Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2833-0951/002
Covid-19 vaccines: An educational, CME review
Advisor in Pediatrics and Pediatric Psychiatry, Children Teaching Hospital of Baghdad Medical City and the National Training and Development Center.
Correspondng Author: Aamir Jalal Al-Mosawi
Citation: Aamir Jalal Al-Mosawi (2022). Covid-19 vaccines: An educational, CME review. Biomedical and Biotechnological Sciences. 1(1). DOI: 10.58489/2833-0951/002
Copyright: © 2022 Aamir Jalal Al-Mosawi, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-07-14, Received Date: 2022-07-14, Published Date: 2022-08-02
Abstract Keywords:
Abstract
Before Covid-19 global pandemic, vaccines were developed within few years, and there have been no vaccine available for preventing corona virus infections in humans. Research aiming at developing vaccines against Coronaviridae viruses family that infect humans and cause disease including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) were conducted only in non-human animals. Therefore, there have been no approved vaccines against these earlier severe corona human infections. However, during, February 2021, eleven vaccines have been approved by at least one national regulatory authority for public use. The aim of this book is to provide an updated overview of covid-19 vaccines research.
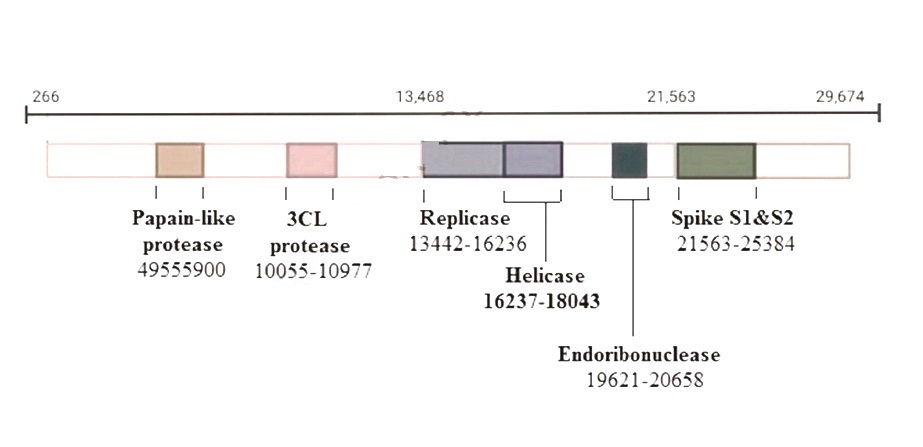
Covid-19 (SARS-CoV-2) is a Beta coronavirus of the Coronaviradae family. It is an enveloped single-stranded RNA virus having a 30 kb genome (Figure-1A) with 4 main viral structure proteins [Spike glycoprotein, membrane protein, envelope protein, and nucleocapsid protein] (Figure-1B).
93% of covid-19 spike gene sequences is a nucleotide sequence of the Rhinolophus affinis bat coronavirus RaTG13 (Figure-1C), and less than 75% covid-19 spike gene sequences is nucleotide sequence of severe acute respiratory syndrome coronavirus (SARS-CoV). The covid-19 spike gene sequences that are not preset in SARS-CoV-2 include 3 short insertions in the N-terminal domain, and 4 five key residues changes in the receptor-binding motif of spike protein receptor binding domain (RBD). The two SARS viruses have the same human cellular angiotensin converting enzyme II (ACE II) receptor.
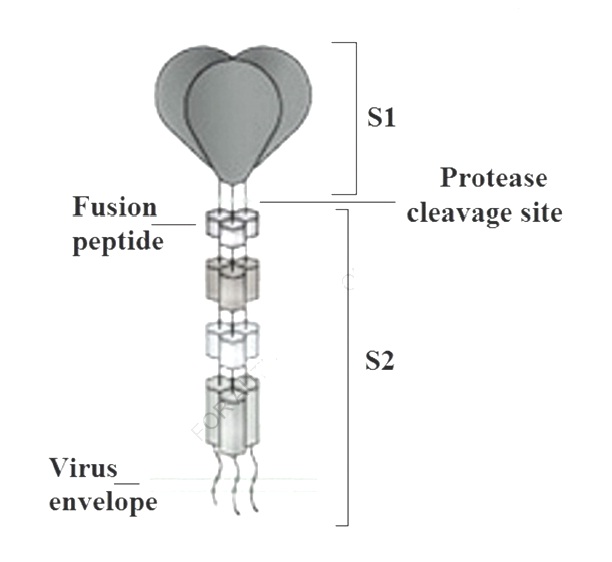
The glycosylated spike (S) protein of SARS-CoV-2 is a fusion protein that facilitates human cell entry by binding to the angiotensin converting enzyme II receptor. The spike uses a glycan shield to stop the human immune response.
Lorenzo Casalino et al (2020) from the University of California emphasized the important structural role of N-glycans at sites N165 and N234 in adjusting the conformational dynamics of the spike’s receptor binding domain, which is responsible for ACEII receptor recognition. They supported their notion by biolayer interferometry experiments, which revealed that deletion of the N-glycans at sites N165 and N234 through N165A and N234A mutations considerably decreased binding to ACE II receptor because of the associated shift of spike’s receptor binding domain conformation toward the a down state.
Figure-1B: Covid-19 has a 30 kb genome with 4 main viral structure proteins
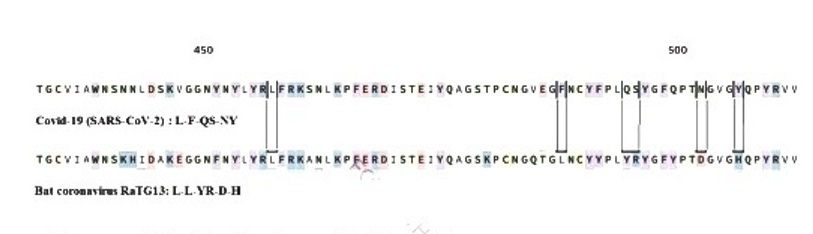
Lorenzo Casalino et al presented end-to-end accessibility analyses showing the vulnerabilities of the glycan shield of the SARS-CoV-2 S protein, and suggested considering them when designing the therapeutic interventions against the virus.
Zunlong Ke et al (2020) from Medical Research Council Laboratory of Molecular Biology in Cambridge, UK explained that the spike (S) protein trimers of SARS-CoV-2 protrude from the covering lipid bilayer and bind to the angiotensin-converting enzyme II receptor and facilitate the entry of virus into to human cells. Thereafter, the spike experiences a complete structural rearrangement to facilitate the fusion of viral membranes with human cells’ membranes. They used cryo-electron microscopy and tomography imaging of intact SARS-CoV-2 virions to show the high-resolution structure, conformational flexibility and distribution of spike’s trimers in situ on the virion surface which mediate the interactions between the spike and the neutralizing antibodies during infection or vaccination.
