Archive : Article / Volume 2, Issue 2
- Research Article | DOI:
- https://doi.org/10.58489/2836-2411/014
Health Indicators: A Review by Thermodynamic Assessment of Acetic Acid in The Presence of Selected Binary Immiscible Solvents
1 Department of Chemistry, Federal University of Technology, P. M. B. 1526, Owerri, Nigeria
2 Department of Chemistry, Chukwuemeka Odumegwu Ojukwu University, Uli Campus Anambra State, 431124, Nigeria
Dr Veronica Ogechi Onyeocha
Dr.V. O. Onyeocha, Professor A. I.Onuchukwu (2023). Health Indicators: A Review by Thermodynamic Assessment of Acetic Acid in The Presence of Selected Binary Immiscible Solvents. Journal of Internal Medicine & Health Affairs (JIMH). 1(1). DOI: 10.58489/2836-2411/014
© 2022 Dr Veronica Ogechi Onyeocha, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 27-12-2022
- Accepted Date: 12-01-2023
- Published Date: 22-02-2023
Health indicator, Partition coefficient, Environment, Binary immiscible solvent, Acetic acid, Analysis
Abstract
Background: This report reviews the thermodynamic assessment of Acetic Acid in binary immiscible solvents as an insidious health indicator that requires the confirmation of amount present and its treatment prior to discharge in effluents and sewage waste management. This measure upholds sustainability for the clean and green environment. Acetic acid is a potent harmful chemical compound. However, it is used in the chemical industry, medicine, household, and foods. Exposure to industrial compounds that contain Acetic acid is hazardous to health. Severe allergic reactions manifest from exposure to Acetic acid include rash, hives, difficulty in breathing, tightness in the chest, swelling of the mouth, face, lips or tongue, irritation, sensitivity, etc. The use of binary immiscible solvents in the analysis of Acetic acid gives the thermodynamic predictive parameter, namely: Partition coefficient (kD), which serves as an indicator that provides the amount of Acetic acid in the environment under suspect. Also, partition coefficient, kD highlights the efficiency of either the removal of Acetic acid from the environment or gives amount of Acetic acid extracted from effluents/sewage assessment by using the technique on the value(s) obtained from partition coefficients.
Methods: Acetic acid was dissolved in three binary immiscible solvents, namely: Carbon Tetrachloride-Water, Diethyl Ether-Water and n-Hexane-Water, at 30â and an atmospheric pressure. Varying quantities of Acetic acid (the solute) were distributed into the respective binary immiscible solvents. The different volumes of AnalaR Grade reagent of Acetic acid were respectively poured into the separating funnel. The separating funnel was shaken vigorously and allowed to stand in order to establish equilibrium of diffusion of the solute in the system, as reported earlier by Onyeocha et. al. Titre-values were obtained for the concentrations of Acetic acid in the two phases of binary immiscible solutions that were formed.
Results: The results in the three systems were analysed, Acetic acid had the partition coefficient, kD = 0.05838 in Diethyl Ether-Water, the dimerization constant, K = 0.0068 while ionization constant, = 0.03998 in the same system. Acetic acid had the kD = 0.05674, K = 2.0281 in magnitude and = 0.0013, in n-Hexane-Water and kD = 0.01643, K = 250.9771 and = 0.0196 in Carbon Tetrachloride-Water.
Conclusion: This work shows that Carbon Tetrachloride-Water, Diethyl Ether-Water and n-Hexane-Water are efficient binary immiscible solvents for the analysis of Acetic acid proven waste treatment management for effluent and sewage disposal to ensure the sustainable clean and green environment devoid of any residual Acetic acid. Indeed, partition function, kD is a novel thermodynamic parameter recently introduced by Onyeocha et. al. for the quantitative evaluation and residual assessment of Acetic acid in the environment.
Thermodynamic parameters
Thermodynamics deals with the concepts of heat and the inter-conversion of heat and other forms of energy during chemical reactions. It deals with heat, work and manifests variation of temperature and their relation to energy, entropy and the physical properties of matter. The behavior of these quantities is governed by the four laws of thermodynamics which show quantitative descriptions using measurable macroscopic physical quantities. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, and also in other complex fields such as meteorology. It deals with the bulk system and does not deal with the molecular constitution of matter. Thermodynamic parameters, are series of physical quantities, which all together/individually, can characterize the observed system. These quantities are: temperature, pressure, volume, concentration, the work done and heat energy. From these functions, other thermodynamic functions such as: entropy, enthalpy, Gibbs energy, Helmholtz energy etc. are derived [1], [thermodynamic parameters - Search (bing.com), https://byjus.com/physics/thermodynamics/] In the attempt to transform matter, thermodynamics plays the important role of rearrangement of the atoms, i.e., bonding, from their initial position to the final stage in the formation of new substances, chemical reactions that may lead to new crystal arrangements. The transformation is influenced by factors such as: the nature of the atoms, the types of bonds being formed and the surroundings. These factors influence the energy of the system during the transformation of matter. The factors make up the thermodynamic parameters. They are called thermodynamic variables [1]. The variables are cardinal parameters that are used in the study of energetics of chemical systems from the view of thermodynamics [1].
