Archive : Article / Volume 2, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-5828/006
Impact of Appropriate Antibiotics within 1hr of Patients Admission
1 Grant Medical College & JJ group Of Hospitals, Mumbai, India
Correspondng Author: Srirupa Biswas
Citation: Srirupa Biswas, Sankha Dasgupta, Moumita Majumder Bhowmick, Smriti Gupta, et.al (2023), Impact of Appropriate Antibiotics within 1hr of Partients Admission, Archives of Urology and Nephrology.2(1). DOI: 10.58489/2836-5828/006
Copyright: © 2023 Srirupa Biswas, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.
Received Date: 2023-02-18, Received Date: 2023-02-18, Published Date: 2023-03-01
Abstract Keywords: antibiotics; patients' outcome; review
Abstract
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections.[1][2] They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity.[3][4] Antibiotics are not effective against viruses such as the common cold or influenza;[5] drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics.
Introduction
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections[1-2. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity [3-4]. Antibiotics are not effective against viruses such as the common cold or influenza; [5] drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics.
Sometimes, the term antibiotic—literally "opposing life", from the Greek roots ἀντι anti, "against" and βίος bios, "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterial (such as sulfonamides and antiseptics) are fully synthetic. However, both classes have the same goal of killing or preventing the growth of microorganisms, and both are included in antimicrobial chemotherapy. "Antibacterial" include antiseptic drugs, antibacterial soaps, and chemical disinfectants, whereas antibiotics are an important class of antibacterial used more specifically in medicine [6] and sometimes in livestock feed.
Antibiotics have been used since ancient times. Many civilizations used topical application of moldy bread, with many references to its beneficial effects arising from ancient Egypt, Nubia, China, Serbia, Greece, and Rome.[7] The first person to directly document the use of molds to treat infections was John Parkinson (1567–1650). Antibiotics revolutionized medicine in the 20th century. Alexander Fleming (1881–1955) discovered modern day penicillin in 1928, the widespread use of which proved significantly beneficial during wartime. However, the effectiveness and easy access to antibiotics have also led to their overuse[8] and some bacteria have evolved resistance to them [1][9][10][11]. The World Health Organization has classified antimicrobial resistance as a widespread "serious threat [that] is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country".[12] Global deaths attributable to antimicrobial resistance numbered 1.27 million in 2019.[13]
Review Of Literature
Sepsis Campaign Guidelines 2012 and 2015 update
- 2015 update: “broad spectrum antibiotics should be administered within 3 hours of the time of presentation”
- 2012 Guideline: “We recommend that empiric antimicrobials be administered within 1 hr of the identification of severe sepsis. Blood cultures should be obtained before administering antibiotics when possible, but this should not delay initiation of antibiotics. The empiric drug choice should be changed as epidemic and endemic ecologies dictate (eg, H1N1, methicillin-resistant S. aureus, chloroquine-resistant malaria, penicillin-resistant pneumococci, recent ICU stay, neutropenia) (grade 1D)”
- As of 2010, only 68% of patients in the SSC registry received antibiotics within 3 hours.
Kumar et al, 2006
- The classic retrospective cohort study of 2,731 septic shock ICU patients showed a strong correlation between delay in effective antibiotic therapy and in-hospital mortality after recurrent or persistent arterial hypotension (P <0>
- Only 50% of the patients received effective antibiotic therapy within the first 6 hours (i.e. appropriate in vitro activity for the isolated pathogenic microorganism or the underlying clinical syndrome).
Gaieski et al, 2010
- A single-center cohort study of 261 patients with severe sepsis undergoing early goal-directed therapy (EGDT) time from triage and qualification for EGDT to appropriate antibiotic therapy was significantly associated with reduced mortality at the <1 xss=removed>P <0>
Puskarich et al, 2011
- A preplanned analysis of a multicenter controlled trial (3 centers) in US EDs of 291 patients with septic shock (EMSHOCK NET) found no change in mortality with hourly delayed antibiotic therapy up to 6 hours after triage or after recognition of shock.
- Antibiotic administration before recognition of shock was associated with a lower mortality as compared with antibiotic administration after recognition of shock (odds ratio = 2.35, 95%CI 1.12 to 4.53).