Before Covid-19 global pandemic, vaccines were developed within few years, and there have been no vaccine available for preventing corona virus infections in humans.
Research aiming at developing vaccines against Coronaviridae viruses family that infect humans and cause disease including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), were conducted only in non-human animals. Therefore, there have been no approved vaccines against these earlier severe corona human infections [1, 2, 3, 4, 5].
There have been vaccines developed to prevent infectious bronchitis of virus chickens have which is a group 3 coronavirus, while the SARS virus are group 4 viruses. Live infectious bronchitis virus attenuated by passage in chicken embryonated eggs was used as vaccine as early as the 1950s. The vaccine can protect chickens from developing clinical signs and loss of ciliary activity in trachea, but 10% of vaccinated chicks do not develop a protective immune response, and protection is short lived, and a decline in productivity is observed after 9 weeks.
Cross-protection associated with vaccine is generally poor, and chickens may need re-vaccination with the same or another serotype after two or three weeks. Single vaccination can protect less than 50% of chickens, and revaccination can be associated with 90 to 100% protection.
In infectious bronchitis virus, the large spike glycoprotein (S) containing a carboxy-terminal S2 subunit (about 625 amino acid residues), which attaches S to the virus envelope, and an amino-terminal S1 subunit (approximately 520 residues) are the parts which induce the viral neutralizing antibody. Differences in S1 of 2 to 3% (10 to 15 amino acids) may produce a different serotype of the virus [7].
Like infectious bronchitis virus, the spike protein, the S antigen of covid-19 virus is the part which induces the viral neutralizing antibody that can contribute to vaccine protection. During March 2020, there was only one DNA-based MERS vaccine completed Phase I clinical trials in humans.
Before the end of the year 2020, 57 covid-19 vaccines have been used in trials including 40 in Phase I-II trials. 17 in Phase II–III trials. Five of theses vaccines have been approved for public use by national regulatory authorities including Tozinameran of Pfizer-Bion-Tech, BBIBP-CorV of Sinopharm, CoronaVac of Sinovac, mRNA-1273 of Moderna, and Gam- COVID-Vac of the Gamaleya Research Institute.
Before March, 2021, there were sixty-six vaccines in clinical studies. Seventeen vaccines were still in Phase I trials, Twenty-three vaccines were in Phase I-II trials, six vaccines were Phase II trials, and twenty vaccines were in Phase III trials [6, 7]. Table-1 summarizes the definitions the phases of medical product development trials.
Table-1:The definitions the phases of medical product development trials | |
| Preclinical studies | During this phase, the product is tested in vitro (test tube or cell culture) and is also tested in experimental studies (In vivo) with use of a wide-range of doses determine the efficacy, toxicity and pharmacokinetics of the product. |
Phase 0 | During this phase, the product is tested in single sub-therapeutic doses in few subjects (10 to 15) to determine the product pharmacokinetics. |
Phase I trials Human testing | During this phase, the product is tested in non-randomized studies on a 20-100 healthy volunteers, to determine the safety, side effects, best dose, and formulation method for the drug. |
Phase II trials | During this phase, the product is tested on 50-300 participants to determine biological effects. |
Phase III trials Pre-marketing phase | During this phase, the product in a randomized controlled multi-center trials on 300-3,000 patients to determine the product clinical effectiveness. |
Phase IV trial | This phase include post-marketing safety surveillance and trials the surveillance to document any possible rare or long-term adverse effects. |
During, February 2021, eleven vaccines haven approved by at least one
national regulatory authority for public use including [6, 7]:
Four conventional inactivated vaccines: BBIBP-CorV, Covaxin, CoronaVac, and CoviVac [ru].
Two RNA vaccines: Pfizer–BioNTech vaccine and Moderna vaccine.
Four viral vector vaccines: Sputnik V, Oxford-AstraZeneca vaccine, Convidicea and Johnson & Johnson vaccine.
One peptide vaccine (EpiVacCorona).
Table-2 shows the date of registration of the eleven vaccines and the first country registered the vaccine.
Table-2: The date of registration of the eleven vaccines and the first country registered the vaccine | ||
| Vaccine | Country | Date |
| Sputnik V (Gam-COVID-Vac) | Russia | August,11, 2020 |
| EpiVacCorona | Russia | October, 14. 2020 |
Pfizer-BioNTech COVID-19 vaccine (Tozinameran) | United Kingdom | December,1,2020
|
Moderna COVID-19 vaccine (mRNA-1273) | United States of America | December, 18, 2020 |
BBIBP-CorV (Sinopharm COVID-19 vaccine) | China
| December,30,2020 |
| Oxford-AstraZeneca COVID-19 vaccine (AZD1222) | United Kingdom | December,30,2020 |
| Covaxin (BBV152) | India | January,3,2021 |
| Convidicea (AD5-nCOV) | Mexico | February,10,2021 |
CoronaVac (Sinovac COVID- 19 vaccine) | Hong Kong, China | February,18,2021 |
| CoviVac | Russia | February,20,2021 |
Johnson & Johnson COVID- 19 vaccine (Janssen COVID- 19 Vaccine) | United States of America
| February,27,2021 |
Three vaccines have been authorized for emergency USA FDA including Pfizer-BioNTech COVID-19 vaccine, Moderna COVID-19 Vaccine, and
Janssen COVID-19 Vaccine
The COVID‑19 vaccines have been credited throughout the world for decreasing the severity and mortality associated with Covid‑19.
Man distribution of covid-19 vaccines was generally prioritized to be given for individuals at high risk of complications and morbidity, including elderly, and also for individuals having high risk of exposure and transmission especially healthcare professionals and workers.
On the 8th of March 2022, 10.9 billion doses of covid‑19 vaccines have been given throughout the world [6, 7].
Sputnik V (Gam-COVID-Vac) is a viral two-vector vaccine which includes two human replication-defective adenovirus serotypes (26 and 5) which were modified to the gene that encodes the full-length spike protein of SARS-CoV-2 (Figure-2), the part which induce the viral neutralizing antibody and contribute to vaccine protection [6, 7, 8].
The vaccine was developed by Gamaleya Research Institute of Epidemiology and Microbiology under the supervision of Denis Logunov.
Sputnik V was registered on the 11th of August, 2020 by the Russian Ministry of Health, and its conditional registration was announced by president Putin at through a video conference. The distribution of Sputnik V started during December, 2020, and during March 2021, the emergency use of the vaccine was authorized in 45 including Russia, Argentina, Belarus, Hungary, Serbia and the United Arab Emirates [7.8].
