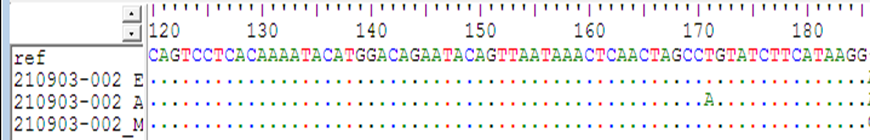Research Article | DOI: https://doi.org/10.58489/2836-497X/006
Detection of Mthfr (C667t) and Mthfd (G1958a) Polymorphisms Among Sudanese Women with The Recurrent Miscarriages
- Sara Elsadig Babiker 1,2
- Maye M. Merghani 6
- Naima osmanyusuf 3
- Babangida Umar 3
- Amr Mohammed Ali ALshahethi 3
- Tyseer A. M. Abdalrahman 2
- Alsadig Gassoum 2,4
- Mohamed Elfatih Abdewadoud 3
- Hassan Hussein Musa 1,5
- Nihad Elsadig Babiker* 1,2,3
- Darfur University College, Sudan
- National Center of Neurological Sciences, Sudan
- University of Medical Science and Technology
- Almadein University College, Sudan
- Faculty of Medical Laboratory Sciences, University of Khartoum, Sudan
- Nahda college, Sudan
*Corresponding Author: Nihad Elsadig Babiker
Citation: S.E. Babiker, M.M. Merghani, N.osmanyusuf, Babangida U., A. Mohammed Ali AL shahethi, Tyseer A. M. Abdalrahman, A.Gassoum, Md. Elfatih Abdewadoud, Hassan H.M., N. E. Babiker, (2023). Detection of Mthfr (C667t) and Mthfd (G1958a) Polymorphisms Among Sudanese Women with The Recurrent Miscarriages. Archives of Gynaecology and Women Health. 2(1). DOI: 10.58489/2836-497X/006.
Copyright: © 2023 Nihad Elsadig Babiker, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 25 January 2023 | Accepted: 03 February 2023 | Published: 06 February 2023
Keywords: recurrent pregnancy loss, dna, gene, mutation and polymorphism
Abstract
Background
Recurrent pregnancy loss affects 1 to 5% of women trying to conceive, I t has a significant impact at individual and social level.
Methodology
case-control study conducted at the research laboratory of the national center of neurological sciences, Khartoum, Sudan. All patients attending Ibrahim Malik teaching hospital and diagnosed with recurrent spontaneous abortion were included as case and healthy women at reproductive age were included as controls. The DNA was isolated by standard phenol chloroform extraction method, The PCR was done by using commercial thermal cycler machine with specific protocol. PCR products were sent for sequencing to Macro gene Europe Laboratory.
Results
494 bp of MTHFR(C677T) and 174 bp of MTHFD (rs2236225) (G19581A) alleles were detected after PCR. The PCR result of MTHFR(C677T) shows; 40 (90%) forMTHFR (C677T) allele was positive in the cases and only12 (24 %) were positive for the control group. For MTHFD (G19581A) allele 42 (95%) were positive in the case group and 14 (28%) were positive in the control. When compared between case and control there was highly significant differences. The sequencing results for MTHFR C677; single Base Exchange was found C>T in the case groups, when used the mutation taster software the mutation was predicted. For MTHFD (G1958A) in the case group tow single Base Exchange were found T>A and G>A, the mutation taster software was predicted T>A mutation.
Conclusion
According to this result the MTHF polymorphisms might become one of the main causes of unexplained diagnosis women with recurrent spontaneous abortion
Introduction
Recurrent pregnancy loss (RPL) is generally affect 1 to 5% of women trying to conceive. Recurrent pregnancy loss (RPL) is defined as two or more consecutive pregnancy losses before the 20th week of gestation [1,2].
The loss of pregnancy at any stage can be a traumatic experience and particular sensitivity is required in assessing and counselling couples with recurrent pregnancy loss. They are more susceptible to experiencing higher levels of depression, anxiety and feelings guilty than the general population. Therefore, these couples may benefit from regular contact and reassurance [3].