Ferrer et al, 2014
- Retrospective analysis of 17,990 patients with severe sepsis and septic shock from the multicenter, multinational Surviving Sepsis Campaign database (Europe, USA and South America) found an hourly increase in mortality with delay in antibiotic administration following recognition of severe sepsis, not just the onset of hypotension
- Differences were statistically significant, as we as clinically significant, beyond 2 hours
De Groot et al, 2015
- Prospective multicenter study in three Dutch Eds,
- 1,168 patients with sepsis (stratified into mild, moderate and severe; overall mortality of 10%)
- in those receiving antibiotics within 6 hours, a reduction in time to antibiotics was not found to be associated with an improvement in relevant clinical outcomes (28 mortality or LOS)
Sterling et al, 2015
- Systematic review of 11 studies that met inclusion criteria, comprising:
- 16,178 patients with severe sepsis/ septic shock who evaluable for antibiotic administration from emergency department triage, and 11,017 patients who were evaluable for antibiotic administration from severe sepsis/septic shock recognition
- There was no significant mortality benefit of administering antibiotics within 3 hours of ED triage or within 1 hour of shock recognition in severe sepsis and septic shock
- The authors suggest that currently recommended timing metrics as measures of quality of care are not supported by the available evidence
- Problems: 7 studies were excluded because authors did not respond to requests for information (selection bias), unclear if antibiotic choice was appropriate in these studies and less than half of patients had confirmed bacteraemia (antibiotics would not be expected to benefit non-bacterial infections or infections with bacteria that are insensitive to the chosen antibiotic)
Garnacho-Montero et al, 2015
- A prospective obsertional study of 928 patients admitted to ICU with severe sepsis/ septic shock (68% with microbiological identification)
- Findings were:
- Inadequate therapy prior to ICU admission was more common in nosocomial sepsis
- Administration of appropriate empirical antimicrobial therapy early was associated with decreased mortality
- Nearly all patients (98.3 %) received at least one dose of antibiotics before ICU admission, however they were inadequate in 31% of patients
- Progression to septic shock in patients with severe sepsis was associated with inadequate antimicrobial therapy prior to ICU admission
Siriwimon Tantarattanapong et al,2021
- The current international sepsis guideline recommends that administration of intravenous broad-spectrum antibiotics should be initiated within 1 hour of emergency department (ED) arrival for sepsis patients.
- Retrospective cross-sectional study, elderly patients (age ¸65 years) diagnosed with sepsis in the ED of a tertiary referral and academic hospital from January were enrolled. Door-to-antibiotic time was defined as the time from ED arrival to antibiotic initiation. The associations of door-to-antibiotic time and each hour delay in first antibiotic initiation with in-hospital mortality were assessed.
- Six hundred patients with the median age of 78.0 (IQR: 72.0-86.0) were studied (50.8
AIMS & OBJECTIVE
- The mantra for timing of antibiotics for serious infections is ‘hit hard, early and appropriately’
- Evaluate outcome of patients admitted in hospital how receive an appropriate antibiotic within 1hour of admission.
- Despite the strong biological plausibility of a need for early antibiotics in patients with serious bacterial infections the importance of antibiotic timing is controversial.
RATIONALE FOR EARLY ANTIBIOTICS
Early antibiotics may:
- prevent injury caused by microbial activity and toxin production
- prevent or ameliorate harmful host responses to infection
- Observational data has shown strong associations between early antibiotics and survival outcomes; however, a recent (flawed) systematic review did not find a benefit for early antibiotics.
- The Sequential Organ Failure Assessment (SOFA) score numerically quantifies the number and severity of failed organs. We examined the utility of the SOFA score for assessing outcome of patients with antibiotics at the time of intensive care unit (ICU) presentation.
- APACHE IV provided the best discrimination and calibration abilities and was useful for quality assessment and predicting mortality in medical ICU patients with antibiotics.
Methods

. - STUDY LOCATION: AMRI HOSPITAL -DHAKURIA,
- Timing of ICU admission - time noted from the patient’s ICU nursing chart when the first vital parameters are noted by the nurse.