Logunov al (2020) reported the development of a COVID-19 vaccine Sputnik V (Gam-COVID-Vac) which include two recombinant adenovirus vectors [serotype 26 (rAd26) and serotype 5 (rAd5)]. Both vectors were modified to (rAd26-S and rAd5-S) which include the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (Figure-2).
Logunov al reported two open, non-randomized phase I/II trials which were conducted at two hospitals in Russia during the period from June 18 to the third of August, 2020.
The two trials included 76 healthy adult volunteers (males and females) aged 18-60 years. There were 38 participants in each trial.
In phase I of the trial, the vaccine was given intramuscularly on day 0 either one dose of rAd26-S or one dose of rAd5-S for 28 days.
In phase II of the trial, which was initiated no earlier than five days after phase I vaccination, a prime-boost vaccination, with rAd26-S given on day 0 and rAd5-S on day 21 were given intramuscularly. Nine volunteers received rAd26-S in phase I, nine received rAd5-S in phase I.
Twenty participants received rAd26-S and rAd5-S in phase II.
Logunov al found the two vaccine formulations were safe and well tolerated. The most common side effects observed were pain at injection site which occurred in 44 participants (58%), hyperthermia which occurred in 38 (50%), headache which occurred in 32 participants (42%), asthenia which occurred in 21 participants (28%), and muscle and joint pain which occurred in 18 participants (24%).
Most side effects were mild and not serious.
The study showed that all participants developed antibodies to SARS-CoV-2 glycoprotein.
At day 42, the titers of receptor binding domain-specific IgG were 14 703 with the use of the frozen formulation, and 11 143 with the use of the lyophilized formulation.
The titers of the neutralizing antibodies were 49・25 with the use of frozen formulation and 45.95 with the use of lyophilized formulation, with a sero-conversion rate of 100%.
Cell-mediated responses were observed in all participants at day 28, with median cell proliferation of 2.5
BBIBP-CorV (Sinopharm COVID-19 vaccine)
is an inactivated vaccine developed by Sinopharm which was developed by rather a traditional technology. Xia et al (2020) reported phase I placebo-controlled study which included 96 participants, aged 18 and 59, and phase II study which included 224 participants, aged 18 and 59 that were performed in Henan Province, China during the period from the 12th April, 2020 to 27th of July, 2020. The ninety-six participants in phase I study were divided into four groups of twenty four participants including three vaccine groups and one placebo group. The participants in the three vaccine group received one of the three doses (2.5, 5, and 10 µg/dose). The 224 adults were participants in phase II study were divided into two vaccine groups (5 μg/dose), each with 84, and two placebo groups, and each with, 28 participants. The 320 participants (mean age, 42.8 years; 200 women [62.5%]), all completed 28 days of the study after the whole-course vaccination.
In phase I and phase II placebo-controlled studies, Xia et al found that the most common side effects associated with vaccination were injection site pain, followed by fever, which were mild and self-limiting. They didn’t report the occurrence of any side effects. The mean titers of neutralizing antibodies in the three dose groups in phase I study, at two weeks were 316 (95% CI, 218-457), 206 (95% CI, 123-343), and 297 (95% CI, 208-424). The mean titers of neutralizing antibodies in phase II study were 121 (95% CI, 95-154) at two weeks, and 247 (95% CI, 176-345) at three weeks. There were no detectable antibody production placebo groups [6, 11].
Xia et al (2021) reported a placebo-controlled, phase I/II studies which were performed at Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan Province, China.
Phase I, study included 192 participants aged 18-80 years, (mean age 53.7 years [SD 15.6]). They were divided into two age groups (18-59 years and ≥60 years), each group of ninety-six participants. Each of the two age groups were divided into three vaccine groups to, each of twenty four participants to receive a 2 μg , 4 μg , or an 8 μg dose , and one placebo group of twenty-four participants. At least one side effect was reported within one week of vaccination in 42 participants (29%) of 144 participants who received the vaccine. Fever was the most common systemic side effect. In the 18-59 years age group, fever occurred in one of the participants (4%) who received a 2 μg dose, in one of the participants (4%) who received a 4 μg dose, and in two of the participants (8%) who received an 8 μg dose.
In the ≥60 years age group, fever occurred in one of the participants (4%) who received an 8 μg group dose.
All side effects were mild or moderate in severity with no serious side effects reported within four weeks following vaccination.
The mean neutralizing antibody titers were higher on day 42 in the 18-59 years age group aged (87.7 [95% CI 64.9-118.6], 2 μg group; 211.2 [158.9- 280.6], 4 μg group; and 228.7 [186.1-281.1], 8 μg group).
The mean neutralizing antibody titers on day 42 the 60 years and older age group were (80.7 [65.4-99.6], 2 μg group; 131.5 [108.2-159.7], 4 μg group; and 170.87 [133.0-219.5], 8 μg group), compared with the participants who received placebo (2.0 [2.0-2.0]).
Phase II study included 448 participants, aged 18-59 years (mean age 41.7 years [SD 9.9]) were divided into four vaccine groups of 84 participants, and one placebo group of 112. The vaccine groups received either 8 μg doses on day 0, or 4 μg dose on days 0 and 14, days 0 and 21, or days 0 and 28.
At least one side effect occurred within the first week in 76 (23%) of 336 vaccine recipients (33 [39%], 8 μg day 0; 18 [21%], 4 μg days 0 and 14; 15 [18%], 4 μg days 0 and 21; and ten [12%], 4 μg days 0 and 28).
One participant who received placebo developed grade 3 fever, which was self-limited and recovered.
All side effects were mild or moderate in severity.
Fever was the most common systematic side effect and occurred in one participant [1%] who received an 8 μg dose. in one participants [1%] who received a 4 μg dose on days 0 and 14, in three participants [4%] who received a 4 μg dose on days 0 and 21, and in two participants [2%] who received 4 μg dose on days 0 and 28.
The vaccine-induced neutralizing antibody titers on day 28 were significantly higher in the 4 μg dose on days 0 and 14 (169.5, 95% CI 132.2-217.1), days 0 and 21 (282.7, 221.2-361.4), and days 0 and 28 (218.0, 181.8-261.3) schedules than in the 8 μg dose day 0 schedule (14.7, 11.6-18.8; all p<0>
Xia et al suggested that the inactivated covid-19 vaccine (BBIBP-CorV) is safe and well tolerated at all tested doses, and can induce humoral responses against covid-19. They found that a two-dose vaccinations with 4 μg vaccine on days 0 and 21 or days 0 and 28 were associated with a greater neutralizing antibody titers than the single 8 μg dose or 4 μg dose on days 0 and 14 [7,11].
Xia et al (2022) reported a double-blind, controlled study (Phase I/II) trial which was conducted during the period from August 14, 2020 to Sept 24, 2020 at Shangqiu City Liangyuan District Center for Disease Control and Prevention in Henan, China.