Partition coefficient
The partition coefficient, kD is the ratio of the concentrations of a molecule in a mixture of two immiscible solvents obtained at equilibrium. This ratio describes the comparison of the solubilities of the solute in the two liquids. It has been used to estimate the distribution of drugs within the body. [Partition Coefficient - an overview | ScienceDirect Topics] Partition coefficient is measured either experimentally in various ways or obtained by calculation based on a variety of methods. [Partition coefficient - Wikipedia] Partitioning is used in geochemistry to describe the equilibrium distribution of chemical species between two coexisting phases. The concentration ratio of this species between the two phases (a and b) is defined as the partition coefficient that quantitatively measures the equilibrium fractionation of the concentration of species in each phase. [(PDF) Partitioning and Partition Coefficients (researchgate.net)] Partition coefficient is an empirically dimensionless property that describes how a chemical substance distributes itself between two phases. [Partition Coefficient - an overview | ScienceDirect Topics] According to Onyeocha et. Al [2,4], partition coefficient technique is an analytical thermodynamic method that is used to evaluate the distribution of species in binary immiscible solvent. The values of partition coefficient resulting from the use of binary immiscible solvent serve as a tool to underscore the efficiency of the beneficiation of solute from solvents. Other parameters such as dimerization constant, K, ionization constant, , etc., depict the properties of solutes in the solvents. Also, the partition coefficient technique is an excellent method of purification and extraction of solutes, from binary immiscible solvents. A high partition coefficient’s value shows high solubility of the solute in the solvent involved and therefore, good separation is assured. Partition coefficient is described as the solubility of a solute in both organic and aqueous layers. It is dependent on the solvent system used. As in thermodynamics, partition coefficient is used to checkmate the efficiency of solute extraction in binary solvents. The concept is in accordance with the predictive parameters such as, Enthalpy, H, and Gibb’s Free Energy, G etc., as described by Onyeocha et. Al [2,3] Partition coefficient, kD, gives the driving force for solute extraction from binary solvent. Given the conditions that are associated with partition coefficient, it is hereby highlighted as “the indicator” that shows:
- the concentration of the solute in a solvent system
- the efficiency of partition coefficient technique
- the criterion for the choice of the best binary immiscible solvent for solvent extraction and the solute beneficiation
According to Onyeocha et. al. [2], the use of binary immiscible solvent in solvent extraction provides the property gradient in partition coefficient. This condition generates the driving force which spontaneously liberates, pushes and distributes the solute molecules into the required solvent (the solvent with greater affinity for the solute). Because of the remarkable property of partition coefficient in giving the degree of the dissolution of the solute in the solvent, it is defined mathematically in the equation:
Concentration of xi in liquid A /Concentration of xii in liquid B = kD (1)
(Where xi and xii are the concentrations of the solute distributed in the solvents ‘A’ and ‘B' that formed the binary immiscible solvents respectively)
Acetic acid: Acetic acid (ethanoic acid) is a colourless liquid that has a distinctive sour taste and pungent smell. It is a weak acid in water, though corrosive when in concentrated form. It has the chemical formula: CH₃COOH. It has two functional groups, the acetyl group (, CH3CO) and hydroxyl group (-OH), it could also be viewed as a methyl group (-CH3) and a carboxyl group together (-COOH). It is a weak monoprotic acid. Acetic acid vapour contains dimers or pairs of molecules that are linked by hydrogen bonds [6]. In solid state acetic acid, the molecules form dimers, being connected by hydrogen bonds. The dimers are detected in vapour at 120. Dimers occur also in the liquid phase, in dilute solutions and non-hydrogen-bonding solvents like Carbon tetrachloride (CCl4). [en.m.wikipedia.org]
Acetic acid is recognized as safe for use in processing foods, it has been a staple of folk medicine for centuries all over the world and it is used in households. It is contained in vinegar. Acetic acid is found in such things as pickles, eardrops, preservatives for foods with related health benefits because of its medicinal properties. [www.livestrong.com/article/467468-what-are-the-health-b…] Acetic acid is a potent compound because of its use in many areas such as industrial processes of foods and food related condiments, medicinal products, and preservatives. [byjus.com/chemistry/uses-of-acetic-acid/ https://EzineArticles.com/expert/Shalini_M/2609777] Acetic acid is a precursor for polyvinyl acetate, cellulose acetate, etc. [www.vdh.virginia.gov] Exposure to industrial compounds that contain acetic acid, which is used in paint, pesticides, plastics and textiles, etc., is hazardous to health. Whether acetic acid will act harmfully depends on the process of manufacture, the other chemicals making up the compound and the degree of dilution. [Acetic acid (ethanoic acid) | National Pollutant Inventory (npi.gov.au), www.livestrong.com/article/467468-what-are-the-health-b…] The side effects of acetic acid are: severe allergic reactions like rash, hives, difficulty breathing, tightness in the chest, swelling of the mouth, face, lips or tongue, irritation, sensitivity etc. [Acetic Acid Side Effects, Side Effects of Acetic Acid (foodsweeteners.com)] The global demand for acetic acid is 6.5 million metric tons per year, of which about 1.5 million metric tons per year is got by recycling and the remaining is manufactured from methanol. [en.m.wikipedia.org] Therefore, there is the need for proper beneficiation method and storage.
