Archive : Article / Volume 2, Issue 2
- Research | DOI:
- https://doi.org/10.58489/2836-2322/019
Chiral Analysis of Sertaconazole by HPLC Method on Polysaccharide Derivatives
1Bioactive Molecules and Chiral Separation Laboratory, Faculty of exact sciences, University TM, Bechar, Bechar, Algeria.
Nasser Belboukhari
Nasser Belboukhari, Aicha Laoufi, Khaled Sekkoum, Layachi Mohammed Abdeldjalil, Ouahabi Abdelbasset (2023). Chiral Analysis of Sertaconazole by HPLC Method on Polysaccharide Derivatives. Pharmacy and Drug Development. 2(2). DOI: 10.58489/2836-2322/019
© 2023 Nasser Belboukhari, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
- Received Date: 29-01-2023
- Accepted Date: 10-07-2023
- Published Date: 12-07-2023
sertaconazole; antifungal agent; HPLC; Chiral Separation; discrimination.
Abstract
Sertaconazole is a pharmaceutical product in the form of a cream, gel, powder, and solution for dermatological use and vaginal cream, tablets, and ovules for gynecological use. The active ingredient is 2% sertaconazole nitrate. Sertaconazole nitrate is an azole antifungal agent, with notable antifungal activity. The active principal structure of sertaconazole has an asymmetric carbon, which makes this molecule chiral and it is in the form of an enantiomeric mixture. Currently, the control of drugs is based on qualitative and quantitative analysis by the use of effective analytical methods for chiral active ingredients. The enantiomeric separation of an antifungal compound, Sertaconazole, using HPLC is described in this work. The columns employed were based on polysaccharide derivatives (Chiralpak IB, Chiralcel OD-H), and the results show that the separations obtained are better, in terms of high resolution and short analysis time. Chiral separation of Sertaconazole was explained by hydrogen bondings and Ï-Ï interactions, between the CSP and this antifungal agent.
Introduction
The treatment of fungal diseases involves several classes of antifungal agents, among which the imidazole- or triazole-based drugs (referred to collectively as the “azoles”) constitute a large and important group. The azoles are, at present, the only antifungal agents with good oral bioavailability and activity against a broad spectrum of fungal pathogens [1, 2].
Sertaconazole is a useful antifungal agent against mycoses of the skin and mucosa, such as cutaneous, genital and oral candidiasis and tinea pedis. Fungistatic and fungicidal activities on Candida are dose-dependent. The antifungal spectrum of sertaconazole includes dermatophytes, Candida, Cryptococcus, Malassezia and also Aspergillus, Scedosporium, and Scopulariopsis. Sertaconazole also shows antimicrobial activity against streptococci, staphylococci, and protozoa (Trichomonas). Sertaconazole has shown an anti-inflammatory effect that is very useful for the relief of unpleasant symptoms [3-7].
These antifungal agents contain one chiral center (Fig.1) and are clinically used as a racemic mixture. The different pharmacological activities of the enantiomers have created an interest to study the pharmacological and toxicological properties of the enantiomers e.g., drugs, pharmaceuticals, agrochemicals, etc. [8-13].
Over the last decade, many analytical and preparative chromatographic and electrophoretic methods (mainly HPLC and capillary electrophoresis (CE)) have been developed to study the biological action and activity of enantiomeric drugs. Among the methods currently used to achieve chiral separation of racemic mixtures, high-resolution chromatographic systems based on chiral stationary phases (CSPs) (direct methods) are more rapid and suitable for racemic mixtures [14-17].
The objective of our research was to obtain enantiomeric separation of Sertaconazole with the HPLC method using two chiral stationary phases Chiralpak IB and chiral cell OD-H.