The study included healthy children (3-5 years, 6-12 years, or 13-17 years) who had no history of SARS-CoV-2 or SARS-CoV infection.
Phase I included 288. 216 children received three doses of BBIBP-CorV (Sinopharm COVID-19 vaccine). There were 24 children in each dose level [2/4/8 μg] in each of the three age cohorts [3-5, 6-12, and 13-17 years) .The control group included 72 children, 24 in each age cohort (3-5, 6-12, and 13-17 years).
Phase II included 720 children. 540 children received the vaccine. There were 60 children in each dose level [2/4/8 μg] in each of three age cohorts [3-5, 6-12, and 13-17 years]). The control group included 180, including 60 in each age cohort (3-5, 6-12, and 13-17 years).
Unwanted effects were mostly mild to moderate in severity, and included fever which occurred in 32 of 251 (12·7%) vaccinated participants, and injection site pain which occurred in 23 of 252 (9·1%) vaccinated participants.
The neutralizing antibody GMT against covid-19 ranged from 105·3 to 180·2 in participants aged 3-5 years , 84·1 to 168·6 in participants aged 6-12 years , and 88·0 to 155·7 in participants aged 13-17 years on day 28 after receiving the second dose of the vaccine.
The neutralizing antibody GMT against covid-19 ranged from 143·5 to 224·4 in participants aged 3-5 years , 127 to 184·8 in participants aged 6-12 years , and 150·7 to 199 in participants aged 13-17 years on day 28 after receiving the third dose of the vaccine.
Xia et al suggested that BBIBP-CorV (Sinopharm COVID-19 vaccine) was well tolerated and safe. The vaccine was associated with vigorous humoral responses against covid-19 infection after receiving two doses. They recommended the use of a 4 μg dose and two-shot vaccination schedule in phase III studies trials in recipients younger than 18 years[7,11].
Oxford-AstraZeneca COVID-19 vaccine (AZD1222) was developed by Oxford University and AstraZeneca. The vaccine development team was led by Sarah Gilbert, and Adrian Hill. The vaccine uses a viral vector which is a chimpanzee adenovirus ChAdOx1.
On the 30th of December 2020, the vaccine was approved for use in the United Kingdom and the first vaccination outside of a trial was administered on 4 January 2021. Thereafter, the vaccine was approved by several medicine regulatory agencies worldwide including the European Medicines Agency, and the Australian Therapeutic Goods Administration, and was also approved for emergency use by the World Health Organization.
Watanabe et al (2021) emphasized that the main target of covid-19 vaccines is the spike glycoprotein of the virus. They suggested that adenovirus-vector vaccines can provide a useful platform for the delivering the viral antigen which contribute to the production of neutralizing antibodies.
They described the structure, conformation and glycosylation of the S protein derived from the adenovirus-vectored ChAdOx1 nCoV-19/AZD1222 vaccine.
They showed the native-like post-translational processing and assembly. They also showed the expression of S proteins on the surface of cells adopting the trimeric pre-fusion conformation. Watanabe et al suggested the use of ChAdOx1 adenovirus vectors as the principal platform for covid-19 vaccines [6, 7, 12].
Voysey et al (2021) reported four ongoing controlled studies performed in the United Kingdom, Brazil, and South Africa during the period from 23rd of April to the fourth of November, 2020, The studies included 23 848 participants, aged 18 years and older wit 11 636 participants (7548 in the UK, 4088 in Brazil) included in the interim primary efficacy analysis.
Participants were divided into a control group and a vaccine group which received ChAdOx1 nCoV-19 vaccine (two doses containing 5 × 1010 viral particles, but a subset of participants in the United Kingdom received a half dose as their first dose (low dose) and a standard dose as their second dose.
The control group received meningococcal group A, C, W, and Y conjugate vaccine or saline.
The vaccine efficacy was generally 70.4% in the vaccine group which included 5807 participants, compared to 1.7% in the control group which included 5829 participants.
Three weeks after the first dose, ten participants were hospitalized for covid-19 disease and all of them were in the control group. Two of the ten hospitalized patients were considered to have a severe illness, and of them died.
175 severe side effects were observed in 168 participants, including 84 in the ChAdOx1 nCoV-19 vaccine group and 91 in the control group.
Three side effects were regarded as possibly related to a vaccine including one in the ChAdOx1 nCoV-19 vaccine group, one in the control group, and one in a participant who remained masked to group allocation.
In an other study published during March, Voysey et al (2021) reported four studies including three single-blind controlled trials which included a phase I/II study in the United Kingdom (COV001), a phase II/III study in the United Kingdom (COV002), and a phase III study in Brazil (COV003); and a double-blind phase I/II study in South Africa (COV005).
The studies were performed during the period from 23rd of April to the sixth of December, 2020. As previously described, individuals 18 years and older were randomly assigned 1:1 to receive two standard doses of ChAdOx1 nCoV-19 (5 × 1010 viral particles) or a control vaccine or saline placebo.
In the UK trial, a subset of participants received a lower dose (2.2 × 1010 viral particles) of the ChAdOx1 nCoV-19 for the first dose. 17178 participants from the four studies were included in the primary analysis including 8597 received ChAdOx1 nCoV-19 vaccine and 8581 received a control vaccine. The vaccine efficacy more than two weeks after the second dose was 66.7% (95% CI 57.4-74.0), with 84 participants developed covid-19 disease (1.0%) of the 8597 participants who received the ChAdOx1 nCoV-19 vaccine. On the other hand 248 participants developed covid-19 disease (2.9%) of the 8581 participants in the control group. No participant received the ChAdOx1 nCoV-19 vaccine was hospitalized because of covid-19 disease after the initial three-week exclusion period. On the other hand, fifteen participants who didn’t receive ChAdOx1 nCoV-19 vaccine (control group) were hospitalized because of covid-19 disease. 108 (0.9%) of 12282 participants who received ChAdOx1 nCoV-19 vaccine, and 127 (1.1%) of 11 962 participants in the control group experienced serious side effects.
Seven deaths occurred, but were considered unrelated to the vaccine including two participants who received the ChAdOx1 nCov-19 vaccine, and five participants in the control group. One of the five deaths in the control group was associated with covid-19 disease. In their, exploratory analyses, Voysey et al suggested that the vaccine efficacy after a single standard dose of vaccine from day 22 to day 90 after vaccination was 76.0% (59.3-85.9).