- Timing of administration of 1st dose of antibiotic will be noted from ICU nursing chart (in minutes from the timing of ICU admission).
- Antibiotic that is administered within the first one hour of admission will be noted from ICU nursing chart.
- Appropriateness of antibiotic - will be assessed from the microbiology culture and sensitivity results once available.
- Outcome measures - will be noted from the ICU database.
- Data will be then recorded systemically in the data collection form and finally entered in the excel sheet for analysis.
- All the data will be then analysed by statistician by appropriate statistical tests.
Search strategy:
The data search was performed on July 22, 2022 till November 28, 2022 in AMRI HOSPITAL -DHAKURIA, we conducted a prospective study of adult patients in ICU ward. Records screened “Patient who are present in ICU for more than 24 hours with antibiotics”. The ICU ward which was included in the study was ICU-2 with 11 beds, ICU -3 with 12 beds, ICU -7 with 9 beds and NS-ICU with 8 beds. Ethical approval was obtained from the institutional review board. Demographic, clinical, and study data were recorded from charts through the electronic medical.
Data extraction and eligibility criteria:
Patients were excluded if they met any of the following criteria
- <18>
- pregnant or post-delivery,
- Excluded If Not Prescribed Antibiotic.
- Excluded If Antibiotic Were Prescribed More Than 24h After Time Zero.
- Discharge against medical advice (DAMA) Excluded
- TSL (Temperament suspension of life support) excluded.
- If a pathogen sample is not sent for none of the culture test like (blood culture, urine culture or sputum culture).
Patient repots inclusion criteria: -
- Patients ≥ 18 years old.
- If a pathogen sample is sent for any of the culture test like (blood culture, urine culture or sputum culture).
Patient characteristics:
- Patients received antibiotic < 1>
- Patients received antibiotic > 1 hour, P value
We recorded information on the selection of patients, inclusion criteria, the duration and time period of the study, the setting (intensive care units), the study design and the total number of patients received antibiotics. We also extracted data on other key study characteristics such as the set point intervals used for assessing the timing and impact of antibiotic therapy, the assessment of the appropriateness of antibiotic therapy and the study endpoints. Criteria used for the analysis of antibiotic appropriateness were based on in vitro susceptibility of causative pathogens in case of microbiologically-documented infections or on antibiotic therapy management guidelines in case of clinically-documented infections.
Outcome measures:
The primary outcome was all-cause mortality and length of stay (ICU & hospital) at the time points reported in the study. Such as INP number, gender, age, admission status (time) cause of admission, date of admission, name of antibiotic ,use of antibiotic number hour of admission, Time of antibiotic received, ITU stay, ITU outcome, Hospital outcome, Total stay, use of mechanical ventilation, Number of day on mechanical ventilation, APACHE IV, SOFA, Co-morbidities (Hypertension, II diabetes, Renal disease, Malignancy, Lung disease, Liver disease, Cancer, Heart disease, Other comorbidities),blood culture, urine culture, sputum culture, appropriate antibiotics , mortality.
Statistical Analysis
The outcome of this study was the association of clinical outcomes of infection with early antibiotics use. For the purposes of this study, early antibiotics use was defined as the time interval from ICU triage to the administration of broad-spectrum antibiotics within one hour. Those who received antibiotic administration more than one hour from ICU triage will be classified as having late antibiotics use. Broad-spectrum antibiotics referred to antibiotics that were effective against both gram-positive and gram-negative bacteria. In the included data, broad-spectrum antibiotics referred to b-lactamase-inhibit penicillin combined with third-generation cephalosporins. We applied the T-test to hospital length of stay and used chi-square to determine the correlation of early antibiotics use to mortality, mechanical ventilation support, and ICU admission. ICU admission was defined as direct admission to the ICU from the ED. We also performed factor analysis regarding the timing of antibiotics administration, including patients’ gender, age, vital signs at ICU triage, clinical symptoms.
The final sample size was 145 patients in the study. Continuous data are demonstrated as median with mean ± standard deviation. Categorical data are presented as number and percentages. The Pearson’s chi-squared test was performed on categorical data for the primary outcome. The chi-square test was used for the analysis and to compare mortality and antibiotic received time interval at ≤1 hour and received beyond the first hour. A two-sided p- value <0>
Ethical approval
- Ethical approval was taken from the AMRI Ethics Committee prior to data collection process.
