Current Issue : Article / Volume 3, Issue 2
- Short Communication | DOI:
- https://doi.org/10.58489/2836-2322/30
High-Dose Methotrexate (Hd-Mtx) : Therapeutic Drug Monitoring Of Patients Followed At The Toxicology Department Of The University Hospital Of Setif, Algeria
1 Toxicology Department, Saadna Abdennour University Hospital, Setif. Algeria
2 Biopharmacy and Pharmaceutical and pharmacotechnics Laboratory, Faculty of Medicine, University Ferhat Abbas of Setif. Algeria
S. Benboudiaf*
S. Benboudiaf. (2024). High-dose Methotrexate (HD-MTX) : Therapeutic drug Monitoring of Patients Followed at the Toxicology Department of the University Hospital of Sétif, Algeria. 3(2). DOI:10.58489/2836-2322/30
© 2024 S. Benboudiaf, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 30-10-2023
- Accepted Date: 05-01-2024
- Published Date: 15-03-2024
Nephrotoxicity, High-Dose Methotrexate (HD-MTX), delayed elimination, Malignant Hematologic Disorder.
Abstract
Therapeutic drug monitoring (TDM) of methotrexate is widely recommended during high-dose administration (> 1 g/m2) in various chemotherapy protocols. This study aims to analyze the blood concentration methotrexate profile to detect delayed elimination in patients monitored at the toxicology department of the University Hospital of Sétif for MTX blood testing. A retrospective descriptive monocentric study was conducted, including all adult patients over 15 years old treated for malignant hematologic disorders with HD-MTX (> 1 g/m2) during their initial therapy. The results revealed that out of 189 monitored patients, only 79 underwent MTX level assessment at 48 hours and 72 hours. Among them, 77.2% exhibited elimination delays at 48 hours and 52% at 72 hours. The analysis of risk factors associated with elimination delay (age, gender, and dosage) did not show any statistically significant correlation. Considering the risk associated with elimination delay in potentiating the toxic effect of MTX, pharmacological therapeutic monitoring remains essential to adjust rescue measures through folinic acid administration and/or intensification of alkaline hydration. This approach aims to enhance the safety and efficacy of HD-MTX-based treatments.
Introduction
Methotrexate (MTX) is an antimetabolite used in oncology for the treatment of certain malignant hematological disorders and osteosarcomas, especially at high doses (greater than 1 g/m2). According to the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT), Therapeutic drug Monitoring (TDM) of high-dose MTX (HD-MTX) is recommended. This monitoring aims to detect delayed elimination, indicating substance accumulation and posing a risk factor for toxic effects, particularly renal toxicity.
The objective of this study is to analyze the blood concentration profile of methotrexate in patients treated at the hematology department of Sétif University Hospital [1] [2].
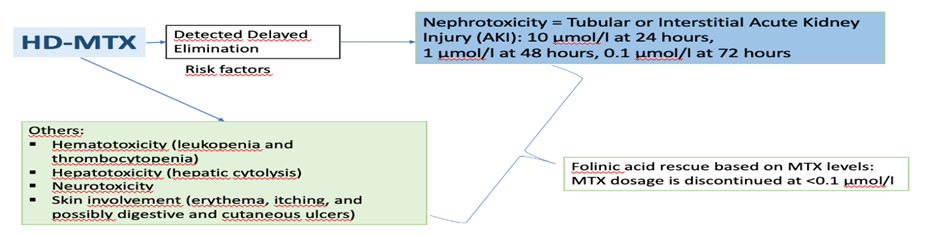
Fig 1: Toxic Effect of MTX at various Tissues
II. Method
This is a retrospective, descriptive, single-center study that included all adult patients over 15 years of age treated for a hematologic malignancy and receiving high-dose methotrexate (HD-MTX) chemotherapy during their first cycle. Data were collected from January 1, 2016, to April 30, 2023, at the Toxicology Department. Blood samples were taken in heparinized tubes 48 hours and 72 hours after the start of the treatment and transported to the laboratory in the dark to measure methotrexate blood concentrations. Analysis was performed using an automated system (EMIT technique), and data were collected in Microsoft Excel and analyzed with SPSS V26. Delayed elimination was defined as a methotrexate concentration exceeding 1 µmol/L at 48 hours and 0.1 µmol/L at 72 hours [1].
III. Results
III.1 Characteristics of the population
A total of 189 patients were included in this study. Over 64% of the patients were treated for non-Hodgkin lymphoma, while 36% had acute lymphoblastic leukemia. Among the patients, 42% were treated with monotherapy, and 48% received combination therapy (the most common combination being with asparaginase and vincristine)

Fig 2: Distribution based on MTX association
The average MTX dose administered was 4.7 g/m2 ± 1.36 g (Fig 03). The average age of the patients was 35 years (ranging from 17 to 66 years), and the male-to-female ratio was 1.5.