Structure
Sertaconazole nitrate (nitrate salt of 7-chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl) ethoxy-methyl] benzo[b]thiophene) (C20H16O4N3Cl3S) [4,14], is an azole group of antifungals with benzothiophene structure similar to tryptophan in the fungal plasma membrane, which facilitates the incorporation of Sertaconazole into fungal cells [4,18,19]. This represents an important difference compared with other azoles used in the treatment of mycoses [9,19]. The lipophilic part of the molecule means that it is soluble in organic solvents such as ethanol (1.7%), chloroform (1.5%), or acetone (0.95%), slightly soluble in N-octanol (0.069%) and practically insoluble in water (<0>
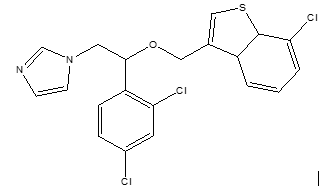
Mechanisms of action
Sertaconazole has two primary effects on cell function. First, it inhibits ergosterol synthesis by blockade of the P450-dependent enzyme pathway that catalyzes the methylation of lanosterol to ergosterol, a major constituent of fungal cell wall membranes. Second, it binds directly to nonsterol lipids in the membrane, which interferes with the regulation of the permeability of fungal cell membranes. Inhibition of ergosterol synthesis interferes with fungal cell growth, whereas direct interaction with the membrane produces subsequent leakage of intracellular components, particularly adenosine triphosphate, thereby contributing to immediate cell death. As a result, sertaconazole is an effective fungicidal and fungistatic agent [4,21].
Material and methods Materials
Chiral columns employed were Chiralpak IBand chiral cell OD-H250 mm×4.6 mm, packed with the 3,5-dimethyl phenyl carbamate derivative of cellulose coated on 10 mm silica-gel support. Both of them were obtained from Chiral Technologies Europe (Illkirch Cedex, France).
Reagents
Cream Dermofix was obtained from the pharmacy (Bechar, Algeria). Organic solvents, methanol, 2-propanol, and hexane were purchased from Sigma-Aldrich (Seelze, Allemagne). All Solvents were HPLC grade.
Recyclage of sertaconazole
We weighed 0.1 g of Cream Dermofix in a beaker after we added 10 ml of Methanol. The mixture obtained was then stirred for 30 minutes and filtered. The resulting filtrate was analyzed by UV and HPLC with C18column.
Analyze the filtrate by UV
The analysis was carried out using a spectrophotometer controlled by WinAspect Plus software. The UV spectrum was recorded in methanol with a scanning range between 190-400 nm.
Analyse by HPLC
The analysis chromatographic by HPLC was realized under the following conditionsː
Column C18, mobile phase, Methanol (100%) filtred and degassed by ultrasonic before use;
flow-rate, 03 mL/min; column temperature, 25 °C; volume injection, 20µl; Detector wavelength, 260 nm. Chiralpak IB and chiral cell OD-H: mobile phase, n-hexane–2-propanol (80/20:V/V), filtered and degassed by ultrasonic before use; flow-rate, 4ml/min; column temperature, 25 °C; volume injection, 20µl; Detector wavelength,260nm.
Results and discussion Analyse by UV
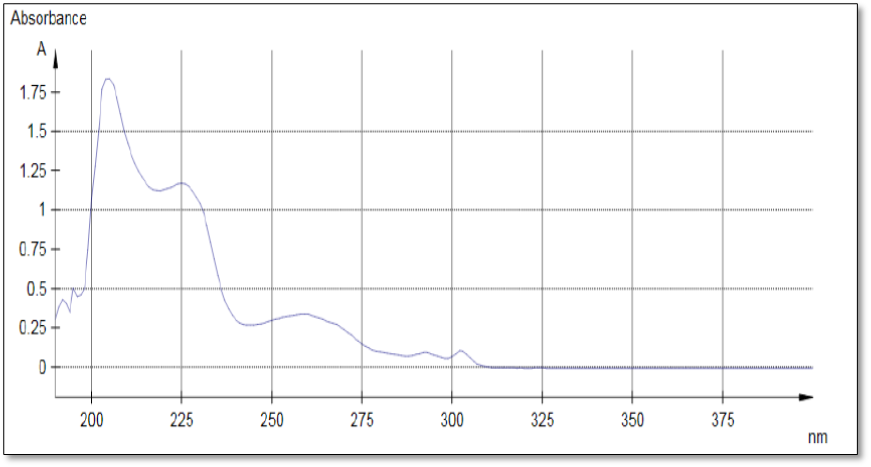
The UV spectrum showed three maximum bands at 260, 292, and 302 nm as illustrated in the following figure.
