Research Article | DOI: https://doi.org/10.58489/2836-2330/009
Importance of glycosylation for SARS-CoV-2 virus, antibodies and COVID-19
*Corresponding Author: Antonis Tsamaloukas
Citation: Antonis Tsamaloukas, (2022). Importance of glycosylation for SARS-CoV-2 virus, antibodies and COVID-19. Journal of Clinical and Medical Reviews. 1(2). DOI: 10.58489/2836-2330/009
Copyright: © 2022 Antonis Tsamaloukas, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 13 December 2022 | Accepted: 22 December 2022 | Published: 29 December 2022
Keywords: SARS-CoV-2, COVID-19, antibody glycosylation, IgG glycosylation, glycomics, biomarkers
Abstract
Summary
In addition to the known endemic respiratory viruses, the pandemic SARS-CoV-2 has been added, with terrible effects on the population worldwide. This virus, like many others, has a glycoprotein structure the so-called spike
protein, which interacts with the ACE-2 receptor as a cellular entry gate and thus initiates the infection. Just as this glycosylation causes infectiousness, the effect of the protective antibodies is also determined by their degree of glycosylation. In particular, the so-called Fc part of the immunoglobulins plays an important role. The type of bound sugar such as fucose, mannose, sialic acid, etc. determines, among other things, which reactions are triggered by binding to immune cells such as NK cells to their existing Fc receptors. These include antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular trogocytosis (ADCT) and antibody-dependent cellular complement deposition (ADCD). In addition to protective phagocytosis, these reactions can trigger the antibody-dependent enhancement (ADE) of the infection and also the dreaded cytokine storm in COVID-19 patients. The functional diversification of IgGs by Fc glycosylation can be determined and tracked both after vaccination and during the disease.
Introduction
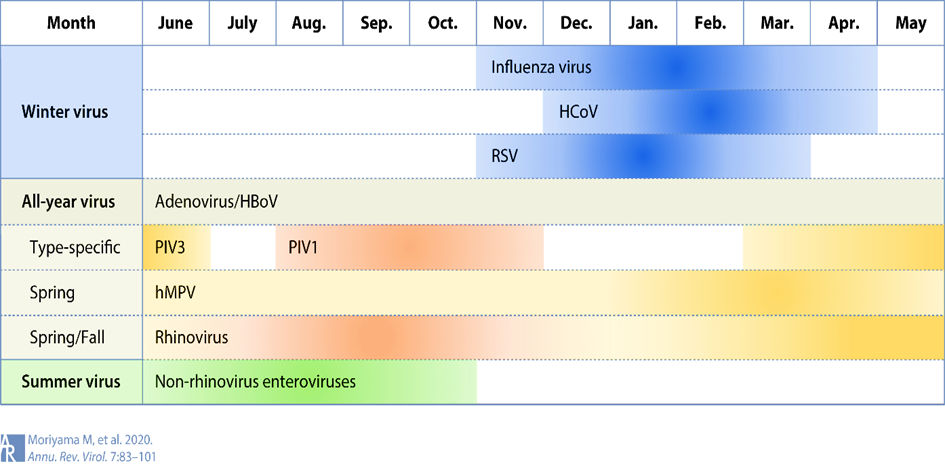
The current global coronavirus pandemic 19 (COVID-19), caused by the novel coronavirus (SARS-CoV-2) and its variants, has led to extensive hospitalizations worldwide [1]. To date, more than 253 million infected people and more than 5 million deaths have been reported [2]. The seasonal cycle of respiratory viral diseases has long been widely recognized, as annual epidemics of influenza and colds hit the human population like clockwork in the winter season in temperate regions. (See Figure 1):
The four endemic human coronaviruses HCoV-229E, -NL63, -OC43 and -HKU1 contribute a significant proportion of upper and lower respiratory tract infections in adults and children [4,5] In addition, epidemics caused by SARS-CoV-1 and the newly emerged SARS-CoV-2 occur during the winter months [3,6]. However, whether this can lead to viral interference in the sense of excluding a "tripledemic" seems likely.
SARS-CoV-2 and other respiratory viruses often "interfere" with each other. The COVID-19 pandemic has been associated worldwide with changes in respiratory virus infections that differed between virus types [7-9].
The decline in respiratory virus infections, including influenza viruses and respiratory syncytial virus (RSV), was most notable at the beginning of the COVID-19 pandemic and continued to varying degrees through subsequent waves of SARS-CoV-2 infections [10, 11].
