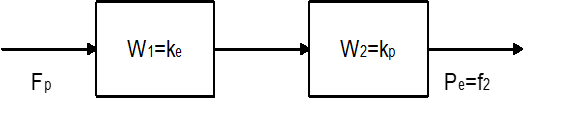Research Article | DOI: https://doi.org/10.58489/2836-2322/007
Development of methods for mathematical modeling of endothelium and smooth muscle cells (MMC). Construction and application of mathematical modeling in silico of basic structures affecting pharmacological targets of the cardiovascular system (CSS)
Department of Pharmacology and Clinical Pharmacology, Medical Institute, Belgorod State National Research University. Russia.
*Corresponding Author: Kanistov Vasil Lyubenov
Citation: Kanistov Vasil Lyubenov, (2022). Development of methods for mathematical modeling of endothelium and smooth muscle cells (MMC). Construction and application of mathematical modeling in silico of basic structures affecting pharmacological targets of the cardiovascular system (CSS). Journal of Pharmacy and Drug Development. 1(2). DOI: 10.58489/2836-2322/007
Copyright: © 2022 Kanistov Vasil Lyubenov, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 09 September 2022 | Accepted: 21 September 2022 | Published: 31 October 2022
Keywords: pharmacological targets, tuning coefficients, mathematical modeling, endothelium, endotheliocytes, smooth muscle cells (MMC), vessel walls, in silico basic structures, cardiovascular system (CCC).
Abstract
Purpose. Development of an adequate and effective mathematical model in silico of basic structures affecting pharmacological targets of the cardiovascular system (CCC).
The article is aimed at researchers in the field of theoretical and experimental pharmacology and medicine.
Materials and methods. We accept the physico-mathematical model "damper-spring" as a theoretical and experimental justification for the development of mathematical modeling methods for the construction and development of a mathematical model: endotheliocytes and smooth muscle cells (MMC). We introduce theoretical and experimental development in silico of basic structures affecting pharmacological targets of the cardiovascular system (CCC), to build and develop a mathematical model: endotheliocytes and smooth muscle cells (MMC),
Outcomes. A real mathematical model of the physico-biological object - endotheliocytes, smooth muscle cells (MMC) and the walls of the vessels of the CCC, presented as pharmacological targets, with an equation in the form of:
Scientific contributions. The constructed mathematical model of the endothelium on mechanical and chemical effects, synthesis and excretion, endothelial factors that cause contraction and / or relaxation of the muscular layer of the vascular wall (constrictors and dilators) is described by the equation:
Conclusion. The model of endothelial function/dysfunction on which experimental studies are still being conducted in pharmacology and medicine in the form of an unbalanced scale is inherently inaccurate.
Application domain. Research processes of fundamental pharmacology and medicine.
Limitations/directions of future research. Models of endothelium and smooth muscle cells (MMC), the construction and application of mathematical modeling in silico of the basic structures acting on pharmacological targets of the cardiovascular system (CCC) in the research processes of fundamental pharmacology and medicine can be done (only) under the supervision of interdisciplinary specialists.
Introduction
In the past few years, it has been argued that clinical trials on the in-silico model are effective as pharmacological targets of the cardiovascular system (CCC). An in silico clinical trial is conducted as an individual computer simulation used in the development or normative evaluation of a drug, device or intervention.
This kind of model (as in silico) makes it possible to study the behavior of the system when internal characteristics and external conditions change, to create and implement scenarios, to solve the optimization problems of the aggregate system. However, in each computer implementation of the model, a specific biological identification corresponds to the constantly changing pharmacological targets of the cardiovascular system (CCC). so-called tuning coefficients. And this, very much complicates on its turn the mathematical model.
Up to the point of this scientific study, there are problems: - A completely simulated clinical trial in silico, the basic structures affecting the pharmacological targets of the cardiovascular system (CCC) are impossible with the application of modern technologies and today's understanding of the ongoing processes in biological structures. But after the application of this new methodology of mathematical modeling of the endothelium and as a result of this study, in silico model development is expected to have significant advantages over current in vitro, ex vivo and in vivo clinical trials, and research in this area is ongoing [1].