Acetic acid pollution: Acetic acid is a base ingredient in the production of chemical products such as insecticides and plastics. Acetic acid mixed solution (as a sewage denitrification carbon source additive) has been used in sewage treatment process. This is due to its environmentally friendliness when it is used under a controlled measure. [CN103387281A - Acetic acid mixed solution as sewage denitrification carbon source additive - Google Patents] However, acetic acid is classified as a hazardous substance. Exposure to toxic amounts causes a lot of health disorders. Inhalation of the vapors expelled by acetic acid can cause dizziness or suffocation. [acetic acid pollution - Search (bing.com)] There are reports of searches to give credence and there are antidotes for treating Acetic acid related burns on humans. Also, regulated amounts of Acetic acid are deployed in the treatment of wastewater, to prevent the leaching of amines in the treated water and prevent odor generation.
There have been researches to give the method and device for treating acetic acid-containing wastewater. Some of these methods include:
- The use of high cost reducing agent. These reducing agents in addition, increase the ions in the solution by the reduction process. This is inappropriate for the recovery and reuse of the water.
- The use of ion exchange resin has high-cost process too. [WO2012018038A1 - Method and device for treating acetic acid-containing wastewater - Google Patents]
- Also, the use of extraction agent in an extractive distillation mode [CN104447275A - Method for purifying acetic acid from acetic acid wastewater - Google Patents], has a high-cost process.
Partition coefficient technique: Partition coefficient has been reported as an indicator to measure drug lipophilicity in an oil-water mixture. The partition coefficient was determined by the shake flask method using two immiscible solvents. The most common hydrophilic solvent is water or phosphate buffer of 7.4, and for oil phase, the good example is octanol. [Partition Coefficient - an overview | ScienceDirect Topics] The partition coefficient is the ratio of the quantity of the solute in moles per litre, in the two phases. It is an effective means of measuring whether the desired goal of beneficiation is achieved. The larger the value of the partition coefficient, the more of the solute is extracted into the organic phase. It is used in purification and concentration of the solute. The basic procedure for liquid-liquid extraction uses two immiscible phases of water and an organic solvent. The two phases are put into the separatory funnel. The solute in the system will distribute between the two phases. [chem.libretexts.org] Partition coefficient gives the ratio of concentration of a compound in a mixture of two immiscible solvents at equilibrium. The ratio (kD), shows the comparison of the solubilities of the solute in the two immiscible liquids [en.m.wikipedia.org]
Background of the study: This review addresses health promotions in relation to the use of Acetic acid in food, medicine, household and chemical industries. It introduces partition coefficient, kD, as the health indicator for the control of the use of Acetic acid in the industries and the control of the ramification of the use of Acetic acid in effluents and sewage management.
Statement of the problem: Health indicators give information on priority issues in health system performance. Health indicator is a quantitative measure of the activities of a system. It gives guidelines for quality monitoring and evaluation of purposes. Acetic acid is defined as a potent chemical substance. Due to the versatile use and associated effects of Acetic acid to life, there is the need to develop an indicator that checkmates the use of this acid for the benefit of the good health of the populace.
Objective of the study: The analysis of Acetic acid in binary immiscible solvents is studied. This write-up presents partition coefficient, kD, as a health indicator for the control of the use of Acetic acid. It highlights the side effects from Acetic acid and recommends the cost effective and efficient measure of removal of the acid from effluents and sewage disposals.
Justification of the study: Public health is a concerned issue for both individuals and organizations. The World Health Organization (WHO) described health indicators as guides for good health performance system. This paper introduces partition coefficient kD, as a health indicator for the use and control of acetic acid in processes and in effluent and sewage management.
Scope of study: Partition coefficient technique is an application that touches all disciplines such as: Geology, Physics, Chemistry, Medicine, Biochemistry, Biology, etc. In this application, binary immiscible solvents are used which give rise to the constant called partition coefficient. Partition coefficient is presented as a health indicator. This principle could apply to all discipline.