A meta-analysis and systematic review showed that up to 19% of patients with COVID-19 co-infections and 24% with super-infections have infections [8]. The presence of co-infection or superinfection was associated with poor outcomes, including increased mortality [8].
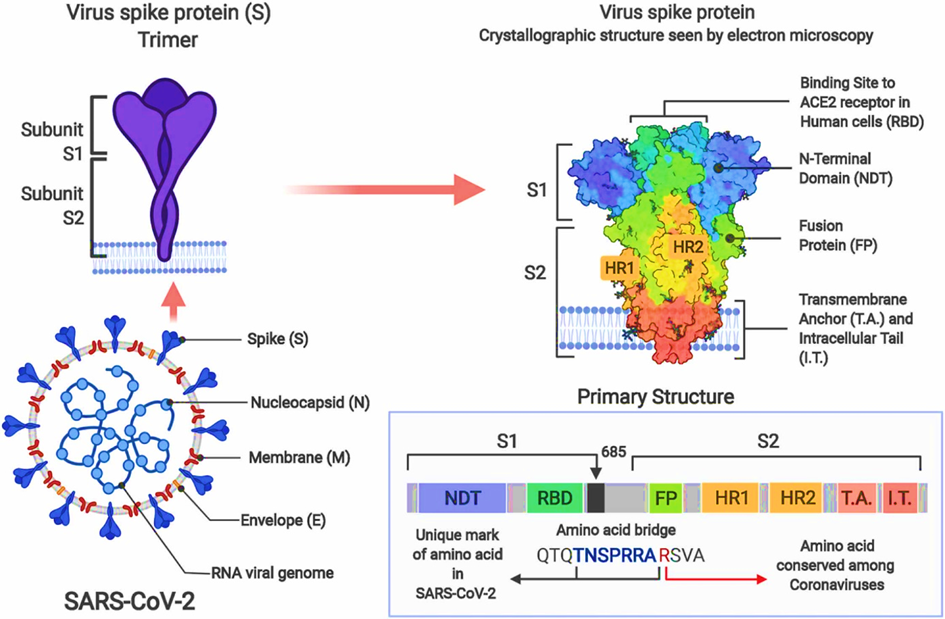
The Coronaviridae (coronaviruses) are a long-known family of enveloped single (+) strand RNA viruses (ss(+)RNA [12, 13]. SARS-CoV-2 is a enveloped virus and the SARS-CoV-2 spike (S) protein mediates virion binding to human cells through its interaction with the ACE2 cell surface receptor and is one of the main immunization targets. The receptor binding domain (RBD) is a critical component of the S-protein subunit (S1) that binds to angiotensin-converting enzyme 2 (ACE2), a recognized receptor for virus entry.
Most mutations occur in the spike (S) protein [14], a surface glycoprotein that plays a crucial role in viral infection (Figure 2).:
Glycosylation of the receptor binding domain of the spike protein of SARS-CoV-2 and the ACE2 receptor leads to stronger and more far-reaching binding interactions between the proteins [15-18]. This also applies to all virus variants. [19].