For the study of the basic structures affecting the pharmacological targets of the cardiovascular system (CCC), it is critically important, the choice of experimental models turned out to be successful. Subsequently, studies of the orientation and effectiveness of potential drugs for the treatment of disorders of the functional states of the cardiovascular system (CVS) depend on this model. Functional states of the cardiovascular system (CCC) are clinically proven in direct connection with the functional states the endothelial system as the main part of the CVS. The endothelium, together with the vascular wall of the artery and vein, is an integral (holistic) organ, capable of responding to mechanical action ("for example, cyclic stretching or fluid shear tension): flowing blood, the amount of blood pressure in the lumen of the vessel and the degree of tension of the muscular layer of the vessel" [2].
Endothelium and vascular wall of the cardiovascular system of the CVS are subjected equally to the effects of: Blood pressure (): - systolic blood pressure (), diastolic pressure (), pulse pressure (), Average dynamic pressure (); Resistance in the vascular system (); Volumetric and linear velocities of blood flow () and (V), etc.
In modern studies of the cardiovascular system, only the strength of blood pressure on the inner walls of blood vessels is taken into account, and anatomical and functional factors caused by the reverse pressure of the blood vessel are not taken into account. In addition, endothelial cells synthesize and release vasoactive mediators in response to various neurohumoral substances (for example, bradykinin or acetylcholine) and physical stimuli (for example, cyclic tension, pressure or shear stress of the liquid). Today, it is accepted that the most well-characterized relaxing factors derived from the endothelium are nitric oxide and prostacyclin.
In the pharmacological study of biological objects, many models have been created for all these processes. It seems that for each study a special model is created to confirm a certain theoretical prediction. Today, at least there is no single universal model on which to conduct all pharmacological experiments!
Materials And Methods
A. The physico-mathematical model of the "damper-spring" as a theoretical and experimental justification for the development of mathematical modeling methods for the construction and development of a mathematical model: endotheliocytes and smooth muscle cells (MMC).
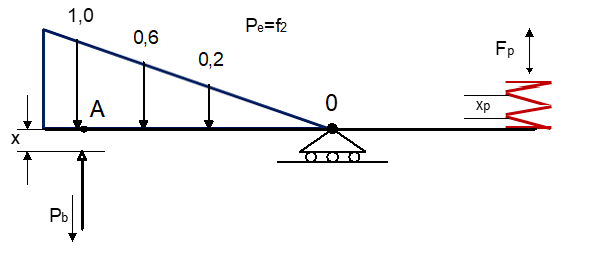
The reaction of endotheliocytes and smooth muscle cells (MMC) vessel wall to the cardiovascular system (CCC) to mechanical and chemical influences leads us to apply the mechanical model "damper-spring" (Figure 1).
At the right end of the flap by the model we have a spring, which is calculated by the equation according to Hooke's law:
Where: - moving the end of the B turns to the spring and - the stiffness of the spring
Since in our case the spring is compressed, the equation takes the form:
This force is called the "blood vessel constriction force", which is right proportional to the value of the resistance of the elastic fibers (the inner elastic membrane and the outer elastic membrane of the vascular wall of the artery and vein) and is directed in the opposite direction to the pressure () of the blood vessels.
At the left end of the flap the model, of t. 0 to t. A have distributed blood pressure forces (mechanical and chemical effects on the walls of blood vessels) and as a result, the value of tuning coefficients according to the in-silico model: (stronger than active), (average active) and (weakly active). And corresponding values (weakly active), (average active) and (stronger active).
The effective force of value , and (, and ) is that acts in t. A flap and is directed in the opposite direction - "the force of narrowing the blood vessel".