Although RPL has significant impact at individual and social level, there is insufficient screening methods for risks of recurrent pregnancy loss. Previous reproductive history is an independent predictor of future pregnancy outcome. The risk of a further miscarriage increases after each successive pregnancy loss, reaching approximately 40
Material and method
This was case-control study conducted at the research laboratory of the national center of neurological sciences (NCNS), Khartoum, Sudan during the period April to September, 2021to detect the possible MTHF gene polymorphisms among women with RSA
All patients attending obstetrics and gynecology unit at Ibrahim Malik teaching hospital and diagnosed with recurrent spontaneous abortion during the aforementioned period were included.Women with more than three recurrent abortions were included in this study as cases and those healthy women at reproductive age were included as controls. Women with chromosomal abnormality, uterine anomalies, genital infections, and endocrinological disorder were excluded. The data was collected by using well stricture questionnaire. Ethical clearance for this study was obtained from ethical review committee, Faculty of Medical laboratory University of Medical Science and Technology, the participants was fully informed about the advantages and disadvantages before participation in the research (verbal informed consent). From each participant 3 ml of venous blood was withdrawn with minimal stasis from the ante-cubital vein using a dry sterile disposable syringe and needle. Blood samples were dispensed into sterile containers with Ethylene Diamine Tetra-acetic Acid (EDTA). They were labeled with subject’s age, sex and identification number and stored at -20°C for molecular analysis.
The DNA was isolated from peripheral blood leukocyte by standard phenol chloroform extraction method. Primers were designed by using Prime3 software. The forward primer for MTHFR 677 was designed as “5- GGTCAGAAGCATATCAGTCATGAG -3”and reverse as “5- CTGGGAAGAACTCAGCGAACTCAG -3” with product size of 494bp fragment. And MTHFD (rs2236225) forward primer “5- CTCCCAAAGTGCTGGGAGTA -3” and reverse as “5- CCTTATGAAGATACAGGCTAGTTGA -3” with product size of 174bp fragment
The PCR was done by using commercial thermal cycler machine (SwiftTMMaxPro SWT-MXP-BLC-4) with specific protocol according to annealing temperature of the primers.
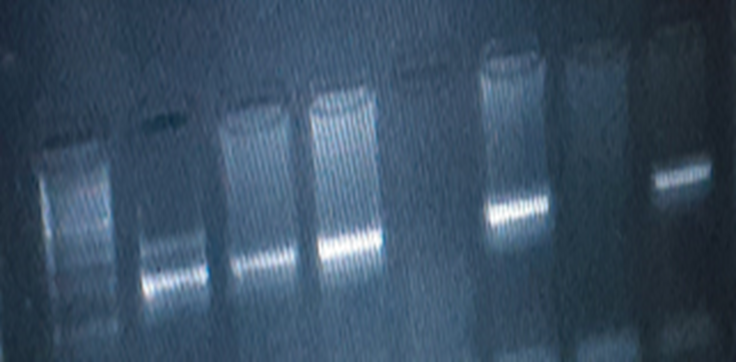
The PCR amplification product was separated on agarose gel and visualized through gel documented system. PCR products were sent for sequencing to Macro gene Europe Laboratory.
Results
In the present study 44 (46.8%) were selected as cases and 50 (53.2%) were selected as control group (Table 1). The most affected age group was 25-34 year 16 (36.4%), followed by 35-40 years 15 (34.1%) (Table2).
