Archive : Article / Volume 2, Issue 1
- Case Report | DOI:
- https://doi.org/10.58489/2836-2217/009
Hyperhomocysteinemia and pregnancy: about a case and literature review
Department of Gynecology and Obstetrics. Mohamed V. Rabat Military Training Hospital. Morocco
Allae eddine BOUCHAIB
Allae eddine BOUCHAIB, Mamadou Alpha BALDE, abdellah BABAHABIB, moulay el mehdi ELHASSANI, jaouad KOUACH. Department of Gynecology and Obstetrics. Mohamed V. Rabat Military Training Hospital. Morocco. Journal of Clinical Case Reports and Trails.2(1). DOI: 10.58489/2836-2217/009
© 2023 Allae eddine BOUCHAIB, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited
- Received Date: 24-01-2023
- Accepted Date: 24-03-2023
- Published Date: 06-04-2023
hyperhomocysteinemia, pregnancy, abortion disease, thrombophilia
Abstract
Homocysteine ââ(Hct) is a substance produced in the metabolism of methionine that can be found in our daily diet. Mutation of the methylenetetrahydrofolate reductase (MTHFR) gene, especially in women with low folate intake. Hyperhomocysteinemia (HHct) can be caused by several factors, such as lack of folic acid, vitamin B6 and B12 deficiency, hypothyroidism, medications, genetic abnormalities, aging and kidney dysfunction. Increased homocysteine ââin peripheral blood can lead to vascular disease, coronary artery dysfunction, atherosclerotic changes, and embolic disease. Thus, upstream of the trophoblastic plugs, any increase in the thrombogenic power of pregnant women will lead to the formation of a clot and the termination of pregnancy. Recent studies have reported that hyperhomocysteinemia is associated with numerous pregnancy complications, including abortion disease, preeclampsia, preterm birth, hematoma placentae, fetal growth restriction, and gestational diabetes. To avoid thrombosis, the treatment will therefore be based on anticoagulants, sometimes combined with low-dose aspirin because of the anti-aggregating action of the latter. In this article, we report the case of a patient with a history of abortive disease on hyperhomocysteinemia who carried a pregnancy to term, while recalling the metabolism of homocysteine, its impact on pregnancy, and the interest of supplementation folic acid to prevent complications due to hyperhomocysteinemia.
Introduction
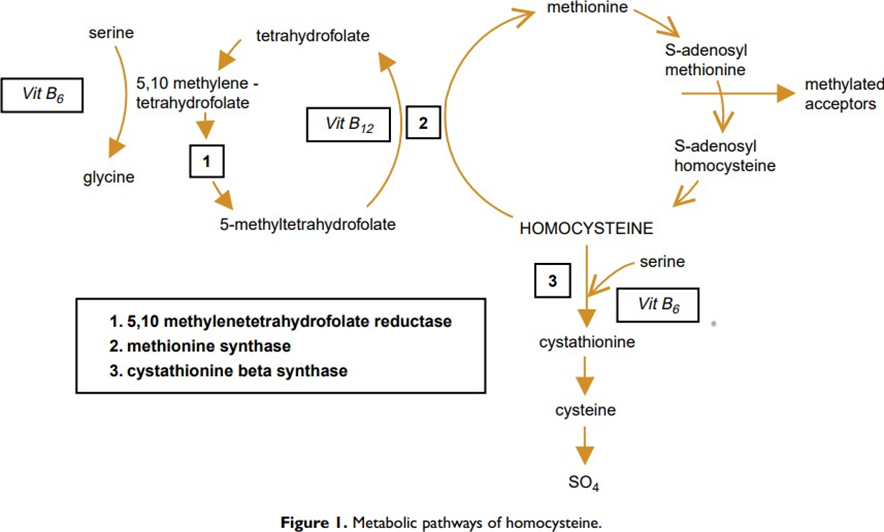
Homocysteine is a sulfur amino acid that represents an intermediate step in the cellular metabolism of an essential amino acid, methionine. However, most homocysteines are remethylated to form methionine. It is formed by the enzymatic synthesis pathway of S-adenosyl-methionine (SAM), the most important methyl group donor involved in many biochemical reactions (synthesis of DNA, proteins, neurotransmitters, hormones, phospholipids ...) (Figure 1). The MTHFR gene mutation is linked to high expressions of total homocysteine, especially in women with low folate intake [1]. MTHFR gene polymorphism is a major cause of hyperhomocysteinemia [2]. Vitamins B6 and B12 and folic acid play an essential role in influencingthe functionality of Hct [3]. Many factors cause hyperhomocysteinemia, including genetic abnormalities, folic acid deficiency, vitamin B6 and B12 deficiency, hypothyroidism, medications, aging andkidney dysfunction [4]. Increased homocysteine in peripheral blood can lead to vascular disease [5], coronary artery dysfunction, atherosclerotic changes [5] and embolic diseases [6]. Hct levels are lower in pregnant women compared to women without Hct [7 ]. Hct levels decrease in early pregnancy; it reaches its lowest value in the second quarter; Thereafter, it increases steadily in late pregnancy until reaching the level of early pregnancy [8]. Hyperhomocysteinemia reduces nitric oxide released by endothelial cells, which will promote the formation of thrombosis, which in turn will affect placental perfusion [9], as a result it can have an impact on pregnancy; and may be responsible for abortive disease, preeclampsia, threat of preterm delivery, retroplacental hematoma, intrauterine growth restriction [9]. The objective of this review is to address the importance of research for hyperhomocysteinemia and its complications during pregnancy by reporting the case of a patient with a history of abortion who led to pregnancy after folic acid supplementation and anticoagulant.