Apps used for sofa calculation is MDCalc
Result
Characteristics of the study population:
There was a total of 145 patients (n=145) admitted during study period. 63, 43.4% patients were admitted receive antibiotic were female and the rest 82, 56.6% male received antibiotic. Male patients received antibiotic within one hour 26, 52.0 % and male patients received more than one hour 56, 58.9%. Female patients received antibiotic within one hour is 24, 48.0% and Female patients received antibiotic more than one hour is 39,41.1%. (Figure 1). There was no significant difference in the gender of the patients as the p value is > 0.05 the P value is 0.422 The patients age group was divided into 7 groups 31-40 (patients received antibiotic within one hour 0,0.0% and patients received more than one hour 5,5.3%) ,41-50(patients received antibiotic within one hour 2,4.0% and patients received more than one hour 6,6.3%) 51-60(patients received antibiotic within one hour 10,20.0% and patients received more than one hour 17,17.9%),61-70 (patients received antibiotic within one hour 13,26.0% and patients received more than one hour 33, 34.7%),71-80 (patients received antibiotic within one hour 17, 34.0% and patients received more than one hour 24,25.3%),81-90 (patients received antibiotic within one hour 8,16.0% and patients received more than one hour 9,9.5%),91-100 (patients received antibiotic within one hour 0,0.0% and patients received more than one hour 1,1.1%). There was no significant difference in the age of the patients as the p value is > 0.05 the P value is 0.378. The mean APACHE IV score of patients received antibiotic within one hour 77.70 and patients received more than one hour 76.28 and the mean SOFA patients received antibiotic within one hour 3.52 and patients received antibiotic more than one hour 3.53. There was no significant difference in the APACHE IV score of the patients as the p value is > 0.05 the P value is 0.469. There was no significant difference in the SOFA score of the patients as the p value is > 0.05 the P value is 0.846. The baseline characteristics of the study population are described in Table 1.
Characteristics Patients received antibiotic < 1> Patients received antibiotic > 1 hour P value Age, mean ± SD 69.66±11.68 66.06±12.50 0.115 Gender, n (%) Male 52.0% 58.9% 0.422 Female 48.0% 41.1% APACHE IV score, mean ± SD 77.70±19.71 76.28±16.05 0.469 OFA score, mean ± SD 3.52±0.93 3.53±0.83 0.846 Table 1. Showing the baseline characteristics of the study population.
GENDER Frequency Percent FEMALE 63 43.4 MALE 82 56.6 Total 145 100.0 AGE Frequency Percent 31-40 5 3.4 41-50 8 5.5 51-60 27 18.6 61-70 46 31.7 71-80 41 28.3 81-90 17 11.7 91-100 1 .7 Total 145 100.0 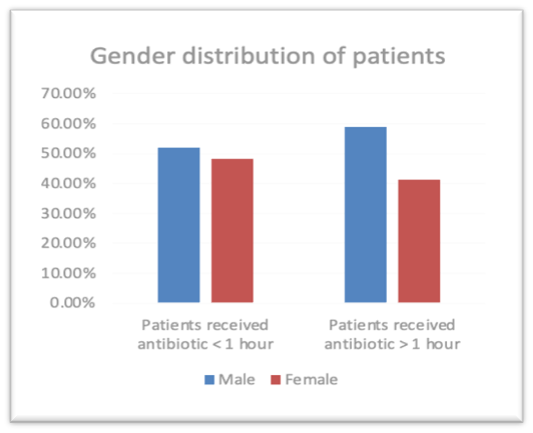
Figure 1 Gender distribution of patients who have received Antibiotic. Co-morbidities