the frequency of the abortion number in the case group; most of them had abortion for three time with frequency about 77.3 %, four times about 9.1%, five time 2.3%, six time 6.8% and nine time about 2.3% (Table 3). The frequency of their gestational age is; 11-14 week about 47.7%, 7-10 weeks about 34.1% and 6 weeks 18 % (Table3)
Table [1]: Distribution of study participant according to study group
| Frequency | Percentage | Valid percent | Cumulative Percent |
Cases | 44 | 46.8 | 46.8 | 46.8 |
Controls | 50 | 53.2 | 53.2 | 100 |
Total | 94 | 100 | 100 |
|
Table [2]: Basic characteristic of study population
Parameters | No (%) | P value |
Sex |
|
0.000 |
Case | 44 (46.8%) | |
Control | 50 (53.2%) | |
Age |
|
0.000 |
18-24 | 6 (13.6%) | |
25-34 | 16 (36.4%) | |
35-40 | 15 (34.1%) | |
>40 | 7 (15.9%) |
Table (3) Distribution of variables
Variables | Frequency | Percent | ||
|---|---|---|---|---|
Number of abortion | ||||
3 | 34 | 77.3 | ||
4 | 4 | 9.1 | ||
5 | 1 | 2.3 | ||
6 | 3 | 6.8 | ||
9 | 1 | 2.3 | ||
Total | 44 | 100.0 | ||
Gestational age | 6 weeks | 8 | 18.2 | |
7-10 weeks | 15 | 34.1 | ||
11-14 weeks | 21 | 47.7 | ||
Total | 44 | 100.0 | ||
Durationb\t mis | 1-3 month | 13 | 29.5 | |
>3 months | 30 | 68.2 | ||
>10years | 1 | 2.3 | ||
Total | 44 | 100.0 | ||
Folic acid | Yes | 44 | 100.0 | |
Smoking | Yes | 1 | 2.3 | |
No | 43 | 97.7 | ||
Total | 44 | 100.0 | ||
Genetic disease f | Yes | 8 | 18.2 | |
No | 36 | 81.8 | ||
Total | 44 | 100.0 | ||
|
| Frequency | Percent |
|---|---|---|---|
Treatment | Yes | 1 | 2.3 |