Observation and clinical case
Patient aged 36 years, positive groupage A, with a history of abortive disease with 7 spontaneous unaspirated and uncureted diaper pits in the first trimester and an upper delivery at 7 months for retroplacental hematoma in a context of preeclapmpsia of a newborn who died on day 2 of life for respiratory distress. During the follow-up of her abortive disease, the patient benefited from a complete assessment having objectified hyperhomocysteinemia without other biological abnormalities; She consulted in our training with a ninth pregnancy estimated at 12 weeks of amenorrhea or an ultrasound was performed objectifying an active monofoetal pregnancy corresponding to gestational age without trophoblastic abruption. The patient was seen in antenatal care on a monthly basis and was placed throughout pregnancy on progestogen plus an antiplatelet agent with a low molecular weight heparin based on lovenox 0.4 per day combined with vitamin supplementation. A prenatal check-up was requested having objectified gestational diabetes on OGGH at 75 g hence its diet alone withcorrect glycemic cycles. Knowing that the patient kept correct temptational figures throughout pregnancy without noticeable uterine bleeding A prophylactic caesarean section was scheduled at 39 weeks of amenorrhea, the clinical examination on the day of the upper route objectified a patient in good general condition normotendue apyretic correct uterine height at 32 cm examination At the speculum objectified a healthy looking cervix without noticeable bleeding. Caesarean section allowed the cephalic extraction of a male newborn apgar at 10 birth weight 3200 grams received by the pediatrician. The suites are simple for the mother and the newborn.
Discussion
Although the mechanisms of vascular changes associated with hypermocysteinemia are not yet fully understood, animal studies have allowed us toprovide specific details [10]. The effect is mainly at the level of the vascular wall with endothelial changes [11]. The lesions observed consist of vascular fibrosis and impaired function of endothelial cells. Endothelial cells are prone to vacuolization and desquamation, exposing subendothelial tissue and activating thrombus formation. Homocysteine decreases in the first trimester of pregnancy, reaches a minimum in the second trimester and increases somewhat to first trimester levels at the end of pregnancy [12]. Therefore, the search for homocysteine during pregnancy is difficult. On the other hand, it is possible to look for mutations in the CBS or MTHFR genes during pregnancy.
Hereditary thrombophilia and hyperhomocysteinemia, or a combination of both, may be responsible for repeated layer fossa, with genetic susceptibilityto venous thrombosis [13]. One third of abortifacient diseases are due to hyperhomocysteinemia [13], as is the case in our patient. A deficiency of vitamin D and folate could raise the level of homocysteine by reducing the enzyme involved in its metabolism. The MTHFR genotype can double or triple the morbidity of fetal loss [14]. Maternal genetic mutations cause embolic kidney disease or heart disease. However, it may not have the same adverse effects on placental circulation in early pregnancy [9]. Quere reports the case of a patient who had 2 fetal losses at 28 and 26 weeks of amenorrhea and three early miscarriages, the only abnormalities of the etiological balance were hyperhomocysteinemia and mutation of the MTHFR gene [15]. Supplementation with folic acid and vitamin B6 in subsequent pregnancies allowed this patient to have a live child, and this is almost the case for our patient who was able to carry a pregnancy to term after having seven fetal losses.