The Co-morbidities are another variable which impact the study frequency and percentage are calculation also hypertension is a common co-morbidities 115 ,79.3%, diabetes 71,49%, renal disease 3,2.1% lung disease 6,4.1% heart disease 9,6.2%. (Table 2) There was no significant difference in the co-morbidities of the patients as the p value is > 0.05 but in case of hypertension the p value is > 0.05, P value is 0.045. (Figure 2)
CO-MORBIDITICS DIABETES
Frequency
Percent
NO
74
51.0
YES
71
49.0
Total
145
100.0
CO-MORBIDITICS HPERTENSION
Frequency
Percent
NO
30
20.7
YES
115
79.3
Total
145
100.0
CO-MORBIDITICS LUNG DISEASE
Frequency
Percent
NO
139
95.9
YES
6
4.1
Total
145
100.0
CO-MORBIDITICS RENAL DISEASE
Frequency
Percent
NO
142
97.9
YES
3
2.1
Total
145
100.0
CO-MORBIDITICS HEART DISEASE
Frequency
Percent
NO
136
93.8
YES
9
6.2
Total
145
100.0
Co-morbidities, n (%)
Patients received antibiotic < 1>
Patients received antibiotic > 1 hour
P value
Hypertension
70.0%
84.2%
0.045
Diabetes
48.0%
49.5%
0.866
Renal disease
2.0%
2.1%
0.966
Lung disease
8.0%
2.1%
0.090
Heart disease
4.0%
7.4%
0.424
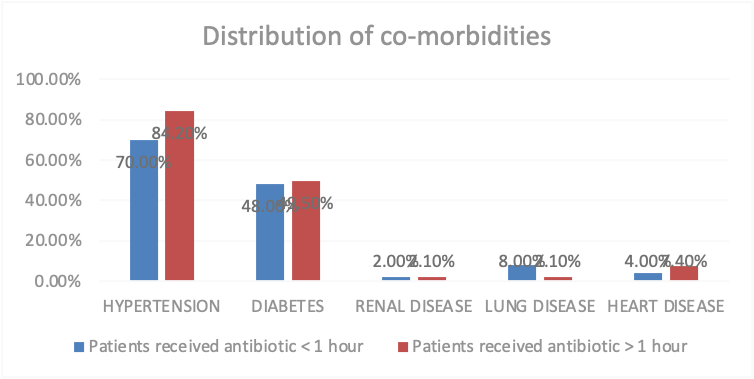
Figure 2: Distribution of co-morbidities of patients who have received Antibiotic Cause of admission In ICU department:
During our study period, the hospital had 145 cases from July 22, 2022 till November 28, 2022 in that female 63, 43.4% and male 82, 56.6%. The cause of admission of patient was maximum was for respiratory and lung disease 30, 2.1% kidney disease 17, 11.9
Discussion
In this prospective observational study patient with age greater then 18 years old were Included and patient from any department to ICU department cause of admission is independent of any disease, door-to-antibiotic time was not associated with in-hospital mortality. Sterling et al. (18) found no significant differences when comparing the antibiotic administration within 3 hours from ED triage and within 1 hour from septic shock recognition. Door-to-antibiotic time and in-hospital mortality were the main focuses of this study, which showed that each extra hour (relative to door-to-antibiotic time ≤1 hour) was not associated with an increase in the mortality rate. The highest mortality rate in this study was in the door-to-antibiotic group of >1hours. Likewise, Peltan et al. (22) found that a door-to-antibiotic time cutoff of 3 hours was associated with mortality, but a cutoff of 1 hour did not show statistical significance. When the door-to-antibiotic times of ≤1 hour and >1hour were compared, the ≤1-hour group had greater severity of illnesses based on the ESI level and NEWS. For this reason, the door-to-antibiotic time of ≤1 hour had a higher mortality rate than the patients who received antibiotics later. The SSC guideline recommends antibiotic initiation within 1 hour. Nonetheless, many studies showed failure to achieve that goal. For instance, Abe et al. (23) found that 30.5% of cases received antibiotics within 1 hour. Ko et al. (24) revealed that the 1-hour target was achieved in 28.6% of septic shock patients treated in the EU. In this study, 48, 96.0%. of the patients received antibiotics within 1 hour and treated in ICU department.