Antibiotics | 1 | 2.3 | |
Heparin | 1 | 2.3 | |
Thyroxin | 1 | 2.3 | |
Vitamin | 1 | 2.3 | |
No | 39 | 88.6 | |
Total | 44 | 100.0 | |
Diagnosis rpb | Unexplained | 42 | 95.5 |
enl uterus | 2 | 4.5 | |
Total | 44 | 100.0 | |
How long they are try for pregnancy | < 2> | 21 | 47.7 |
3 years | 1 | 2.3 | |
5 years | 11 | 25.0 | |
> 5 years | 11 | 25.0 | |
Total | 44 | 100.0 | |
Outcome of the pregnancies | Miscarriage | 44 | 100.0 |
If the outcome miscarriage | Only miscarriage | 20 | 45.5 |
Miscarriage b\wterm and preterm | 24 | 54.5 | |
Total | 44 | 100.0 | |
They were planned pregnancy | Yes | 1 | 2.3 |
No | 43 | 97.7 | |
Total | 44 | 100.0 | |
Genetic disease male side | Yes | 7 | 15.9 |
No | 37 | 84.1 | |
Total | 44 | 100.0 |
Molecular study
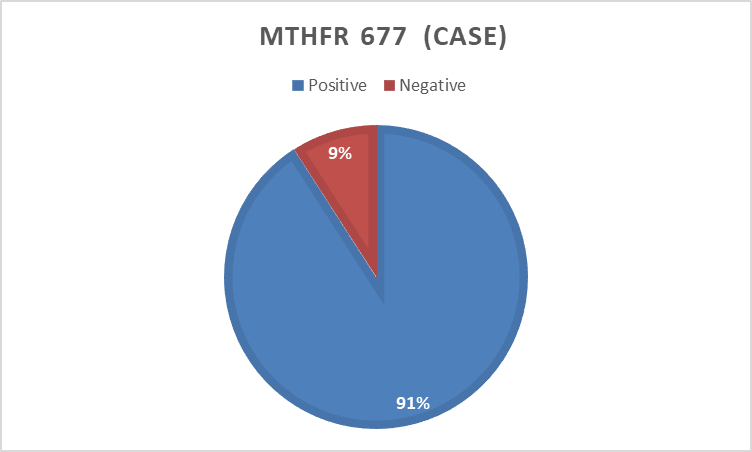
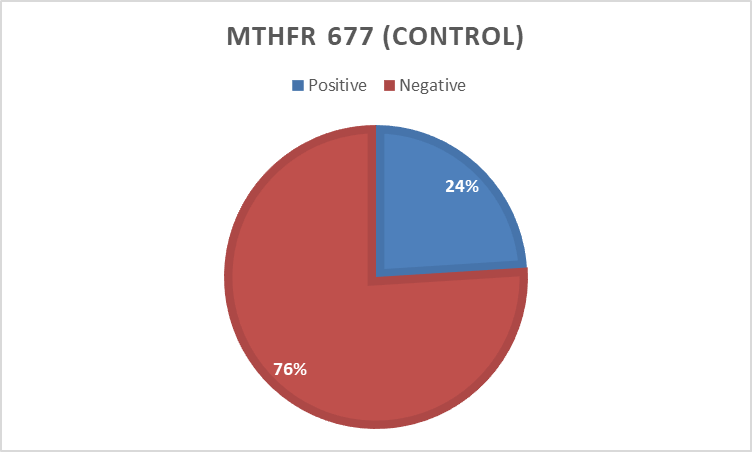
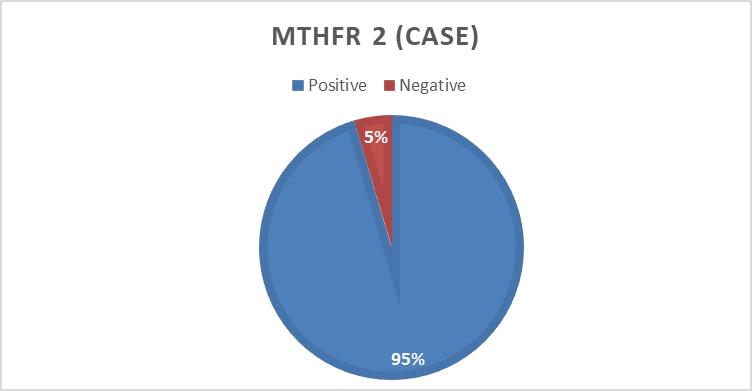
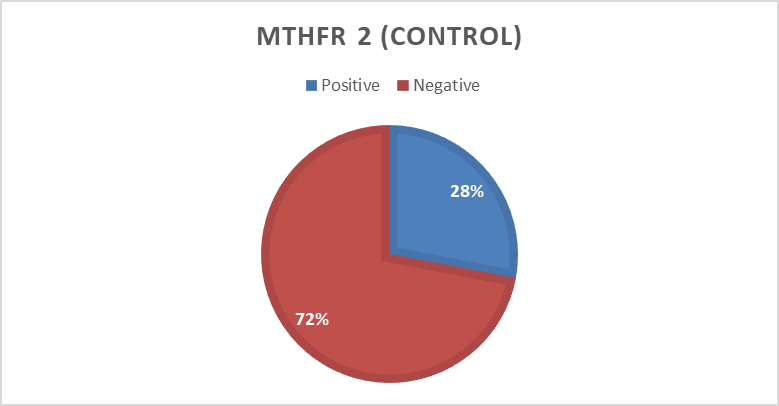
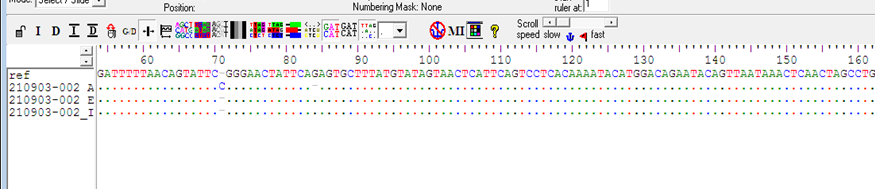
In the present study 494 bp of MTHFR(C677T) and 174 bp of MTHFD (rs2236225) (G19581A) alleles were detected with gel electrophoresis after PCR (Figure 1 and 2). The PCR result shows that about 40 (90%) forMTHFR (C677T) allele was positive in the cases and only12 (24 %) were positivefor the control group (table4). For of MTHFD (G19581A) allele 42 (95%) were positive in the case group and about 14 (28%) were positive in the control (table4). (Figure 3, 4,5and 6)
When compared between case and control for the tow alleles there was highly significant differences (p. Value 0.000) (table5).