Recent studies have reported that homocysteine expression levels in women with preeclampsia are significantly higher than those in normotensive pregnant women [16]. However, a significant difference in its levels between PE patients and non-pregnant women has not been very common [17], implying hyperhomocysteinemia in PE is the change in blood volume rather than a mutation in the MTHFR gene [17]. Several studies aim to determine whether hyperhomocysteinemia can be used to predict the risk of preeclampsia inlow-risk pregnant women [18]. High levels of homocysteine have been detected during the first or second trimester of pregnancy without being directly involved in preeclampsia. The reason for the high level of homocysteine in early pregnancy could be due to the alteration of vascular endothelial cells during the first trimester. As pregnancy progresses, this lesion worsens placental ischemia, eventually leading to preeclampsia [19]. However, the level of homocysteine expression during the second trimester does not help predict the risk of preeclampsia in pregnant women with chronic hypertension [20]. Homocysteine levels in patients with retroplacental hematoma were significantly higher than in patients with normal pregnancies [21]. A comparative retrospective case-control study of patients with retroplacental hematoma compared to other patients who had normal pregnancies without signs or symptoms of bleeding objectified a significant increase in homocysteine expression in retroplacental hematoma [22 On the other hand, in other studies, the authors revealed that hyperhomocysteinemia was not necessarily associated with retroplacental hematoma [23]. In our patient, the tentional figures were correct throughout pregnancy with no detectable signs of abnormal bleeding.
Among the actions of homocysteine on endothelial cells is vascular obstruction, which can be the cause of premature delivery. However, the prevalence of decidual angiopathy is not significantly related topre-term delivery [24]. An association between neural tube defects (NSFNA) and hyperhomocysteinemia has been reportedby several authors [25]. In fact, AFTN is not due to a direct effect of homocysteine, but to an abnormal functioning of methionine synthase (MS) which is a key enzyme in remethylation, and also involved in the production of the myelin base protein.
Hyperhomocysteinemia may impact the transfer of amino acids in the placenta, which could cause intrauterine growth restriction [26]. Some authors reveal that compared to normal pregnancies, homocysteine levels are higher in patients who have had pregnancies with intrauterine growth restriction [27], this could be due to damage to endothelial cells that can cause impaired uterine and placental blood flow. In our patient who had a full-term pregnancy the birth weight was eutrophic, it seems premature to retain the direct causal link between hyperhomocysteinemia and intrauterine growth restriction.
Hyperhomocysteinemia is congenital thrombophilia, along with antithrombin deficiency, protein C deficiency, S protein deficiency, activated protein C resistance (due to Leiden mutations in the V gene) and factor II mutations [28]. Pregnancy itself is a risk factor for thrombosis. Therefore, the combination of hyperhomocysteinemia and pregnancy represents a situation with a high risk of thromboembolic events. It is even advisable to prescribe anticoagulants in addition to vitamin therapy to patients once congenital thrombotic tendencies have been identified. This prophylaxis consisted in our case of treatment with calciparin in early pregnancy, low molecular weight heparin in late pregnancy, then calciparin resumption in late pregnancy, during delivery and 1 month after delivery.
Conclusion
The activity of homocysteine during normal pregnancies its impact is a widely debated topic. According to the literature, homocysteine levels are not constant during pregnancy. The genetic polymorphisms associated with hyperhomocysteinemia and its metabolism are clearly associated with complications during pregnancy. However, clinical trials aimed at showing this require a lot of resources. High-dose folic acid supplementation during pregnancy may help women with complications from hyperhomocysteinemia
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Yamada K., Chen Z., Rozen R., Matthews R. G. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):14853–14858.
- Long S., Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Australian Family Physician. 2016;45(4):237–240
- Refsum H. Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. British Journal of Nutrition. 2001;85(Supplement 2):S109–S113.
- Alvares Delfino V. D., de Andrade Vianna A. C., Mocelin A. J., Barbosa D. S., Mise R. A., Matsuo T. Folic acid therapy reduces plasma homocysteine levels and improves plasma antioxidant capacity in hemodialysis patients. Nutrition. 2007;23(3):242–247.
- Graham I. M., Daly L. E., Refsum H. M., et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA: The Journal of the American Medical Association. 1997;277(22):1775–1781.
- den Heijer M., Koster T., Blom H. J., et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. The New England Journal of Medicine. 1996;334(12):759–762.
- Cikot R. J. L. M., Steegers-Theunissen R. P., Thomas C. M., de Boo T. M., Merkus H. M., Steegers E. A. Longitudinal vitamin and homocysteine levels in normal pregnancy. The British Journal of Nutrition. 2001;85(1):49–58.
- Walker M. C., Smith G. N., Perkins S. L., Keely E. J., Garner P. R. Changes in homocysteine levels during normal pregnancy. American Journal of Obstetrics and Gynecology. 1999;180(3):660–664.