During our study period, the hospital had 145 cases from July 22, 2022 till November 28, 2022 in that female 63,43.4% and male 82, 56.6%. The patients age group was divided into 7 groups 31-40,41-50,51-60,61-70,71-80,81-90,91-100 in which 61-70 maximum patient were admitted in hospital 46,31.7% and minimum patient admitted in hospital age group is 91-100 in hospital 1,0.7%. The cause of admission of patient was maximum was for respiratory and lung disease 30,2.1% kidney disease 17, 11.9
Conclusion
In my study it is demonstrated that gram-negative bacteria remains the major pathogen as has been demonstrated in most ICUs in India. Mortality of the patients were grouped into two groups, antibiotic received within one hour and other major group is antibiotic received more than one hour. The antibiotic received within one hour is sub group into antibiotic (death 2,4.0% and discharge 48,96.0%) and antibiotic received within more than one hour antibiotic (10,10.5
Limitations
Number of patient population is small. The Sensitive % was not calculated. The study says the impact of appropriate antibiotics within 1hour of patient’s admission and the culuture report takes 3 days so Antibiotics were compared with antibiotic policy it complies with the antibiotic drug list provide with the list with organism (Document: AMRI/DHK/HIC/POL/02 effective 15/09/2022) all are appropriate so we can conclude that antibiotic which were received against the Organism Antibiotic susceptibility.
References
- Aronin SI, Peduzzi P, Quagliarello VJ. (1998). Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Annals of internal medicine. 129(11):862-9.
- Auburtin M, Wolff M, Charpentier J. (2006). Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Critical care medicine. 34(11):2758-65.
- Gaieski DF, et al, (2010). Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med;38(4):1045-53.
- Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Fernández-Delgado E, López-Sánchez JM. (2015). Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Critical care (London, England). 19:302.
- Kumar A, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96.
- Lepur D, Barsić B. (2007). Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 35(4):225-31.
- Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, (2010) et.al, The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med;36(2):222-31.
- Lu CH, Huang CR, Chang WN. (2002). Community-acquired bacterial meningitis in adults: the epidemiology, timing of appropriate antimicrobial therapy, and prognostic factors. Clinical neurology and neurosurgery. 104(4):352-8.
- Miner JR, Heegaard W, Mapes A, Biros M. (2001). Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. The Journal of emergency medicine. 21(4):387-92.
- Pines JM. (2008) Timing of antibiotics for acute, severe infections. Emerg Med Clin North Am;26(2):245-57.
- Pines JM, Isserman JA, Hinfey PB. (2009). The measurement of time to first antibiotic dose for pneumonia in the emergency department: a white paper and position statement prepared for the American Academy of Emergency Medicine. J Emerg Med;37(3):335-40.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. (2005). Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM: monthly journal of the Association of Physicians. 98(4):291-8.
- Nazarian DJ, Eddy OL, Lukens TW, Weingart SD, Decker WW. (2009) Clinical policy: critical issues in the management of adult patients presenting to the emergency department with community-acquired pneumonia. Ann Emerg Med;54(5):704-31.
- Puskarich MA,(2011)et al; Emergency Medicine Shock Research Network (EMSHOCKNET). Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med;39(9):2066-71.
- Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. (2015). The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Critical care medicine. 43(9):1907-15.
- van Zanten AR. (2014) The golden hour of antibiotic administration in severe sepsis: avoid a false start striving for gold*. Crit Care Med;42(8):1931-2. 17.
- Villar J, Clement JP, Stotts J. (2014) Many emergency department patients with severe sepsis and septic shock do not meet diagnostic criteria within 3 hours of arrival. Annals of emergency medicine. 64(1):48-54.
- Vilella AL, Seifert CF. (2014) Timing and appropriateness of initial antibiotic therapy in newly presenting septic patients. The American journal of emergency medicine. 32(1):7-13.
- P. Naucler; A. Huttner; C.H. van Werkhoven; M. Singer; P.Tattevin; (2020) et.al, :Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship.Clinical Microbiology and Infection.
- Rational antibiotic utilization in selected pediatric conditions. Malaysian Health Technology Assessment Unit,2002.
- Gaur AH, English BK: (2006) The judicious use of antibiotics - An investment towards optimised health care. Indian Paediatr, 73, 343-50.
- WHO global strategy for containment of antimicrobial resistance. World Health Organization, 2001.