Table (4) Distribution of MTHFR polymorphisms results among study population
|
|
| Frequency | Percent |
|---|---|---|---|---|
MTHFR 677 | Case | Positive | 40 | 90.9 |
Negative | 4 | 9.1 | ||
Total | 44 | 100.0 | ||
Control | Positive | 12 | 24.0 | |
Negative | 38 | 76.0 | ||
Total | 50 | 100.0 | ||
MTHFD(G19581A) | Case | Positive | 42 | 95.5 |
Negative | 2 | 4.5 | ||
Total | 44 | 100.0 | ||
Control | Positive | 14 | 28.0 | |
Negative | 36 | 72.0 | ||
Total | 50 | 100.0 |
Table (5) Associationbetween MTHFR polymorphism and study population
Variables | Study population | Total | Chi squire | P. value | Odd Ratio | ||
|---|---|---|---|---|---|---|---|
Case | Control | ||||||
MTHFR C677T | Positive | 40 (76.9%) | 12(23.1%) | 52(100.0%) | 42.4 | 0.000 | 31.7 |
Negative | 4 (9.5%) | 38 (90.5%) | 42 (100.0%) | ||||
Total | 44(46.8%) | 50 (53.2%) | 94 (100.0%) | ||||
MTHFR (G19581A) | Positive | 42 (75.0%) | 14 (25.0%) | 56 (100.0%) | 44.2 | 0.000 | 54.0 |
Negative | 2 (5.3%) | 36 (94.7%) | 38 (100.0%) | ||||
Total | 44 (46.8%) | 50 (53.2%) | 94 (100.0%) | ||||
Sequencing result