Methods: As reported earlier by Onyeocha et. al. [2-4] different volumes of acetic acid are respectively pippeted into the separating funnel, at 30℃ and an atmospheric pressure. The separating funnel is shaken vigorously and allowed to stand in order to establish equilibrium in the system. Equilibrium concentrations are calculated from the titre values generated from the two phases of the solutions from the binary immiscible solvent. Partition coefficient is given as:
kD = Concentration of Acetic acid in the organic phase (CxA)/Concentration of Acetic acid in Water (CxB)
(i.e.) kD = (C_X^A)/(C_X^(*B) ) (1)
Analysis of results: Observing the Nernst Distribution Law (Equation I) and in cognizance of the interfering reactions of dimerization and ionization reactions of Acetic acid in the organic solvents and water respectively, the equations as described by Rastogi et. Al [5], and as used in Onyeocha et. al. [2,4], are used for the analysis of the results, as shown below.
The equations below are used:
(C_X^A)/(C_X^(*B) ) = kD + 2k_D^2KC_X^(*B) (2)
From equation (2), a plot of (C_X^A)/(C_X^(*B) ) vs C_X^(*B) is a straight line with intercept kD and slope equal to 2k_D^2K. This is used for the calculation of the dimerization constant, K.
Furthermore, when the solute undergoes ionization in the solvent, the equation (3) below is used:
(C_X^A)/(C_X^(*B) ) = kD + (KkD)1/2(C_X^(*B))-1/2 (3)
A plot of (C_X^A)/(〖 C〗_( X)^(*B) ) vs 1/〖〖(C〗_( X)^(*B))〗^(1/2) is a straight line with intercept kD, and a slope equal to (KkD)1/2. The ionization constant K ≡ α is calculated.
When both dimerization and ionization reactions are taking place in solvents A and B respectively, then equation (4) is used [5]:
(C_X^A)/(C_X^(*B) ) = kD (1- α) + 2kD2K (1- α)2CX*B (4)
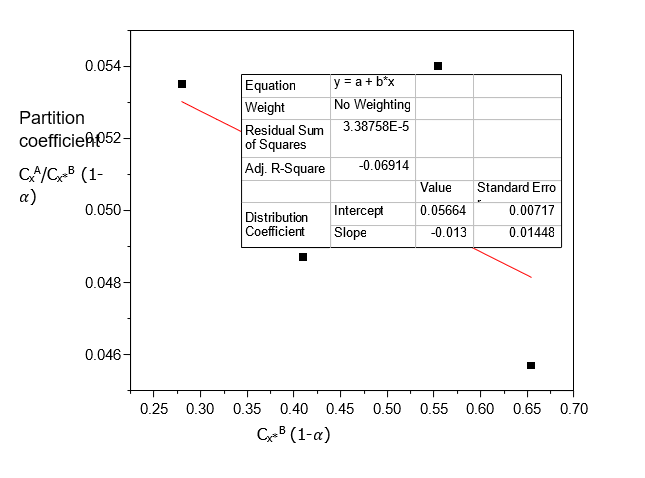
The plot of (C_X^A)/(C_X^(*B) )(1-α) vs Cx*B (1-α) gives a straight line with 2kD2K as the slope.
The equations (2), (3) and (4) are applied in all the tables and figures below to calculate the partition coefficient, dimerization constant and ionization constant for the analysis of the binary solvents and the efficiency of the partition coefficient technique for Acetic acid in binary immiscible solvent(s).
Results
The results from this research are shown in Tables 1 – 10 and Figures 1 – 9 below.