Voysey et al thought that the protection against covid-19 disease did not wane during this initial three months. They found that the antibody levels were maintained during the initial three months, with minimal waning by day 90. In the participants who received two standard doses of the vaccine, after the second dose, the vaccination efficacy was higher in participants with a longer prime-boost interval (vaccine efficacy 81.3% [95% CI 60.3-91.2] at ≥12 weeks) than in participants with a short interval (vaccine efficacy 55.1% [33・0-69・9] at <6>
Voysey et al also reported immunogenicity data which showed that the binding antibody responses were more than two-fold higher after an interval of twelve or more weeks, compared with an interval of less than six weeks in participants aged 18-55 years (GMR 2.32 [2.01-2.68]).
Voysey et al suggested that a 3-month dose interval can be superior to a short dose interval in protecting the largest number of people in the population as early as possible when supplies are inadequate, and can also improves protection after receiving a second dose [7,12].
Kalaska et al (2022) highlighted the association of ChAdOx1 nCoV-19 vaccine of AstraZeneca with thrombosis and thrombocytopenia, particularly in young female who can develop unusual localized thrombosis following vaccination.
In an experimental model of electrically-induced arterial thrombosis in the carotid artery of female rats. Kalaska et al found that at four weeks following vaccination, ChAdOx1 nCoV-19 vaccine was associated with covid-19 specific neutralizing antibody responses in all rats.
There was slight luminal narrowing of the carotid artery with extravasation of blood in vessel/thrombus area in vaccinated rats. The changes were not associated with differences in thrombus weight and composition.
The vaccinated rats experienced a slight increase (14-24%) in platelet aggregation.
ChAdOx1 nCoV-19 vaccination was not associated with significant changes in blood coagulation, platelet counts, and their activation markers.
Kalaska et al suggested that ChAdOx1 nCoV-19 vaccination is not associated with higher risk of arterial thrombosis in female rats, because it didn’t affect affected thrombus formation, and was not associated with thrombocytopenia or changes in hemostasis parameters [12].
Covaxin (BBV152) is a whole-virion, inactivated vaccine developed by Bharat Biotech in collaboration with the Indian Research Council of Medical Research and the National Institute of Virology. It is formulated with a tolllike receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG) or alum (Algel).
Ella et al (2021) reported a multi-centre, controlled phase I study which was performed in eleven hospitals in India. The study included 375 participants, aged 18-55 years, including 300 in three vaccine groups of 300, and seventy-five participants the control group (received Algel only).
Participants in the three vaccine groups received one of three formulations (3 μg with Algel-IMDG, 6 μg with Algel-IMDG, or 6 μg with Algel).
The vaccine was given in two intramuscular doses on day 0 and day 14. Following the receive of the two doses, local and systemic side effects were observed in 17 participants (17%; 95% CI 10.5-26.1) who received 3 μg with Algel-IMDG, 21 participants (21%; 13.8-30.5) who received 6 μg with Algel-IMDG, 14 participants (14%; 8.1-22.7) who received 6 μg with Algel , and ten participants (10%; 6・9-23・6) who received Algel-only.
The most common side effects were pain injection site which occurred in seventeen of 17 of 375 participants (5%), headache which occurred in 13 participants (3%), fatigue which occurred in 11 participants (3%), fever which occurred in 9 participants (2%), and nausea or vomiting which occurred in seven (2%).
All the side effects were mild or moderate and were more common following the first dose. One side effect of viral pneumonitis occurred in a participant who received 6 μg with Algel, but was considered to be unrelated to the vaccine.
Seroconversion rates were 87・9% in participants who received 3 μg with Algel-IMDG, 91.9%, in participants who received 6 μg with Algel-IMDG, and 82.8% in participants who received 6 μg with Algel.
CD4+ and CD8+ T-cell responses were found in a subset of sixteen participants from both Algel-IMDG groups [6, 7, 13].
In an other paper, published during March, Ella et al (2021) reported a multi centre controlled phase II study which performed in nine hospitals in India. The study included 380 healthy adults and adolescents participants (aged 12 -65 years).
190 participants received a 3 μg with Algel-IMDG vaccine, and 190 participants receive a 6μg with Algel-IMDG vaccine. The vaccine was given in two intramuscular doses of vaccine on day 0 and day 28. The mean titers (GMTs; PRNT50) at day 56 were significantly greater in the 6 μg with Algel-IMDG vaccine than with the 3 μg with Algel-IMDG vaccine.
Seroconversion based on PRNT50 at day 56 was available for 171 participants (92.9% [95% CI 88.2-96.2] of 184 participants who received the 3 μg with Algel-IMDG vaccine, and was available for 174 participants (98.3% [95.1-99.6]) of 177 participants who received the 6 μg with Algel- IMDG vaccine. GMTs (MNT50) at day 56 were 92・5 (95% CI 77.7-110.2) in the participants who received 3 μg with Algel-IMDG vaccine, and were 160.1 (135.8-188.8) in the participants who received 6 μg with Algel-IMDG vaccine.
Seroconversion based on MNT50 at day 56 was available for 162 participants (88.0% [95% CI 82.4-92.3]) of 184 participants who received the 3 μg with Algel-IMDG vaccine, and available for 171 participants (96.6% [92.8- 98.8]) of 177 participants who received the 6 μg with Algel-IMDG vaccine.
Ella et al found no important difference in the percentage of participants who received the 3 μg with Algel-IMDG vaccine, and the participants who received the 6 μg with Algel-IMDG vaccine and experienced local or systemic side effects. They didn’t report the occurrence of serious side effects in the study.
Ella et al emphasized that in phase I study, BBV152 vaccine resulted in high neutralizing antibody responses that continued to be elevated in all participants at three months after the second vaccination.
In the phase II study, BBV152 vaccine resulted in better reactogenicity and safety outcomes, and improved humoral and cell-mediated immune responses compared with the phase I study. They also stressed the 6 μg with
Algel-IMDG vaccine formulation was chosen for the phase III efficacy study [13].
In an other paper, published during December, Ella et al (2021) reported a double-blind, placebo-controlled, phase III clinical trial which was conducted during the period from November 16, 2020 to January 7, 2021 in 25 Indian hospitals or medical clinics. The study included 24419 participants aged 18 years or older including health participants and participants having stable chronic disorders except immune deficiency condition (Including immunosuppressive treatment).
12221 participants received two intramuscular doses of Covaxin (BBV152), vaccine, and 12 198 participants received placebo 4 weeks apart.
130 of 16973 participants whom were followed for least 14 days after the second dose, developed symptomatic covid-19 disease.
24 of 8471 (0·3%) vaccine recipients developed symptomatic covid-19 disease, and 106 of 8502 (1·2%) placebo recipients, developed symptomatic covid-19 disease.
The overall efficacy of Covaxin (BBV152) was 77·8% (95% CI 65·2-86·4).
The safety population included 25753 participants. 5959 unwanted effects were reported in 3194 participants. Covaxin BBV152 was well tolerated 1597 of 12 879 (12·4%) vaccine recipients experienced unwanted effects, and 1597 of 12 874 (12·4%) of placebo recipients experienced unwanted effects.