The sequencing results were analyzed using different bioinformatics soft-wares and tools for MTHFR C677T. The obtained sequences aligned using BioEdit-ClustalW software with a normal sequence from GenBank gene (accession number NC_000001.11in NCBI).
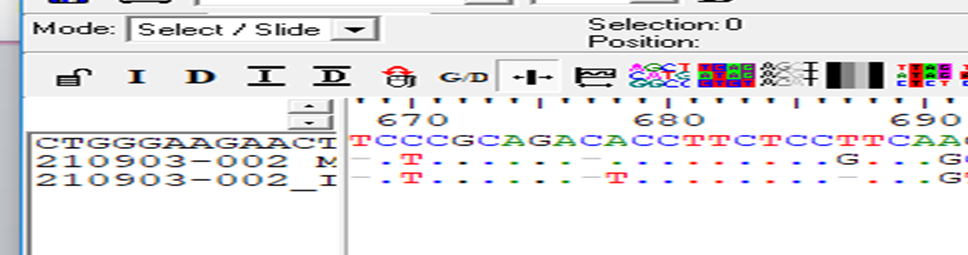
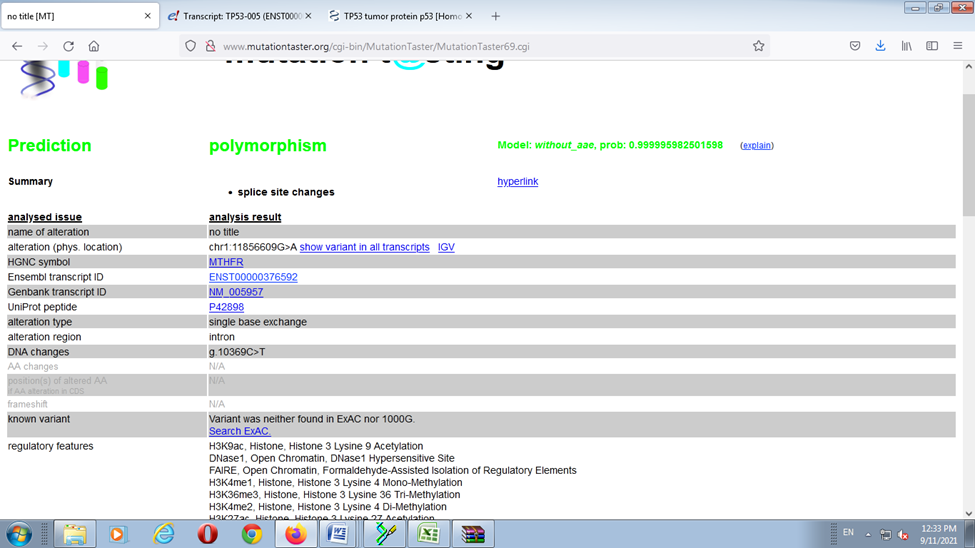
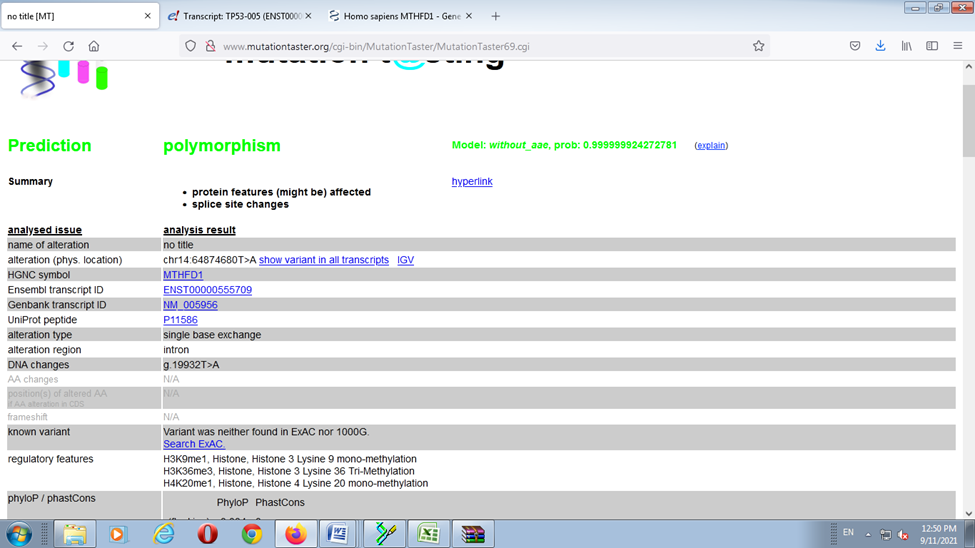
When the cases were compared with the normal reference the single Base Exchange was found C>T While when the controls were compared with normal reference, no any single base exchange was found among the all control groups (figure 7,8 ). when used the mutation taster software the mutation was predicted (figure 9 ).
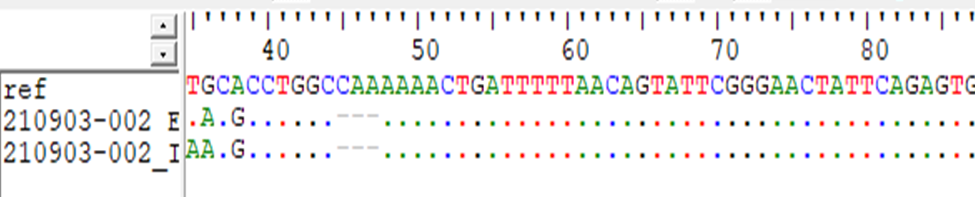
For MTHFD (G1958A) sequences aligned using BioEdit-ClustalW software with a normal sequence from GenBank gene (accession number NC_0000014.91in NCBI). When the cases were compared with the normal reference tow single Base Exchange were found T>A and G>A While when the controls were compared with normal reference, no any single base exchange was found among the all-control groups (figure 10,11) and the mutation taster soft warewas predicted T>A mutation (figure 12).