Table 1. Data for the partition coefficient (kD) of Acetic acid in Carbon Tetrachloride-Water at 30°C and an atmospheric pressure, equation (2)
Volume of Acetic acid (ml) | Concentration in Carbon tetrachloride (A) (CxA) (mole/litre) | Concentration in Water (B) (Cx*B) (mole/litre) | (kD) |
0.4 | 0.0100 | 0.2200 | 0.0455 |
0.6 | 0.0350 | 0.3950 | 0.0886 |
0.8 | 0.0250 | 0.4700 | 0.0532 |
1.0 | 0.0650 | 0.6050 | 0.1074 |
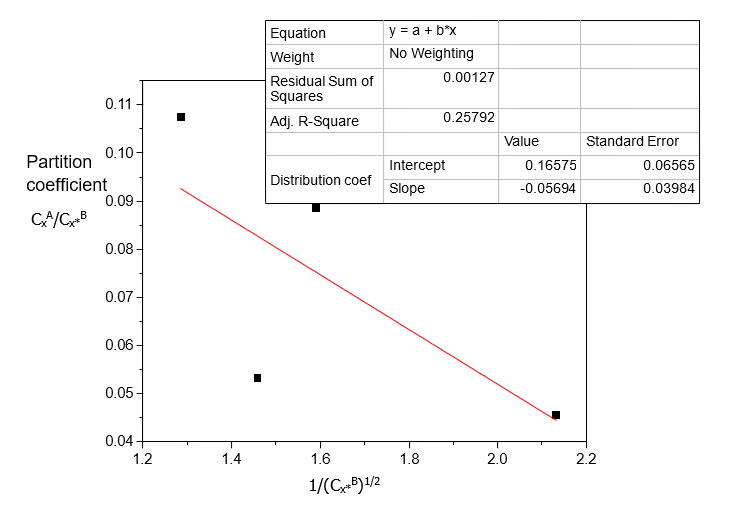
Table 2. Data for the partition coefficient of Acetic acid in Carbon Tetrachloride-Water at 30°C and an atmospheric pressure, equation (3)
CxA/Cx*B | 1/ Cx*B (mole/litre)-1/2 |
0.0455 | 2.1322 |
0.0886 | 1.5911 |
0.0532 | 1.4586 |
0.1074 | 1.2857 |
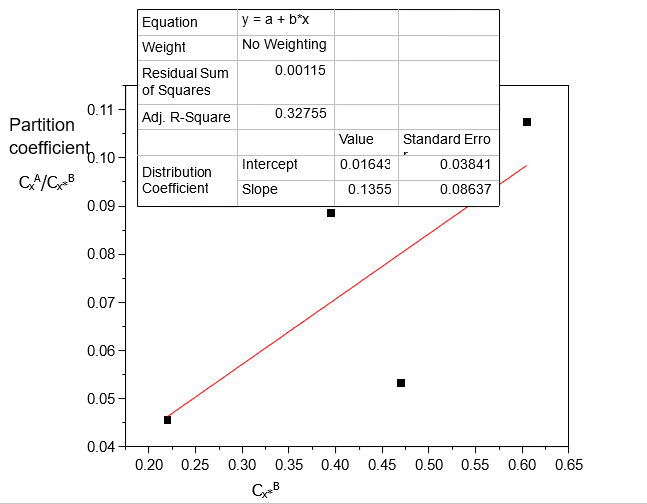
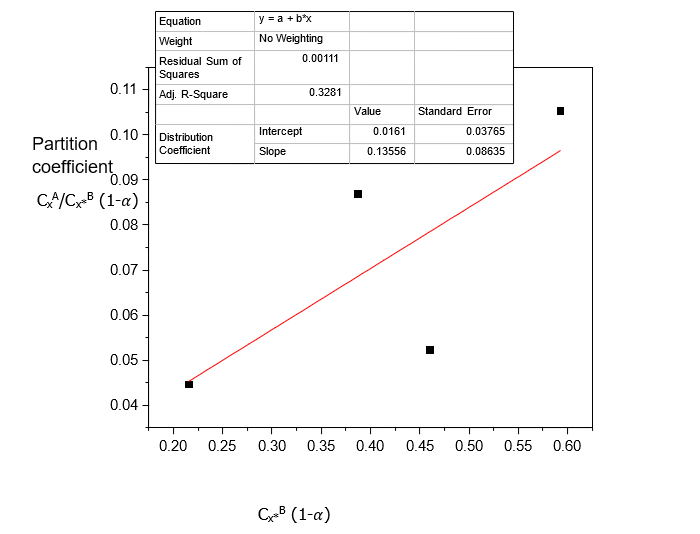
Table 3. Data for the partition coefficient of Acetic acid in Carbon Tetrachloride-Water at 30°C and an atmospheric pressure, equation (4)
CxA/Cx*B | CxA/Cx*B (1-) | Cx*B (mole/litre) | Cx*B (1-) (mole/litre) |
0.0455 | 0.0446 | 0.2200 | 0.2157 |
0.0886 | 0.0869 | 0.3950 | 0.3873 |
0.0532 | 0.0522 | 0.4700 | 0.4608 |
0.1074 | 0.1053 | 0.6050 | 0.5931 |
Table 1, shows that Acetic acid dissolves more in Water than in Carbon tetrachloride. This corrects the statement in “The Dimerization Effects of Some Solutes on the Partition Coefficient kD in Binary Immiscible Solvent” [4], where there was misplacement and wrong interchange of data in the case of Acetic acid in Carbon Tetrachloride-Water. Generally, the increase in the concentration of Acetic acid in Carbon Tetrachloride-Water increases its dissolution and distributions in the solvents.
Table 2 shows that distribution coefficient values increase with increasing concentration of Acetic acid in Carbon Tetrachloride-Water and decreasing ionization constant values. Table 3 shows that the distributions of Acetic acid increase with increasing concentration.