There was no important difference in the distributions of serious adverse effects between vaccine and placebo recipients. There was no case of anaphylaxis or vaccine-related deaths reported in this study.
This study shoed that Covaxin BBV152 was well tolerated, and very effective against symptomatic covid-19 disease [13].
CoronaVac (Sinovac COVID-19 vaccine) is an inactivated virus vaccine developed by Sinovac Biotech Sinovac Life Sciences, Beijing, China. It was developed using a traditional technology similar to BBIBP-CorV and BBV152 vaccines. CoronaVac does not need to be stored frozen, and can be stored and transported at 2-8 °C. The vaccine used in phase I was produced using a cell factory process (CellSTACK Cell Culture Chamber 10, Corning, Wujiang, China) in a dose escalating manner, while the vaccine used in phase II was manufactured using a bioreactor process (Ready To Process WAVE 25, GE, Umea, Sweden) [6,7].
Zhang et al (2021) reported a placebo-controlled, phase I/II study which included participants aged 18-59 years who were enrolled from the community in Suining County of Jiangsu province.
During the period from the 16th of April to the 25th April, 2020, 144 participants were joined phase I study, and during the period from the 3rd of May to the 5th May, 2020, 600 participants joined phase II study.
The vaccine was given in two vaccination schedules, the days 0 and 14 vaccination group and the days 0 and 28 vaccination group.
In phase I study, the first thirty-six participants in each group were divided into vaccine group of 24 participants who received low dose CoronaVac 3 μg per 0.5 ml of aluminum hydroxide diluent per dose, and a placebo group.
The first thirty-six participants in each group were divided into vaccine group of 24 participants who received high-dose CoronaVac 6 μg per 0.5 ml of aluminum hydroxide diluent per dose, and a placebo group.
In the phase II study, at screening, participants were initially divided into two groups; the days 0 and 14 vaccine group and the days 0 and 28 vaccine group.
Thereafter, the participants were randomly divided into three groups (2:2:1) to receive two doses of either low-dose CoronaVac, high-dose CoronaVac, or placebo.
143 participants received at least one dose of the vaccine for phase I study, and 600 for phase II study.
In phase I study, side effects for the days 0 and 14 group occurred in seven (29%) participants of 24 participants who received 3 μg dose, in nine participants (38%) of 24 participants who received 6 μg dose, and in two participants (8%) of 24 participants who received placebo.
Side effects for the days 0 and 28 group, occurred in three participants (13%) of 24 participants who received 3 μg dose, in four participants (17%) of 24 participants who received 6 μg dose , and in three participants (13%) of 23 participants who received placebo.
The sero-conversion of neutralizing antibodies on day 14 following the days 0 and 14 vaccine group occurred in 11 participants (46%) of 24 participants who received 3 μg dose, in 12 participants (50%) of 24 participants who received 6 μg dose , and in none (0%) participants who received placebo.
The sero-conversion of neutralizing antibodies on day 28 after the days 0 and 28 vaccine group occurred in 20 participants (83%) of 24 participants who received 3 μg dose, in 19 participants (79%) of 24 participants who received 6 μg dose, and in one participant (4%) of 24 participants who received placebo.
In the phase II study, side effects for the days 0 and 14 group occurred in 40 participants (33%) of 120 participants who received 3 μg dose, in 42 participants (35%) of 120 participants who received 6 μg doses, and in 13 participants (22%) of 60 participants who received placebo.
Side effects for the days 0 and 28 vaccine group occurred in 23 participants (19%) of 120 participants who received 3 μg dose, in 23 participants (19%) of 120 participants who received 6 μg doses, and in 11 participants (18%) of 60 participants who received placebo.
Seroconversion of neutralizing antibodies occurred in 109 participants (92%) of 118 participants who received 3 μg dose, in 117 participants (98%) of 119 participants who received 6 μg dose, and in two participants (3%) of 60 participants who received placebo at day 14 after the days 0 and 14 vaccine group, while at day 28 after the days 0 and 28 vaccine group, sero-conversion occurred in 114 participants (97%) of 117 participants who received 3 μg dose, 118 participants (100%) of 118 participants who received 6 μg dose , and occurred in no (0%) participant of 59 participants who received placebo [7,13].
Zhang et al recommended the use of the 3 μg dose of CoronaVac for efficacy evaluation in phase I study.
Wu et al (2021) reported a placebo-controlled, phase I/II study of CoronaVac which include healthy adult participants, aged sixty years and older and was performed in Renqiu (Hebei, China).
During the period from the 22nd of May to the first of June, 2020, seventy two participants were enrolled in phase I study including 24 participants in each vaccine group and 24 participants in the placebo group.
During the period from the 12th June to the 15th of June, 2020, 350 participants were enrolled in phase II study including 100 participants in each of three vaccine group and 50 participants in the placebo group.
Vaccine or placebo was given by intramuscular injection in two doses (days 0 and 28).
Phase I included a dose-escalation study, where participants were divided into to two groups to receive 3 μg inactivated virus in 0.5 ml of aluminum hydroxide solution per injection or 6 μg per injection.
Two thirds of each group received CoronaVac or placebo (aluminium hydroxide solution only). In phase II study, the participants were divide into four groups (2:2:2:1) to receive CoronaVac at 1・5 μg, 3 μg, or 6 μg per dose, or placebo.
In the two phases, within 28 days after injection, side effects occurred in 20 participants (20%) of 100 participants who received 1.5 μg dose, in 25 participants (20%) of 125 participants who received 3 μg dose, in 27 participants (22%) of 123 participants who received 6 μg dose, and in 15 (21%) of 73 participants who received placebo group.
All side effects were mild or moderate in severity and pain at the injection site was the most common side effect occurring in 39 participants (9%) of 421 participants. As of 28th of August, 2020, eight side effects occurred in seven participants (2%), but were considered to unrelated to the vaccine.
In phase I, sero-conversion after the second dose occurred in 24 of 24 participants (100.0% [95% CI 85.8-100.0]) who received 3 μg dose and in 22 of 23 participants (95.7% [78.1-99.9]) who received 6 μg dose. In phase II, sero-conversion occurred in 88 of 97 participants who received 1.5 μg dose (90.7% [83.1-95.7]), in 96 of 98 participants who received 3 μg dose (98.0% [92.8-99.8]), and in 97 of 98 (99.0% [94.5-100.0]) participants who received 6 μg dose. Antibody responses didn’t occur in the placebo groups.
Wu et al suggested that CoronaVac was safe and well tolerated in older adult participants. As the neutralizing antibody titers produced by the 3 μg dose vaccine were similar to those produced by the 6 μg dose vaccine, and higher than those produced by the 1.5 μg dose vaccine, they suggested the use of the 3 μg dose CoronaVac in phase II trials study [12,13].