Discussion
several studies have evaluated whether a correlation between the C677T and A1298C polymorphisms of the methylenetetrahydrofolate reeducates (MTHFR) gene and a higher risk of recurrent pregnant loss (RPL), only few studies that evaluated the correlation between the MTHFD (rs2236225) (G1958A) and recurrent miscarriage. This study was aimed to detect the MTHFR (C667T) and MTHFD (G1958A) polymorphisms among Sudanese women with the recurrent miscarriages
In the present study 44 women were selected as cases and 50 apparently healthy women were selected as control group. The most affected age group was 25-34 year 16 (36.4%), followed by 35-40 years 15 (34.1%). The frequency of the abortion number in the case group; most of them had abortion for three time with frequency about 77.3 %, the frequency of their gestational age is; 11-14 week about 47.7%, 7-10 weeks about 34.1% and 6 weeks 18 %, 97%of theses pregnancies were un planned, this highlight lack of pre pregnancy counseling for these women generally this big issue in Sudan but at least it should be provided for high-risk group like women with RPL.
The PCR result shows that about 40 (90%) for MTHFR (C677T) allele was positive in the cases and only12 (24 %) were positive or the control group. For MTHFD (G1958A) allele 42 (95%) were positive in the case group and about 14 (28%) were positive in the control. When compared between case and control for the tow alleles there was highly significant differences.
The sequencing results revealed; when the cases were compared with the normal reference the single Base Exchange was found C>T While when the controls were compared with normal reference, no any single base exchange was found among the all-control groups, in addition to that when used the mutation taster software the mutation was predicted.
Park et al reported; MTHFR catalyzes irreversible conversion of 5,10‑methylentethrahydrofolate to 5‑methiltethrahydrofolate (CH3‑THF). The normal activity of MTHFR aids to maintain folate and methionine in the bloodstream at constant levels, preventing Hcy accumulation. Polymorphisms in the gene encoding MTHFR may lower its enzymatic activity. The variant C677T leads to a substitution of a cytosine into a thymine at position 677 within exon 4 of the MTHFR gene. This genetic variant leads to an amino acidic substitution in position 222 [6]
In the other hand Domenico et al said; the C677T variant of the MTHFR gene does not appear to influence predisposition to miscarriage in the first or second trimester of pregnancy. Thus, it may be unadvantageous to analyze them for diagnostic aims. However, daily intake of folic acid remains an important therapeutic practice for pregnant women in order to reduce the risk of congenital defects among other complications [8]. This study finding disagree with the study by Al-Achkar et al; in 206 women with URPL versus healthy women showed an increased risk for RPL in individuals carrying the MTHFR 677T allele and the homozygous genotype 677TT. Also, in the other study which used larger samples size showed that; the frequency of the T allele of the MTHFR 677 locus in the URPL group was also statistically significantly higher than that in the control group, indicating that the MTHFR 677T allele is a risk factor for URPL, which is consistent with the results of the study by Al-Achkar et al [9].
Mtiraoui et al; analyzed genetic polymorphisms in women with RPL and found that the risk of developing URPL in women carrying the homozygous mutant genotype (CC) was increased. Klai et al.; also found that women with the mutated gene have an increased risk of miscarriage and other pregnancy complications [10,11].
For MTHFD (G1958A) gene sequences results showed; when the cases were compared with the normal reference tow single Base Exchange were found T>A and G>A, while when the controls were compared with normal reference, no any single base exchange was found among the all-control groups and the mutation taster software was predicted T>A mutation.
Parle-McDermott et al reported; a polymorphism [1958G→A (R653Q, dbSNP rs2236225] within the gene encoding the trifunctional enzyme MTHFD1 (5,10-methylenetetrahydrofolate dehydrogenase, 5,10-methenyltetrahydrofolate cyclohydrolase, 10-formyltetrahydrofolate synthetase) is a maternal risk for severe placental abruption, a maternal risk for having a pregnancy affected by a neural tube defect (NTD) and is possibly involved in fetal in viability [12].
in addition, Anne Parle-McDermott etal said; identified the MTHFD1 1958AA genotype as an independent maternal risk factor for unexplained pregnancy loss during the second trimester of pregnancy [13].