In the specific case, the numerical values and (mechanical and chemical effects) is the collection of suma from all coefficients indicating the degree and direction of activity of the pharmacological targets of the cardiovascular system (CCC) on the functions of endotheliocytes, smooth muscle cells (MMC) and the vessel wall to deterioration or to improvement. We can write:
For a mechanical model in the form of a "damper-spring" on a physico-biological control object (FBOU) (Figure 1), we will make a mathematical model in dynamics, with a differential equation, in the form of:
Looking at equation (3) and equation (4), equation (5) takes the form:
Note: The values - with a conditionally negative effect on the functions of endotheliocytes, smooth muscle cells (MMC) and the vessel wall include both cyclic stretching and fluid (blood) shear tension. The values include all elements with a conditionally positive effect on the functions of endotheliocytes, smooth muscle cells (MMC) and vessel walls, including both cyclic stretching and fluid (blood) shear tension.
Thus, the cost of effective strength depends on the potentially positive and/or negative effect of endotheliocyte function, smooth muscle cell (MMC) and vessel wall on the state of homeostasis and the activity of pharmacological targets of the cardiovascular system (CVS).
B. Theoretical and experimental development in silico of basic structures acting on pharmacological targets of the cardiovascular system (CCC), for the construction and development of a mathematical model: endotheliocytes and smooth muscle cells (MMC),
Assuming that the relationship between the change in effective strength and the change in the distance between the flap and the A-point on the interaction of the membrane wall of endotheliocytes and smooth muscle cells (MMC) is linear, we can write:
Where: - positive constant. (The tuning factor depends on the value and )
- variable distance in the functional dependence of the value and
- Future considerations coincide with an embarrassing process
We can easily see that with equality, endotheliocytes and smooth muscle cells (MMC) are not in dysfunction, but function normally - for example, the state of homeostasis and normal activity of pharmacological targets of the cardiovascular system (CVS)
In this equilibrium mode of operation, endotheliocytes and smooth muscle cells (MMC) work in "normal mode". The level of mechanical pressure of the endothelial cell does not exceed the permissible values. Smooth muscle cells (MMCs) of the vascular wall receive signals for normal functioning without actively participating in the endothelial system reaction – for example: the production of NO and prostacyclin from endotheliocytes.
Under initial equilibrium conditions, we can write:
Where is:
The transfer function between the control pressure and the error signal can be obtained using Mason's formula as follows:
Where: – Convert Laplace to a function
= Laplace conversion to function
- values and (mechanical and chemical effects) as a collection of suma from all coefficients indicating the degree and direction of activity of pharmacological targets of the cardiovascular system (CCC) on the functions of endotheliocytes, smooth muscle cells (MMC) and vessel walls to deterioration or improvement.
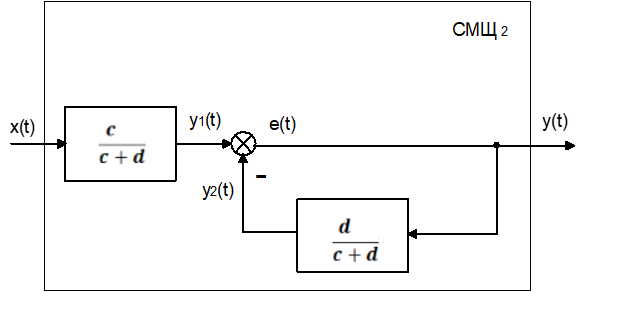
Up to this point, in all research models that simulate the response of the endothelium to mechanical and chemical influences, synthesis and excretion, endothelial factors that cause contraction and / or relaxation of the muscular layer of the vascular wall (constrictors and dilators) are considered as unbalanced scales. But the real model is different - the isolated endothelial factors have more complex effects of the system on blood flow and smooth muscle cells (MMC) vessel wall. A mathematical model of this process is shown through an equation and an equation, and a structural-functional scheme of the physico-biological control object (FBOU) endotheliocytes and smooth muscle cells (MMC) of the vessel wall is constructed Figure 2 and Figure 3.