Analyse by C18 column
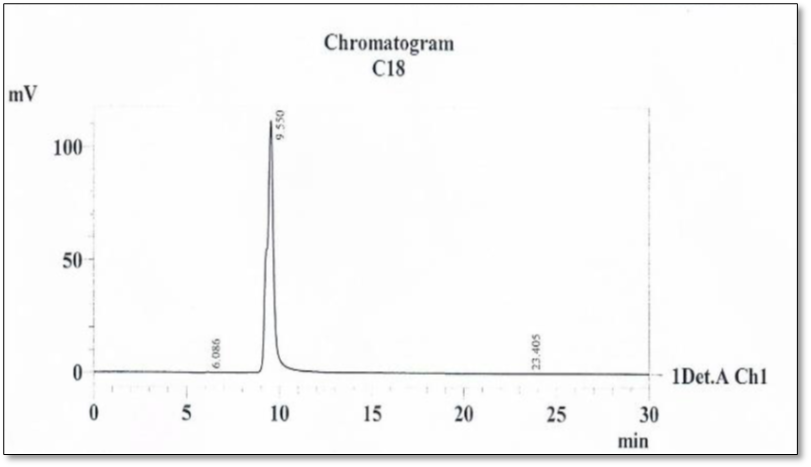
Analysis of the filtrate by C18 in the polar organic phase gave a single peak of 99.85%, indicating that the filtrate contained only one compound, which was Sertaconazole Fig.3. Therefore, Sertaconazole is pure.
Analyse by CSPs Chiralpak IB and Chiralcel OD-H
The chromatographic parameters, Tr1, Tr2, α, and Rs for the resolved enantiomers of Sertaconazole, with Chiralpak IB and chiral cell OD-H, are given in Table 1. The typical chromatogram of the resolved enantiomers of this antifungal agent on the Chiralpak IB column is shown in Fig.4

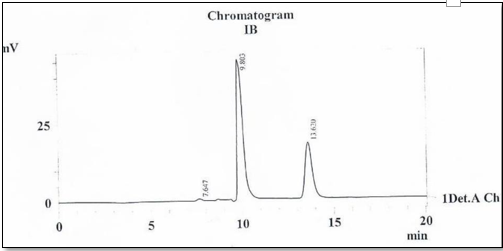
The enantiomeric separation of Sertaconazole was successfully performed under normal phase conditions with a good value of selectivity 2.77, 3.49 and better value of resolution of 6.14,8.52 for both of CSPs based on polysaccharide derivative, Chiralpak IB and Chiralcel OD-H, respectively. The result indicates that the chiral stationary phases used are suitable for the resolution of enantiomers of Sertaconazole.
- Retention time of the enantiomers of Sertaconazole is shorter on chiral pak IB and chiral cell OD-H.
- Resolution of the enantiomers of these antifungal agents on two CSPs containing the “same” chiral selector either coated (Chiralcel OD-H) or immobilized (Chiralpak IB) onto the surface of silica is better but it is best on the coated CPS (Chiralcel OD-H). This could be explained by the different conformations or rigidity of coated and covalently immobilized polysaccharide derivatives. However, the more likely reason for such differences seems to be the different chemical composition of these two types of polysaccharide-based CSPs [22, 27-30].
- The chiral discrimination mechanism at the molecular level on polysaccharide CSPs is not clear. But it has been reported that the chiral resolution by these CSPs is achieved as a result
of different hydrogen-bonding, π-π and dipole-dipole-induced interactions between the chiral
stationary phase and the enantiomers [22, 31-34]. The molecule of Sertaconazoleis an antifungal agent contains electronegative atoms namely nitrogen, oxygen, sulfur, and chlorine together with four aromatic rings in their chemical structure. Therefore, the resolution of the enantiomers of Sertaconazole is achieved due to the different hydrogen bondings between the electronegative atoms of Sertaconazole and cellulose CSP and to the π–π interactions between phenyl rings of cellulose CSP and the aromatic rings of Sertaconazole. The four aromatic rings of each enantiomer of Sertaconazole form a complex with cellulose CPS which is stabilized by π-π interactions of different magnitudes for the enantiomers and, hence, resolution of enantiomers occurs.