Conclusion
In the conclusion our research result showed highly significant association between the MTHFR C667T, MTHFD G 1958 A polymorphisms and RSA in the Sudanese women, according to this result the MTHF polymorphisms might become one of the main causes of unexplained diagnosis women with recurrent spontaneous abortion.
Conclusion
In the conclusion our research result showed highly significant association between the MTHFR C667T, MTHFD G 1958 A polymorphisms and RSA in the Sudanese women, according to this result the MTHF polymorphisms might become one of the main causes of unexplained diagnosis women with recurrent spontaneous abortion.
References
- Pillarisetty LS, Mahdy H. (2022), Recurrent Pregnancy Loss. [Updated 2022 Sep 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan-.
View at Publisher | View at Google Scholar - Chen H, Yang X, Lu M. (2016), Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: a systematic review and meta-analysis. Arch Gynecol Obstet. 293(2):283-90.
View at Publisher | View at Google Scholar - Schwerdtfeger KL, Shreffler KM. (2009), Trauma of Pregnancy Loss and Infertility for Mothers and Involuntarily Childless Women in the Contemporary United States. J Loss Trauma.14(3):211-227.
View at Publisher | View at Google Scholar - ESHRE Guideline Group on RPL; Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. (2018), ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open; 6;2018(2): hoy004.
View at Publisher | View at Google Scholar - Khan S, Dickerman JD. (2006), Hereditary thrombophilia. Thromb J.; 12; 4:15.
View at Publisher | View at Google Scholar - Park WC and Chang JH: (2014), Clinical Implications of Methylenetetrahydrofolate Reductase Mutations and Plasma Homocysteine Levels in Patients with Thromboembolic Occlusion. Vasc Spec Int; 30: 113‑119.
View at Publisher | View at Google Scholar - Sah AK, Shrestha N, Joshi P, Lakha R, Shrestha S, Sharma L, Chandra A, Singh N, Kc Y, Rijal B. (2018), Association of parental methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with unexplained recurrent pregnancy loss. BMC Res Notes. 5;11(1):233.
View at Publisher | View at Google Scholar - Domenico Dell'edera, Antonella L'episcopia, Francesca Simone, Maria Giovanna Lupo, Annunziata Anna Epifania And Arianna Allegretti. (2018), Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms and susceptibility to recurrent pregnancy loss. BIOMEDICAL REPORTS 8: 172-175.
View at Publisher | View at Google Scholar - Al-Achkar W, Wafa A, Ammar S, Moassass F, Jarjour RA. (2017), Association of methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms with recurrent pregnancy loss in Syrian women. Reprod Sci; 24:1275–9.
View at Publisher | View at Google Scholar - Mtiraoui N, Zammiti W, G hazouani L, Braham NJ, Saidi S, Finan RR, et al. (2006), 31- Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy losses. Reproduction; 131:395–401.
View at Publisher | View at Google Scholar - Klai S, Fekih-Mrissa N, El Housaini S, Kaabechi N, Nsiri B, Rachdi R, et al. (2011), Association of MTHFR A1298C polymorphism (but not of MTHFR C677T) with elevated homocysteine levels and placental vasculopathies. Blood Coagul Fibrin; 22:374–8
View at Publisher | View at Google Scholar - Parle-McDermott A, Mills JL, Kirke PN, Cox C, Signore CC, Kirke S, Molloy AM, O’Leary VB, Pangilinan F, O’Herlihy C et al. (2005), The MTHFD1 R653Q polymorphism is a maternal genetic risk factor for severe Abruptio Placentae. Am J Med Genet; 132A,365–368.
View at Publisher | View at Google Scholar - Anne Parle-McDermott, Faith Pangilinan, James L.Mills 3, Caroline C. Signore , Anne M.Molloy, Amanda Cotter5,8 , Mary Conley , Christopher Cox , Peadar N.Kirke , John M.Scott 1 and Lawrence C.Brody. (2005), A polymorphism in the MTHFD1 gene increases a mother’s risk of having an unexplained second trimester pregnancy loss. Molecular Human Reproduction Vol.11, No.7 pp. 477–480.
View at Publisher | View at Google Scholar