On the basis of equation (10), we will draw up a structural and functional scheme of the physico-biological control object (FBOU) - endotheliocytes and smooth muscle cells (MMC) of the vessel wall (Figure 2)
In case of imbalance - the presence of an increase in the cost (values and / or) levels of mechanical and chemical effects on the endothelium and smooth muscle cells (MMC) on the walls of blood vessels, we come to a new pneumatically mechanical model of a physico-biological object in the form of a "nozzle-flap-bellows"
Now let's point out the way to build a mathematical model with equation (5): and according to the structural diagram on Figure 3.
After replacing in with and we get the equation
Results And Discussion
Consider Figure 1, Figure 3, the equation and the equation . See also the formulas: :
Let's note that
Then it takes the form:
Convert the structural diagram Figure 3. Obtains a transfer function by connection, with negative feedback in a closed system of physico-biological control object (FBOU):
Looking for a transfer function of embarrassing effects (the effective force of potentially positive and/or negative effects of endotheliocyte function, smooth muscle cells (MMC) and vessel walls on the state of homeostasis and the activity of pharmacological targets of the cardiovascular system (CVS)):
Replace and
Get:
- is a pharmacological coefficient - with a potentially negative or positive effect of pharmacological targets of the cardiovascular system (CCC).
See equation (5):
We get:
Where: - Potential negative effect of pharmacological targets of the cardiovascular system (CVS) on the function of endotheliocytes, smooth muscle cells (MMC) and the vessel wall.
– Potential positive effect of pharmacological targets of the cardiovascular system (CCC). on the function of endotheliocytes, smooth muscle cells (MMC) and vessel walls.
It's easy to notice what makes sense when i.e.,
Thus, the potential negative effect of pharmacological targets of the cardiovascular system (CVS) on the functions of endotheliocytes, smooth muscle cells (MMC) and the vessel wall should not exceed half the absolute value of the potential positive effect of pharmacological targets of the cardiovascular system (CVS).
Consistently and step by step we introduce and - degrees and direction of activity pharmacological targets of the cardiovascular system (CCC) (Figure 3) the embarrassing effect of SMS. 2 (), in the form of pharmacological coefficients (Table 1 and Table 2) are represented by the equation:
Where: and - degrees and direction of activity pharmacological targets of the cardiovascular system (CCC), numerical coefficients (0.2, 0.6, 1.0)
- Pharmacological coefficients, calculated by the formula equation () are presented as an embarrassing effect of SMSh. 2 () in Figure 3.
- low degree of deterioration of endothelial function.
- a high degree of deterioration in endothelial function.
- "at the boundary of stability” = moderate deterioration of endothelial function
Table 1 – Numerical values pharmacological coefficients depending on the values of in silico coefficients and as pharmacological targets of the cardiovascular system (CCC).
in silico коэффициенты иcidi | d3 = 1,0 stronger active | d2 = 0,6 average active | d1 = 0,2 inactive |
c3 = 0,2 stronger active | Kf27= 0.333333 …. | Kf25 = 0.6 | Kf24 = 0,71428571428 …. |
c2 = 0,6 average active | Kf22 = 0,142857142857… | Kf27 = 0.333333 …. | Kf26 = 0.45454545 … |
c1 = 1,0 inactive | Kf21 = 0.09900990099 … | Kf23 = 0,230769230769 … | Kf27 = 0.333333 …. |
Table 2 – Numerical values pharmacological coefficients and associations of the degree of deterioration in the function of endotheliocytes, smooth muscle cells (MMC) and vessel walls, as pharmacological targets of the cardiovascular system (CVS).
Pharmacological coefficients
conclusionThus, as a result of the development of methods for mathematical modeling of endothelium and smooth muscle cells (MMC),it was found that the main featuresof the basic structures affectingthe pharmacological targetsof the cardiovascular system (CCC) are thefollowing: 1. In the construction and application of mathematical modeling in silico have reached a numerical value, References
|