- Chambers H: (2001) Antimicrobial agents: General considerations. Hardman JG, Limbird LE: The pharmacological basis of therapeutics. New York: MacGraw-Hill, 1143-69.
- Okeke IN; Aboderin OA; Byarugaba DK; (2007) et al: Growing problem of multidrug-resistance enteric pathogens in Africa. Emerg Infect Dis, 13, 1640-6.
- Archibald LK, Reiller LB: (2001) Clinical microbiology in developing countries. Emerg Infect Dis, 7, 302-4.
- French GL (2005): Clinical impact and relevance of antibiotic resistance. Adv Drug Deliv Rev, 57, 1514-27.
- Davey P; Brown E; Fenelon L; ( 2005) et al: Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev, (4), CD003543.
- Lodise TP; McKinnon PS; Swiderski L; Rybak M (2003): Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis , 36, 1418-23.
- Harbarth S; Garbino J; Pugin J; (2003) et al: Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med, 115,529-35.
- Gonzales R; Bartlett JG; Besser RE; (2001) et al: Principles of appropriate antibiotic use for treatment of non-specific upper respiratory tract infections in adults: background. Ann Intern Med, 134, 490-4.
- Matthias Adorka; MitongaKabwebweHonore; MartieLubbe; Jan Serfontein; Kirk Allen (2013): The Impact of Appropriate Antibiotic Prescribing on Treatment Evaluation Parameters. J Public Health Africa, 4(1).
- Antimicrobial resistance: Does stopping a course of antibiotics early lead to antibiotic resistance? World Health Organization, 2020
- E Oakley-Hannibal; H Gawda (2015): G142(P) Are antibiotics administered within one hour in suspected neonatal sepsis as per nice guidelines? British Association of Perinatal Medicine, 100(3).
- Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. (2004) Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med;164:637-44.
- Joo YM, Chae MK, Hwang SY, Jin S-C, Lee TR (2014) et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency dept. ClinExpEmerg Med;1:35-40.
- Buckman, S. A., Turnbull, I. R., &Mazuski, J. E. (2018) Empiric Antibiotics for Sepsis. Surgical Infections,, 19(2), 147–154.
- Vilella, A. L., & Seifert, C. F. (2006) Timing and appropriateness of initial antibiotic therapy in newly presenting septic patients. The American Journal of Emergency Medicine, 32(1), 7–13.
- Baré, M., Castells, X., Garcia, A., Riu, M., Comas, M., (2006) et.al, Importance of appropriateness of empiric antibiotic therapy on clinical outcomes in intra-abdominal infections. International Journal of Technology Assessment in Health Care, 22(02), 242-248.
- Lancaster, J. W., Lawrence, K. R., Fong, J. J., Doron, S. I., Garpestad, E., (2008) et.al, Impact of an Institution-Specific Hospital-Acquired Pneumonia Protocol on the Appropriateness of Antibiotic Therapy and Patient Outcomes. Pharmacotherapy, 28(7), 852–862.
- Pierre Emmanuel Charles, Claire Tinel, Saber Barbar, SergoAho, SebastienPrin, (2009) et.al, Procalcitonin kinetics within the first days of sepsis: relationship with the appropriateness of antibiotic therapy and the outcome. Critical Care.
- Roberta Capp, MD; YuchiaoChang ,Phd; David F. M. Brown, MD (2011): Effective Antibiotic Treatment Prescribed By Emergency Physicians in Patients Admitted to the Intensive Care Unit With Severe Sepsis or Septic Shock: Where is the Gap? The Journal of Emergency Medicine, Vol. 41, No. 6, pp. 573–580.
- SurbhiLeekha, MBBS; Christine L. Terrell, MD, Randall S. Edson, MD (2011): General Principle of Antimicrobial Therapy. Mayo Clinic Proceedings, 86(2), 156-167.
- Christopher J. Grace, MD, FACP; Brad Robinson, MPH, MD: Empiric Antibiotic Selection. Infectious Disease Antimicrobial agents.
- Antibiotic Resistance. World Health Organization. 2020.
- Antibiotic police (2022)
- Philip S Stewart, William Costerton (2001): Antibiotic resistance of bacteria in biofilms. The Lancet, 358(9276), 135-138.