Conclusion
The enantiomeric resolution of the antifungal agent, Sertaconazole on chiral stationary phases based on cellulose derivatives (Chiral pak IB and Chiralcel OD-H) is achieved in the present study. The Result indicates that the separation is better(high resolution and short analysis time). Chiral separation of Sertaconazole was explained by hydrogen bondings and π-π interactions, between the CSP and this antifungal agent.
References
- Catalán-González, Mercedes, and Juan Carlos Montejo-González.
- Aicha Laoufi, Nasser Belboukhari, Khaled Sekkoum, Hassan Y. Aboul-Enein, (2021) Synthesis and chiral separation of atropisomers of 4,5-Dimethyl Δ4 N-phenyl N-aryl imidazoline-2-thione derivatives, Chirality.;1–10.
- A. J. Canilo-Mufloz, C. Tur, and J. Torres. (1996) Journal of Antimicrobial Chemotherapy 37, 815-819.
- Yuanyuan Wang, Lulu Pang, Manying Wu and Ning Ou. (2009) Journal of Chromatography B877, 4047–4050.
- Aggarwal, A. K., and P. Jindal.
- N Bounoua, K Sekkoum, M Gumustas, N Belboukhari, SA Ozkan, Development of stability indicating HPLC method for the separation and validation of enantiomers of miconazole, Chirality 30 (6), 807-815
- Imran Ali, Nadia Boumoua, Khaled Sekkoum, Nasser Belboukhari, Ayman Ghfar, Mohamed Ouladsmane, Bayan Ahmed AlJumah; (2021) A comparison of chiral resolution of antifungal agents on different polysaccharide chiral columns under various mobile phase modes: Application in the biological samples, Journal of Chromatography B 1175 ,122738
- A. Thienpont, J. Gal, C. Aeschlimann and GFélix. (1999) ANALUSIS 8 (27) 713-718.
- Alfonso Javier, Carrillo-Muñoz, Cristina Tur-Tur, Gustavo Giusiano, Cristina MarcosArias, Elena Eraso, Nerea Jauregizar and Guillermo Quindós. (2013) Expert Rev. Antiinfection. Ther 11(4) 347-358.
- Nasser Belboukhari1, Khaled Sekkoum1, Zaid Mohammed Elamine1 and Abdelkrim Cheriti Chiral Analysis of Captopril Derivatives by Hplc Methods, Acta Scientific Medical Sciences 3.7 (2019): 187-192.
- MN Rebizi, K Sekkoum, N Belboukhari, A Cheriti, HY Aboul-Enein, Liquid Chromatographic Enantioseparation of Some Fluoroquinolone Drugs Using Several Polysaccharide-Based Chiral Stationary Phases, Journal of chromatographic science
- A Kraimi, N Belboukhari, K Sekkoum, A Cheriti, HY Aboul-Enein Liquid chromatographic chiral separation of acenocoumarol and its hemiketal form, Journal of chromatographic science 55 (10), 989-991
- MN Rebizi, K Sekkoum, N Belboukhari, A Cheriti, HY Aboul-Enein Chiral separation and determination of enantiomeric purity of the pharmaceutical formulation of cefadroxil using coated and immobilized amylose-derived and cellulose-derived, Egyptian Pharmaceutical Journal 15 (2), 88
- Michael A. Pfaller, Deanna A. Sutton. (2006) Diagnostic Microbiology and Infectious Disease 56 ,147–152.
- Nasser Belboukhari; Abdelkrim Cheriti; Christian Roussel; Nicolas Vanthuyne, (2010) Chiral separation of hesperidin and naringin and its analysis in a butanol extract of Launeae arborescens, Natural Product Research: Formerly Natural Product Letters, 1478-6427, Volume 24, Issue 7, Pages 669 – 681
- Mohamed Nadjib Rebizi, Khaled Sekkoum, Antonella Petri, Gennaro Pescitelli, Nasser Belboukhari,(2021)Synthesis, enantioseparation, and absolute configuration assignment of iminoflavans by chiral high-performance liquid chromatography combined with online chiroptical detection, J Sep Sci;1–11.