Han et al (2021) reported a double-blind, controlled, phase 1/2 clinical trial of CoronaVac which included 550healthy children (3-17 years). The study was conducted at Hebei Provincial Center for Disease Control and Prevention in Zanhuang (Hebei, China).
Phase I was conducted during the period from October 31, 2020 to Dec 2, 2020 and included 71 participants who received at least one dose of vaccine (in 0·5 ml aluminum hydroxide adjuvant) or aluminum only.
Phase II was conducted during the period from December 12, 2020 to Dec 30, 2020 and included 480 participants who received at least one dose of vaccine (1·5 μg /3 μg in 0·5 ml aluminum hydroxide adjuvant) or aluminum only
In the safety profile of both phases, unwanted effects were reported in 56 (26%) of 219 participants who received 1·5 μg vaccine, 63 (29%) of 217 in participants who received 3 μg vaccine, and 27 (24%) of 114 participants who received aluminium only, without significant difference (p=0·55).
Most unwanted effects were mild and moderate in severity. Pain at the injection site was the most common unwanted effects occurring in 73 of 550 participants (13%). Pain at the injection site occurred in 36 of 219 participants (16%), occurred in 35 of 217 participants (16%) who received 1·5 μg vaccine, occurred in 35 of 217 participants (16%) who received 3 μg vaccine, and two participants who received only aluminium (2%).
As of June 12, 2021, only one serious adverse effect of pneumonia not related to the vaccine occurred in a participant who received only aluminium.
In phase I, sero-conversion of neutralizing antibody following the second dose occurred in 27 of 27 participants who received 1·5 μg vaccine, and in 26 of 26 participants who received 3 μg vaccine, with the geometric mean titers of 55·0 and 117·4.
In phase II, sero-conversion occurred in 180 of 186 participants (96·8%) 1·5 μg group and 180 of 180 participants (100·0% [98·0-100·0]) who received 3 μg vaccine with the geometric mean titers of 86·4 (73·9-101·0) and 142·2 (124·7-162·1). There were no detectable antibody responses in participants who received only aluminium.
The work of Han et al suggested that CoronaVac was safe and well tolerated. The vaccine was associated with induction humoral responses in children. Neutralizing antibody titres associated with 3 μg vaccine were higher than those associated 1·5 μg vaccine. Therefore, Han et al recommended the use of 3μg vaccine with a two-dose in future studies in children (3-17 years) [7, 13].
Johnson & Johnson COVID-19 (Ad26.COV2.S) vaccine is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector vaccine. It contains a full-length and stabilized covid-19 spike protein. It was developed by Janssen Vaccines in Leiden (Netherlands), and Belgian Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson. The vaccine is given in only one dose and does not need to be frozen when stored and during transportation. The vaccine was authorized for emergency uses by the US Food and Drug Administration and was authorized for a conditional marketing by the European Medicines Agency [6, 7].
Sadoff et al (2021) reported a multi-center, placebo-controlled, phase I-IIa study which included healthy adult participants aged ages of 18-55, 65 years of age or older. The participants received low dose Ad26.COV2.S vaccine (5×1010 viral particles/ml) or high dose vaccine (1×1011 viral particles/ml) or placebo, in a single-dose or two-dose schedule.
The most frequent side effects were fatigue, headache, myalgia, and pain injection-site. The most frequent systemic side effect was fever. Systemic side effects were less common in the older age group, and also less in participants who received the low vaccine.
Reactogenicity was less after the second dose. Neutralizing-antibody titers against wild-type virus were found in 90% or more of all participants on day 29 after the first vaccine dose (Mean titer [GMT], 224 to 354), and reached 100% by day 57 with more elevation in titers (GMT, 288 to 488), regardless the age group or the dose of the vaccine.
Titers continued to be stable until at least day 71.
The second dose was associated with an elevation in the titer by a factor of 2.6 to 2.9 (GMT, 827 to 1266).
Spike-binding antibody responses were similar to neutralizing-antibody responses. On day 14, CD4+ T-cell responses were observed in 76 to 83% of the participants in the younger age group and in 60 to 67% of the older age group, with an obvious skewing toward type 1 helper T cells. CD8+ T-cell responses were generally strong, but were lower in the older age group [7, 12].
Sadoff et al (2021) reported an international, double-blind, placebo-controlled, phase III trial which included 19,630 covid-19 negative adult participants who received Ad26.COV2.S and 19,691 who received placebo.
Sadoff et al found that Ad26.COV2.S had a protective effect against moderate to severe-critical covid-19 disease with onset at least 14 days after vaccination.
116 vaccinated participants developed covid-19 disease, while, 348 participants who received placebo developed covid-19 disease. Vaccine efficacy was 66.9%.
The vaccine was more protective against severe-critical Covid-19 (Efficacy: 76.7%) for onset at ≥14 days and Efficacy was 85.4%.
86 of 91 participants (94.5%) in South Africa were infected with 20H/501Y.V2 variant, vaccine efficacy was 52.0% against moderate to severe-critical Covid-19 with onset at least 14 days and 64.0% at least 28 days after vaccination. The efficacy against severe-critical Covid-19 was 73.1% with onset at least 14 days and 81.7%, at least 28 days after vaccination.
Reactogenicity was higher in vaccinated participants than in participants who placebo, but was mostly mild to moderate and short-lived.
Three deaths (none were Covid-19-related) were reported in the vaccinated participants, and 16 deaths occurred in participants who received placebo including five 5 Covid-19-related deaths.
The study of Sadoff et al suggested that a single dose of Ad26.COV2.S vaccine can protect against symptomatic Covid-19 disease and asymptomatic infection and can also protect against severe-critical disease, including hospitalization and death [7, 13].
Sadoff et al (2022) emphasized that Johnson & Johnson COVID-19 (Ad26.COV2.S) vaccine was very protective against severe-critical covid-19 disease, hospitalization, and death in the primary phase III efficacy analysis. They reported a final assessment in the double-blind phase of a multinational, placebo-controlled study which included 8940 participants followed for least six months.
In the per-protocol population of 39,185 participants, the vaccine was 56.3
Covid-19 vaccines: an overview
Rogliani et al (2021) studied covid-19 vaccines’ efficacy inducing neutralizing antibodies against covid-19 disease .They studied 836 healthy adult vaccine recipients from 11 studies.
BBIBP-CorV (Sinopharm COVID-19 vaccine), Oxford-AstraZeneca COVID-19 vaccine (AZD1222), Pfizer/BioNTech vaccine (BNT162b2), and Sputnik V were associated with a significant effect on the level of neutralizing antibodies (SMD > 1.3).