- Zammiti W., Mtiraoui N., Mahjoub T. Lack of consistent association between endothelial nitric oxide synthase gene polymorphisms, homocysteine levels and recurrent pregnancy loss in Tunisian women. American Journal of Reproductive Immunology. 2008;59(2):139– 145.
- Wilcken DEL, Dudman NPB. Mechanism of thrombogenesis and accelerated atherogenesis in homocysteinemia. Haemos- tasis 1989; 19(suppl 1): 14-23.
- Woo KS, Chook P, Lolin YI, Cheung ASP, Chan LT, Sun YY, et al. Hyperhomocystinemia is a risk factor for arterial endo- thelial dysfunction in humans. Circulation 1997; 96: 2542-4.
- Walker MC, Smith GN, Perkins SL, Keely EJ, Garner PR. Changes in homocysteine levels during normal pregnancy. Am J Obstet Gynecol 1999; 180: 660-4.
- Raziel A., Friedler S., Schachter M., Ron-El R., Kornberg Y., Sela B. A. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. American Journal of Reproductive Immunology. 2001;45(2):65–71.
- Nelen W. L., Steegers E. A., Eskes T. K., Blom H. J. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet. 1997;350(9081):p. 861.
- Quere I, Bellet H, Hoffet M, Janbon C, Mares P, Gris JC. A woman with five consecutive fetal deaths: case report and retrospective analysis of hyperhomocysteinemia prevalence in 100 consecutive women with recurrent miscarriages. Fertil Steril 1998; 69: 152-4.
- Şanlıkan F., Tufan F., Göçmen A., Kabadayı C., Şengül E. The evaluation of homocysteine level in patients with preeclampsia. Ginekologia Polska. 2015;86(4):287–291.
- Raijmakers M. T., Zusterzeel P. L., Steegers E. A., Peters W. H. Hyperhomocysteinaemia: a risk factor for preeclampsia? European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2001;95(2):226–228.
- Hogg B. B., Tamura T., Johnston K. E., Dubard M. B., Goldenberg R. L. Second-trimester plasma homocysteine levels and pregnancy- induced hypertension, preeclampsia, and intrauterine growth restriction. American Journal of Obstetrics and Gynecology. 2000;183(4):805–809.
- Sun F., Qian W., Zhang C., Fan J. X., Huang H. F. Correlation of maternal serum homocysteine in the first trimester with the development of gestational hypertension and preeclampsia. Medical Science Monitor. 2017;23,
- Zeeman G. G., Alexander J. M., McIntire D. D., Devaraj S., Leveno K. J. Homocysteine plasma concentration levels for the prediction of preeclampsia in women with chronic hypertension. American Journal of Obstetrics and Gynecology. 2003;189(2):574–576.
- Goddijn-Wessel T. A., Wouters M. G., Molen E. F. v.d., et al. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1996;66(1):23–29. doi: 10.1016/0301-2115(96)02383-4.
- Budde M. P., de Lange T. E., Dekker G. A., Chan A., Nguyen A. M. Risk factors for placental abruption in a socio-economically disadvantaged region. The Journal of Maternal- Fetal & Neonatal Medicine. 2007;20(9):687– 693. doi: 10.1080/14767050701482738
- Chaudhry S. H., Taljaard M., MacFarlane A. J., et al. The role of maternal homocysteine concentration in placenta - mediated complications: findings from the Ottawa and Kingston birth cohort, BMC Pregnancy Childbirth. 2019;19(1): p. 75. doi: 10.1186/s12884-019-2219-5.
- Kramer M. S., Kahn S. R., Rozen R., et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. International Journal of Epidemiology. 2009;38(3):715–723
- D'Anna R., Baviera G., Corrado F., Ientile R., Granese D., Stella N. C. Plasma homocysteine in early and late pregnancies complicated with preeclampsia and isolated intrauterine growth restriction. Acta Obstetricia et Gynecologica Scandinavica. 2004;83(2):155–158
- Tsitsiou E., Sibley C. P., D'Souza S. W., Catanescu O., Jacobsen D. W., Glazier J. D. Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta. The Journal of Physiology. 2009;587(16):4001–4013. doi: 10.1113/jphysiol.2009.173393
- Furness D., Fenech M., Dekker G., Khong T. Y., Roberts C., Hague W. Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Maternal & Child Nutrition. 2013;9(2):155–166. doi: 10.1111/j.1740- 8709.2011.00364.x.
- Rao AK, Kaplan R, Sheth S. Inherited thrombophilic states. Semin Thromb Hemost 1998; 24 suppl 1: 3-12.


