayo Balogun Omolere. M and Alagbe, J.O (2020). Probiotics and medicinal plants in poultry nutrition: A review. United International Journal for Research and Technology, 2(1): 7-13.
- Alagbe, J.O. (2020). Effect of dietary supplementation of Cymbopogon Citratus oil on The Performance and Carcass characteristics of broiler chicks. European Journal of Biotechnology and Bioscience. 8(4): 39-45.
- Tapsell, L.C., Hemphill, I., and Cobiac, L., Patch, C.S., Sullivan, D.R., Fenech, M., Roodenrys, S., Keogh, J.B., Clifton, P.M., Williams, P.G., Fazio, V.A. and K.E. Inge. (2006). Health benefits of herbs and spices: the past, the present, the future. Medicinal Journal of Australia, 185(4): S4–24.
- Agubosi, O.C.P., Oluwafemi, R.A and Alagbe, J.O. (2021). The effect of processing on the proximate, mineral and vitamin composition of Neem leaves (Azadirachta indica) grown in Gwagwalada, FCT, Abuja. Abuja Journal of Agriculture and Environment, 1(1): 293-299.
- Orwa, C., Mutua, A, Kindt, R., Jamnadass, R. and Simons, A. (2009). AgroforestreeDatabase:a tree reference and selection guide. Version 4.0
- Soladoye, M.O., Chukwuma, E.C. and F.P. Owa. 2012. An ‘Avalanche’ of plant species for the traditional cure of diabetes mellitus in South-Western Nigeria. Journal of Natural Product and Plant Resources, 2:60–72
- Burkill, H. M (1985).
- Obodo, B.N., Iweka, F.K., Obhakhan, J.O., Dada, F.L., Festus, O.O. and Onoyovwi, A.O. (2013) Hepatic Potentials of Xylopia aethiopica Leaves in Adult Wistar Rats. International Journal of Herbs and Pharmacological Research, 2, 36-41.
- Konan, N.D., Bosson, A.K., Mamyrbekova-Bekro, J.A., Jean, N. and Bekro, Y.A. (2009) Chemical Composition and Antioxidant Activities of Essential Oil of Xylopia aethiopica (Dunal) A. Rich. European Journal of Scientific Research, 2, 311-318.
- Kiran, S.R. and Devi, P.S. (2007) Evaluation of Mosquitocidal Activity of Essential Oil and Sesquiterpenes from Leaves of Chloroxylon swietenia DC. Parasitology Research, 101, 413-418
- Boampong, J.N., Ameyew, E.O., Aboagye, B., Asare, K., Kyei, S., Donfack, J.H and Woode, E. (2013). The curative and prophylactic effect of Xylopia acid on Plasmodium berghei infection in mice. Journal of Parasitology, 2013: 1-7.
- Boyom, F.F., Ngouana, V., Zollo, P.H., Menut, C., Bessiere, J.M., Gut, J., Rosenthal, P.J. (2003). Composition of antiplasmodial activities of essential oils from some Cameroonian medicinal plants. Phytochemistry, 64: 1269-1275.
- Germolec, D.R., Kashon, M., Nyska, A., Kuper, C.F., Portier, C., Kommineni, C., Johnson, K.A and Luster, M.I. (2004). The accuracy of extended histopathology to detect immunotoxic chemicals. Toxicological Science, 82: 504-514.
- Kuefe, W. (2014). The discovery of Aspirin: A reappraisal. BMJ Clinical Research 321(7276): 1591-1594.