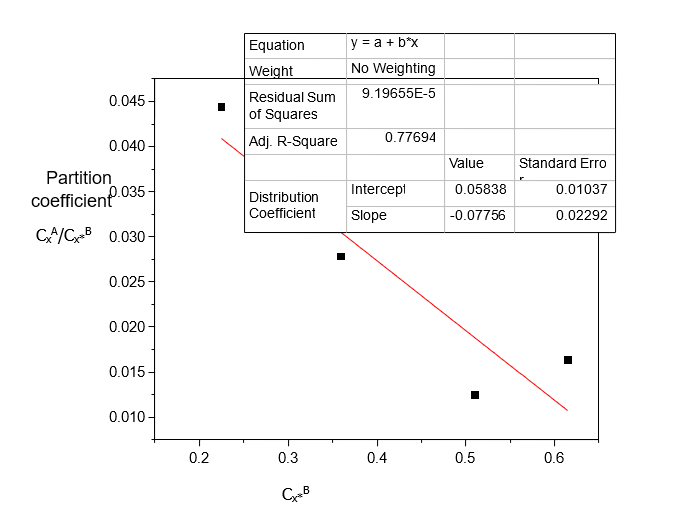
Table 4. Data for the partition coefficient (kD) for Acetic acid in the binary solvents: Diethyl Ether-Water at 30°C and an atmospheric pressure, equation (2)
Volume of Acetic acid (ml) | Concentration in Diethyl ether (CxA) (mole/litre) | Concentration in Water (Cx*B) (mole/litre) | (kD) |
0.4 | 0.0100 | 0.2250 | 0.0444 |
0.6 | 0.0100 | 0.3600 | 0.0278 |
0.8 | 0.0063 | 0.5100 | 0.0124 |
1.0 | 0.0100 | 0.6150 | 0.0163 |
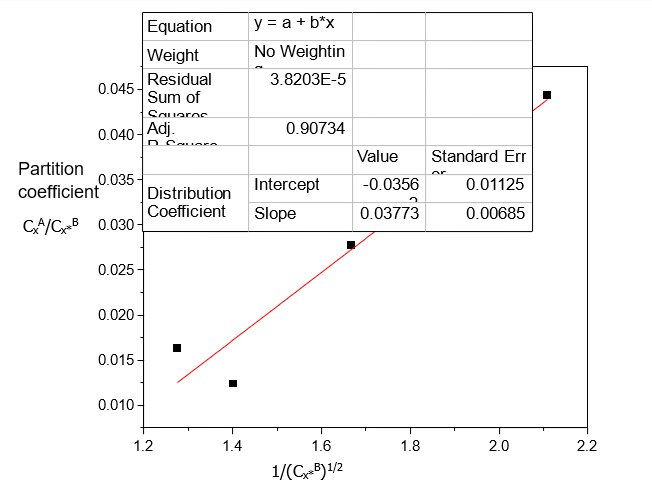
Table 5. Data for the partition coefficient of Acetic acid in Diethyl Ether-Water at 30°C and an atmospheric pressure, from equation (3)
CxA/Cx*B | 1/ Cx*B (mole/litre)-1/2 |
0.0444 | 2.1084 |
0.0278 | 1.6667 |
0.0124 | 1.4004 |
0.0163 | 1.2752 |
Table 6. Data for the partition coefficient of Acetic acid in Diethyl Ether-Water at 30°C and an atmospheric pressure, equation (4)
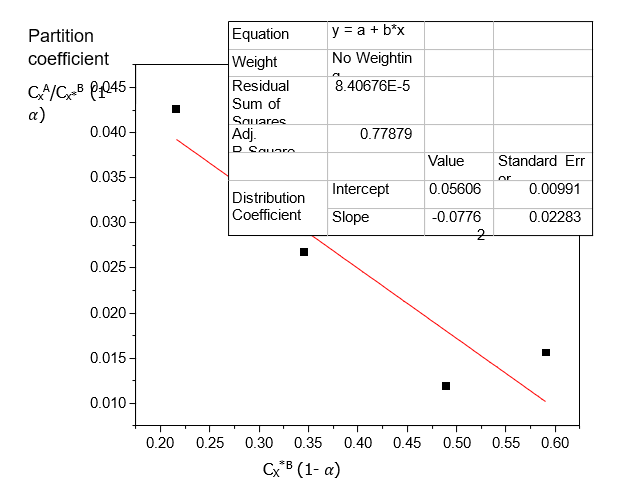
CxA/Cx*B | CxA/Cx*B (1-) | Cx*B (mole/litre) | Cx*B (1-) (mole/litre) |
0.0444 | 0.0426 | 0.2250 | 0.2160 |
0.0278 | 0.0267 | 0.3600 | 0.3456 |
0.0124 | 0.0119 | 0.5100 | 0.4896 |
0.0163 | 0.0156 | 0.6150 | 0.5904 |
Table 4 shows that Acetic acid dissolves more in Water than in Diethyl ether. The dissolution of Acetic acid in Diethyl ether decreases with increase in concentration. Table 5 shows that the distribution coefficient decreases as the concentration of Acetic acid increases, also the ionization of Acetic acid decreases with increasing concentration. From Table 6, the distribution coefficient value of Acetic acid decreases as the concentration of Acetic acid increases in Water.