- Mohammed El Amin Zaid, Nasser Belboukhari, Khaled Sekkoum, Bousmaha Ibtissam and Hassan Y. Aboul Enein, (2021) Synthesis and Chiral Separation of Some 4-thioflavones, Journal of Chromatographic Science, 1–7.
- Hassan Y. Aboul-Enein, Imran Ali. (2002) Journal of Pharmaceutical and Biomedical Analysis 27 ,441–446.
- J.L. Bernal, L. Toribio, M.J. del Nozal, E.M. Nieto and M.I. Montequi. J. (2002) Biochem. Biophys. Methods 54, 245–254.
- R. Ferretti, B. Gallinella, F. La Torre, L. Turchetto. (1997) Journal of Chromatography A 769 231–238.
- Anuvat Roongpisuthipong, Amphan Chalermchockcharoenkit, Korakot Sirimai, Prapat Wanitpongpan, Atthapon Jaishuen, Suporn Foongladda, Nisit Kongkergkiat and Jeerawan Prymanee. (2010) Asian Biomedicine 4 (3) 443-448.
- Mahima Manian, Kumpal Madrasi, Ayyappa Chaturvedula and Ajayk Banga, (2016) Pharmaceutic 8(21) 1-14.
- Jamie D. Croxtall and Greg L. Plosker. (2009) Drugs 69 (3) 339-359.
- Aboul-Enein, H. Y. and Ali, I. Fresenius J. Anal. (2001) Chem370 ,951–955.
- Bezhan Chankvetadze, (2013) Enantioseparations by High-Performance Liquid Chromatography Using Polysaccharide-Based Chiral Stationary Phases: An Overview, Chiral separations: Methods and Protocols, Edition Gerhard K. E. Scriba, 81-111
- Toshimasa Toyo’oka, (2013) Chiral Benzofurazan-Derived Derivatization Reagents for Indirect enantioseparations by HPLC, Chiral separations: Methods and Protocols, Edition Gerhard K. E. Scriba, 233-248
- Mohammed El Amin Zaid · Nasser Belboukhari · Khaled Sekkoum · Jose Carlos Menendez Ramos, Hassan Y. Aboul‑Enein (2019) Analysis of different factors affecting a liquid chromatographic chiral separation of some imino‑hesperetin compounds, SN Applied Sciences 1:1444
- Ismahan Rahou, Sekkoum Khaled and Belboukhari Nasser, 2019 Antibacterial activities of Substituted 4-Iminoflavanes, Journal of Advanced Research in Science and Technology, 6(2),978-984.
- M Bouanini, N Belboukhari, JC Menéndez, K Sekkoum, A Cheriti Chiral separation of novel iminonaringenin derivatives, Chirality 30 (4), 484-490
- I Rahou, K Sekkoum, N Belboukhari, A Cheriti, HY Aboul-Enein, Liquid chromatographic separation of novel 4-amino-flavanes series diastereomers on a polysaccharide-type chiral stationary phase, Journal of chromatographic science 54 (10), 1787-1793
- K Addadi, K Sekkoum, N Belboukhari, A Cheriti, HY Aboul‐Enein, Screening approach for chiral separation of β‐aminoketones by HPLC on various polysaccharide‐based chiral stationary phases, Chirality 27 (5), 332-338
- N Belboukhari, N Lahmar, K Sekkoum, A Cheriti, H Y Aboul-Enein, Chiral separation of several flavanones by liquid chromatography, Current Pharmaceutical Analysis 11 (3), 201-209
- K Addadi, K Sekkoum, N Belboukhari, A Cheriti, HY Aboul‐Enein, Screening approach for chiral separation of β‐aminoketones by HPLC on various polysaccharide‐based chiral stationary phases, Chirality 27 (5), 332-338
- N Belboukhari, N Lahmar, K Sekkoum, A Cheriti, H Y Aboul-Enein, Chiral separation of several flavanones by liquid chromatography, Current Pharmaceutical Analysis 11 35.