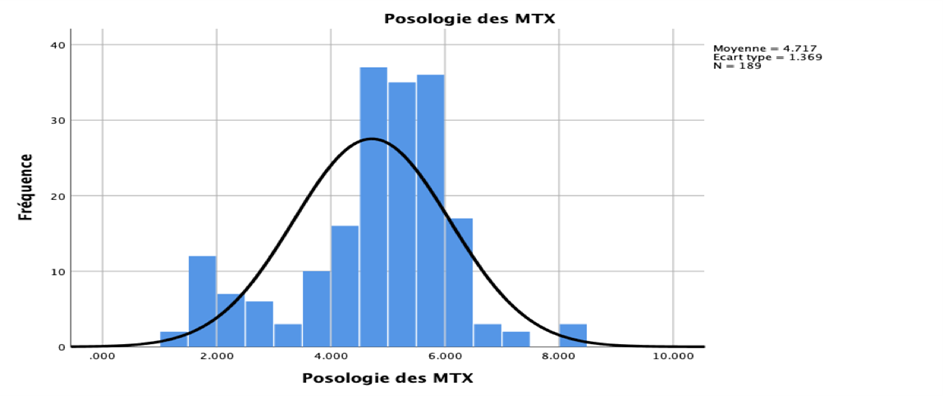
Fig 3: Distribution based on MTX Dose

Fig 4: Distribution based on Age
III.2/ Methotrexemia and delayed elimination (DE)
Among the 79 patients who underwent MTX level testing at 48 and 72 hours, 77.2% showed delayed elimination at 48 hours and 52% at 72 hours. The correlation study between risk factors represented by age, gender, and administered dose, and the occurrence of delayed elimination did not reveal any statistical significance (tabI, tabII).
Table I: Patients with Delayed Elimination (DE) at 48 hours and 72 hours
DE | Effectif 48h | Percentage valid % (48h) | Effective (72h) | Percentage valid %(72h) |
| With DE | 61 | 77,2 | 43 | 51,8 |
| Without DE | 18 | 22,8 | 40 | 48,2 |
| Total | 79 | 100,0 | 83 | 100,0 |
Table 2: Patients with Delayed Elimination (DE) at 48 hours and 72 hours
Variables | OR/IC 95% (48H) | P | OR/IC 95% (72h) | P |
Age | 1,058 | 0,15 | 1,01 | 0,15 |
Sexe | 0,45 | 0,6 | 0,824 | 0,81 |
Dose HD-MTX | 1,07 | 0,42 | 0,19 | 0,81 |
Discussion
The therapeutic drug monitoring of MTX, coupled with hyperhydration measures and the use of folinic acid rescue, helps limit the incidence and/or severity of toxic manifestations during HD-MTX treatments [3]. Our results indicate that 77.2% of our patients exhibit delayed elimination, which can worsen MTX-induced nephrotoxicity and its metabolite. This delay could be attributed to genetic factors, patient physiopathological characteristics (such as age or pre-existing nephropathy), as well as drug interactions (NSAIDs and Beta-lactams) and pharmacological factors (high dosage and infusion duration) [3] [5]. However, our analyses did not reveal a statistically significant correlation between the studied risk factors (dose, gender, and age) and the occurrence of delayed elimination. It's also noteworthy that among our patients, 48% were treated with combination therapy (MTX in combination with asparaginase and vincristine). In the case of polychemotherapies, these combinations can sometimes lead to unpredictable serious accidents.
Conclusion
Given the risk of delayed elimination in potentiating the toxic effects of MTX, TDM, including folinic acid rescue, remains essential to enhance the safety and effectiveness of HD-MTX treatment. Methotrexemia remains the best marker of exposure, but comparing creatinine levels during the treatment to baseline values helps identify clinically asymptomatic patients at risk. Before each administration of MTX-HD, it is essential to assess the patient's renal function (in addition to at least checking the CBC, platelet count, liver function, and albumin level). If the patient has impaired renal function before starting treatment, or in elderly individuals, methotrexate should be used with caution, and dose adjustment should be considered. All drug interactions involving methotrexate should be considered comprehensively before initiating treatment [5].
References
- Garcia, H., Leblond, V., Goldwasser, F., Bouscary, D., Raffoux, E., Boissel, N., ... & Joly, D. (2018). Toxicité rénale du méthotrexate à haute dose. Néphrologie & Thérapeutique, 14, S103-S113.
- Wiczer, T., Dotson, E., Tuten, A., Phillips, G., & Maddocks, K. (2016). Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. Journal of oncology pharmacy practice, 22(3), 430-436.
- Le Guellec, C., Blasco, H., Benz, I., & Hulin, A. (2010). Niveau de preuve du suivi thérapeutique pharmacologique du méthotrexate au décours de son administration à haute-dose. Thérapie, 65(3), 163-169.
- Bagarry-Liégey, D., Nicoara, A., Duffaud, F., Guillet, P., Pignon, T., Catalin, J., ... & Favre, R. (1996). Adaptation individuelle de posologie du méthotrexate à haute dose (MTX-HD) en pratique clinique courante. La Revue de médecine interne, 17(8), 689-698.
- ansm. Méthotrexate haute dose (MTX-HD) : l’ANSM rappelle les mesures générales de prévention du risque de néphrotoxicité. risques medicamenteux - médicaments - publié le 03/01/2022 - mis à jour le 13/07/2023