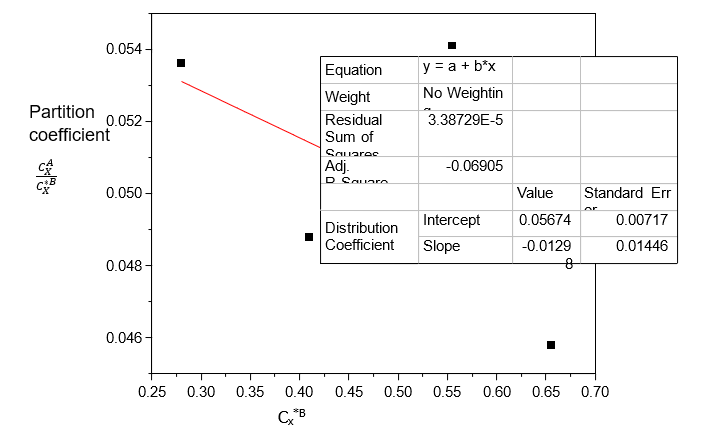
Table 7. Data for the partition coefficient (kD) for Acetic acid in n-Hexane-Water at 30°C and an atmospheric pressure, equation (2)
Volume of Acetic acid (ml) | Concentration in n-Hexane (CxA) (mole/litre) | Concentration in Water (CxB) (mole/litre) | CxA/Cx*B |
0.4 | 0.0150 | 0.2800 | 0.0536 |
0.6 | 0.0200 | 0.4100 | 0.0488 |
0.8 | 0.0300 | 0.5550 | 0.0541 |
1.0 | 0.0300 | 0.6550 | 0.0458 |
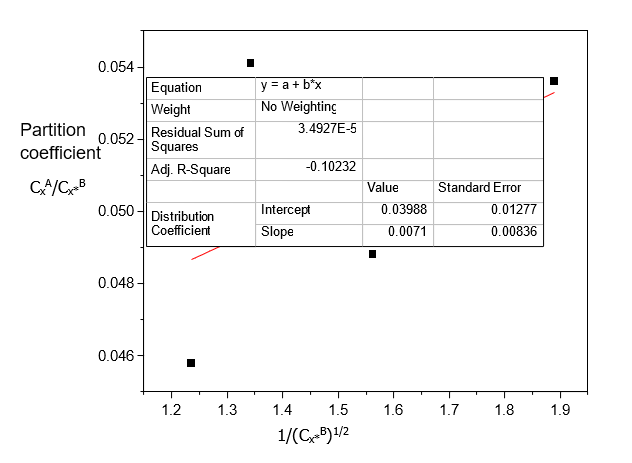
Table 8. Data for the partition coefficient of Acetic acid in n-Hexane-Water at 30°C and atmospheric pressure, equation (3)
CxA/Cx*B | 1/ Cx*B (mole/litre)-1/2 |
0.0536 | 1.8896 |
0.0488 | 1.5618 |
0.0541 | 1.3423 |
0.0458 | 1.2356 |
Table 9. Data for the partition coefficient of Acetic acid in n-Hexane-water at 30°C and an atmospheric pressure, equation (4)
CxA/Cx*B | CxA/Cx*B (1-) | Cx*B (mole/litre) | Cx*B (1-) (mole/litre) |
0.0536 | 0.0535 | 0.2800 | 0.2796 |
0.0488 | 0.0487 | 0.4100 | 0.4095 |
0.0541 | 0.0540 | 0.5550 | 0.5543 |
0.0458 | 0.0457 | 0.6550 | 0.6541 |
Table 7 shows that the dissolution of Acetic acid increases with increasing concentration in both n-Hexane and Water; Acetic acid dissolves more in Water than in n-Hexane. Table 8 shows that the ionization of Acetic acid decreases with increasing concentration in Water. From Table 9, the distribution coefficient value decreases as the concentration of Acetic acid dissolves more in Water.
Table 10. The partition coefficient, kD, dimerization constant, K, and ionization constant, , for Acetic acid in the binary immiscible solvents: Carbon Tetrachloride-Water, Diethyl Ether-Water and n-Hexane-Water respectively at 30°C and an atmospheric pressure.