CoronaVac of Sinovac and Convidicea (Ad5-nCoV) were also associated with a significant effect (SMD > 0.8 to ≤1.3).
Ad26.COV2.S (JOHNSON & JOHNSON COVID-19) was associated with a medium effect (SMD > 0.5 to ≤0.8).
BBIBP-CorV (Sinopharm COVID-19 vaccine), and Oxford-AstraZeneca COVID-19 vaccine (AZD1222) were more effective (p < 0> (Ad5-nCoV), Moderna COVID-19 vaccine (mRNA-1273), and CoronaVac of Sinovac.
CoronaVac was more effective (p < 0>
Sputnik V and Pfizer/BioNTech vaccine (BNT162b2) were more effective (p < 0>
In recipients aged ≤60 years, Oxford-AstraZeneca COVID-19 vaccine (AZD1222), Sinopharm COVID-19 vaccine), BBIBP-CorV, and mRNA-1273 of Moderna were the most effective vaccines.
Rogliani et al found that all covid-19 vaccines produced considerable levels of SARS-CoV-2 neutralizing antibodies. However, only AZD1222 and mRNA-1237 were studied in patients aged ≥70 years.
Pfizer/BioNTech vaccine BNT162b and mRNA-1237 of Moderna can be more rapidly re-engineered to mimic new mutations of covid-19 [7, 13].
Liu X et al (2021) reported a study which was conducted during the period from February 11 to February, 26, 2021 in the United Kingdom ,and included 463 adult participants, aged 50 years and older (Mean age: 57·8 years), with no or well controlled comorbidities, and had no prior covid-19 disease. The participants were randomly assigned to receive heterologous vaccination schedules given at 28-day prime-boost intervals:
1-ChAdOx1 nCoV-19 of AstraZeneca / ChAdOx1 nCoV-19 of AstraZeneca.
2-ChAdOx1 nCoV-19 of AstraZeneca / Pfizer/BioNTech vaccine (BNT162b).
3-Pfizer/BioNTech vaccine (BNT162b) /Pfizer/BioNTech vaccine (BNT162b).
4-Pfizer/BioNTech vaccine (BNT162b) / ChAdOx1 nCoV-19 of AstraZeneca.
Pfizer/BioNTech vaccine (BNT162b)/ ChAdOx1 nCoV-19 of AstraZeneca and ChAdOx1 nCoV-19 of AstraZeneca / Pfizer/BioNTech vaccine (BNT162b) heterologous vaccination schedules were associated with higher levels of covid-19 anti-spike IgG than that of ChAdOx1 nCoV-19 of AstraZeneca alone.
Liu X et al supported flexibility using heterologous prime-boost vaccination using ChAdOx1 nCoV-19 of AstraZeneca and Pfizer/BioNTech vaccine (BNT162b) [7-12].
Yu et al (2022) reported a study which included 200 recipients vaccinated with three doses of a covid-19-inactivated vaccine.
Vaccination was associated with 95.5% positive neutralization activity for the Omicron covid-19 variant (B.1.1.529).
Vaccination was associated with 99.5% for covid-19 prototype.
Vaccination was associated with 98.5% for covid-19 Delta variant.
The geometric mean titers (GMT) for the Omicron covid-19 variant (B.1.1.529) was 49 and the immune levels remained sustained for two months.
Thereafter, the immune levels declined by 4.9-fold when compared with the prototype (GMT, 239), and declined by 3.0-fold when compared with the Delta variant (GMT, 148).
The study of Yu et al suggested that using three doses of a covid-19-inactivated vaccine can be effective and is associated with cross-neutralizing activity against Omicron covid-19 variant (B.1.1.529) at two months following the third dose of the vaccine [7,12].
Acknowledgement
Some of the figures in this paper were used in previous author’s publications, but he has their copyright.
Conflict of Interest
None.
References
- Al-Mosawi AJ. Bat-Human Coronaviruses: A Global Health Problem and a Therapeutic Challenge. Journal of Medical Clinical Case Reports 2020; 2(2) 1-3. Doi: 10. 5281/zenodo.3878405
- Al-Mosawi AJ. The Use of the Available Research Evidence to Crack the Padlock of Sars-CoV-2. Journal of Virology Research & Reports 2020; 1 (1):1-8 Doi: 10.5281/ zenodo.3970844
- Al-Mosawi AJ. Bat-human coronaviruses: Keys to the therapeutic challenge.1st ed., Saarbrücken; LAP Lambert Academic Publishing: 2020 (ISBN: 978-620-0-47386-8).
- Al-Mosawi AJ. Using research evidence to crack the padlock of SARS CoV-2. 1st ed., LAP Lambert Academic Publishing, Saarbrücken; Germany: 2020 (ISBN: 978-620-2-67319-8).
- Al-Mosawi AJ. The use of the available research evidence to crack the padlock of SARS-CoV-2.1st ed., Baghdad; Iraq Headquarter of Copernicus Scientists International Panel Publishing: 2020 (ISBN: 9798655618800).
- Al-Mosawi AJ. Covid-19 vaccines research progress (Ed). LAP Lambert Academic Publishing, Saarbrücken: 2021 (ISBN: 978-620-3-57458-6).
- Al-Mosawi AJ. Covid-19 vaccines: An updated overview. LAP LAMBERT Academic Publishing: March, 2022 (ISBN: 978-613-9-44833-3).
- Al-Mosawi AJ. Вакцины Covid-19: Обновленный обзор (Russian edition). Sciencia Scripts :2022-04-28 (ISBN-13:978-620-4-65076-0, ISBN-10: 6204650769).
- Al-Mosawi AJ. Covid-19-Impfstoffe: Ein aktueller Überblick (German edition). Verlag Unser Wissen: 2022-04-28 (ISBN-13: 978-620-4-65064-7, ISBN-10: 6204650645).
- Al-Mosawi AJ. Vaccins Covid-19: Une vue d'ensemble actualisée (French edition). Editions Notre Savoir: 2022-04-28 (ISBN-13: 978-620-4-65069-2, ISBN-10: 6204650696).
- Al-Mosawi AJ. Vacunas Covid-19: Una visión general actualizada (Spanish edition).Ediciones Nuestro Conocimiento: 2022-04-28 (ISBN-13: 978-620-4-65066-1, ISBN-10: 6204650661).
- Al-Mosawi AJ. Vaccini Covid-19: Una panoramica aggiornata (Italian edition). Edizioni Sapienza: 2022-04-28 (ISBN-13: 978-620-4-65074-6. ISBN-10: 6204650742).
- Al-Mosawi AJ. Vacinas Covid-19: Uma visão actualizada (Portuguese edition). Edições Nosso Conhecimento: 2022-04-28 (ISBN-13: 978-620-4-65075-3, ISBN-10: 6204650750).