Acetic acid | Distribution coefficient kD | Dimerization constant, K | Ionization constant | Association & ionization constant |
Carbon tetrachloride-Water | 0.01643 | 250.9771 | 0.0196 | 261.37 |
Diethylether-Water | 0.05838 | 0.0068 | 0.03998 | 12.35 |
n-Hexane-Water | 0.05674 | -2.0281 | 0.0013 | -2.03 |
Table 10 summarizes the result for the analysis of Acetic acid. Acetic acid has a high dimerization constant of 250.9771 and therefore a low distribution coefficient value of 0.01643 and ionization constant of 0.0196 in Carbon Tetrachloride-Water. Acetic acid has the appreciable distribution coefficient value of 0.05838 and the dimerization constant of 0.0068 with the ionization constant of 0.03998 in Diethyl Ether-Water. Acetic acid has the distribution coefficient value of 0.05674 and the dimerization constant of 2.0281 and the ionization constant value of 0.0013. In this analysis, the three binary immiscible solvents: Carbon Tetrachloride-Water, Diethyl Ether-Water and n-Hexane-Water are recommended for the beneficiation of Acetic acid.
The figures are shown below:
Thus; 1-0.9987
Conclusion
Partition coefficient technique is a good analytical method for the beneficiation of Acetic acid from contaminants. From this analysis, the three binary immiscible solvents: Carbon Tetrachloride-Water, Diethyl Ether-Water and n-Hexane-Water are recommended for the beneficiation of Acetic acid. This analysis has shown that Acetic acid could be extracted with the three binary solvents that are recommended, to determine the concentration that is present in the system, to checkmate the efficiency of the beneficiation process, etc. Partition coefficient therefore is emboldened as a health indicator for the control of the use of Acetic acid.
Introduction
Health indicators summarize information about a given priority topic for health system performance. It gives comparable and actionable data across different geographic, organizational or administrative boundaries. Health indicators support health authorities, institutions and organizations in monitoring the health of their populations and in tracking the viability of the local health system. It gives room for key performance dimensions as described in the Health System Performance Measurement Framework which allows a common approach for managing health system performance across the country. [www.cihi.ca] Health indicators could be divided into two: (i) direct measures indicators that measure health phenomena such as: diseases, deaths, use of services and (ii) indirect measures with examples as: social development, education and poverty indicators. [Global health indicators: an overview - PMC (nih.gov)] Health indicators could express lifestyles, individual behaviours such as physical exercise, diet, smoking, or substance and alcohol abuse, morbidity, mortality and important precursors of these factors. Measurement of health status is defined in terms of the Global Disease Burden, GDB, which is seen as the impact of a health problem as expressed in mortality, morbidity and financial cost. [Measuring Global Health - Physiopedia (physio-pedia.com)] [Measuring Global Health - Physiopedia (physio-pedia.com)] Assessing the quality of health services, especially in primary health care is important. Primary health care is taken at the cost the community can maintain at every stage of their development. This gives rise to the need to develop methods for quality assessment and monitoring. The use of quality indicators, which are defined as quantitative measure of the activities can serve as a guideline for quality monitoring and evaluation of relevant patient care and support services. [Primary health care quality indicators: An umbrella review | PLOS ONE]. According to the World Health Organization (WHO), primary health care gives the persons’ health needs throughout the lifetime. This includes the physical, mental and social well-being. This health approach includes health promotion, disease prevention, treatment, rehabilitation and palliative care. The scheme is defined in three categories [www.who.int]:
- Meeting people’s health needs throughout their lives
- Addressing the broader determinants of health through multisectoral policy and action
- Empowering individuals, families and communities to take charge of their own health
References
- Onuchukwu AI (2016): Chemical Thermodynamics for Science Students. Owerri: FUTO Press; 4th ed.; 1-18.
- Onyeocha VO (2021): Comparisons of the effects of solute interactions on partition coefficient, kD, in selected binary immiscible solvents: a case of oxalic acid and succinic acid. Publisher Full Text
- Onyeocha VO (2022): Analyses of Ethylenediaminetetraacetic Acid (EDTA) in Selected Binary Immiscible Solvents: The Role of Partition Coefficient, kD. DOI: doi.org/10.47363/JMSMR/2022(3)124
- Onyeocha VO, Akpan OD, Onuchukwu IA, (2018) et al.: The Dimerization Effects of Some Solutes on the Partition Coefficient (kD) in Binary Immiscible Solvents. Intern. Lett. Chem. Phys. Astrono.; 80: 40–52.
- Rastogi RP, Misra RR (1980): An Introduction to Chemical Thermodynamics. India:Vikas Publishing House Pvt Ltd; Second Edition.; pp 296–300.
- Morrison RT, Boyd RN (2005): Organic Chemistry. Sixth Edition; 375.