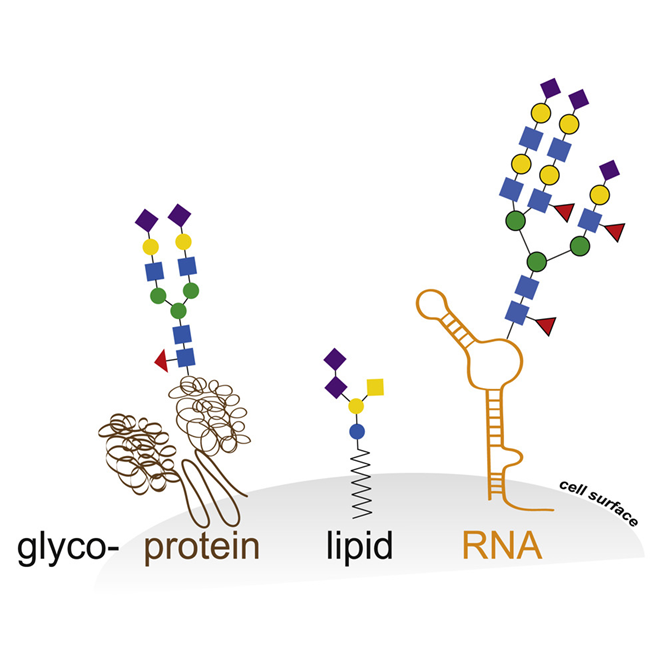
The viruses use the host cell machinery to glycosylate their own proteins during replication [20]. These viral host cell-derived glycans facilitate various structural and functional roles, from immune evasion by glycan shielding to enhancing immune cell infection [16, 20-22]. With regard to the role of SARS-CoV-2 glycosylation in viral replication, infectivity and immune response, glycosylation has major potential implications for therapeutic and vaccination strategies as well as serological testing [23]. Historically, the role of glycosylation in the dichotomous immune system has always been the focus of research [24-29]. The innate and adaptive immune response [30] in which glycosylation both plays a protective role and contributes to immune evasion by masking viral polypeptide epitopes and contributing to the cytokine cascade via non-fucosylated IgG, interact with a pronounced glycan epitope on the SARS-CoV-2 spike protein. Glycosylation is the most common and complex post-translational protein modification. In addition to proteins, many lipids are glycosylated, and only recently it has been shown that elaborated glycan structures can also be bound to RNA [31], as shown schematically in the following figure:
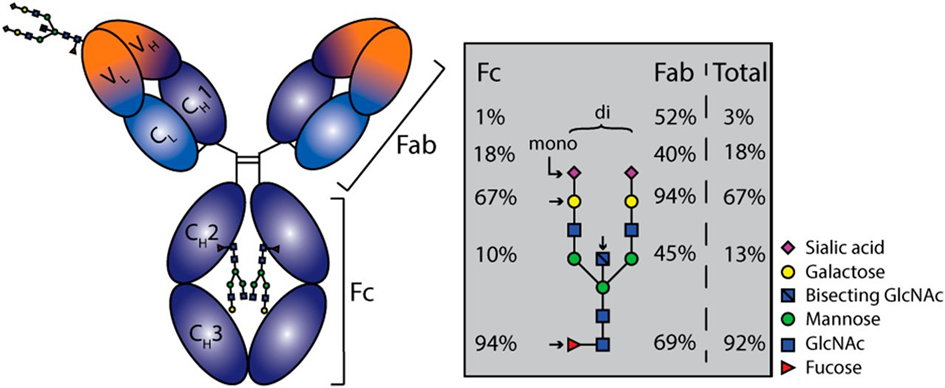
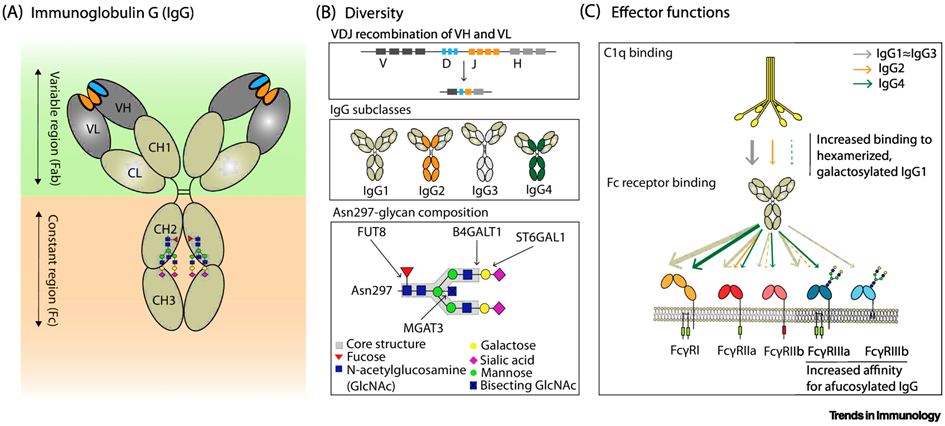
The most intensively studied example of Importance of alternative protein glycosylation for biological functions are immunoglobulins [32,33], which are among the main weapons of humoral immunity. Immunoglobulin G (IgG) antibodies play an important role in the immune response against SARS-CoV-2. Antibodies are Y-shaped molecules (see Fig. 5, 6). In the past, research on humoral responses to viral infection focused mainly on the V-end of the Y: the antigen binding regions, or Fab (fragment antigen binding). Conversely, the tail of Y (also known as fragment crystallizable or Fc domain) has numerous effector functions, such as antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular trogocytosis (ADCT) and antibody-dependent cellular complement deposition (ADCD). Antibody-dependent enhancement (ADE) also takes place via FCγR:
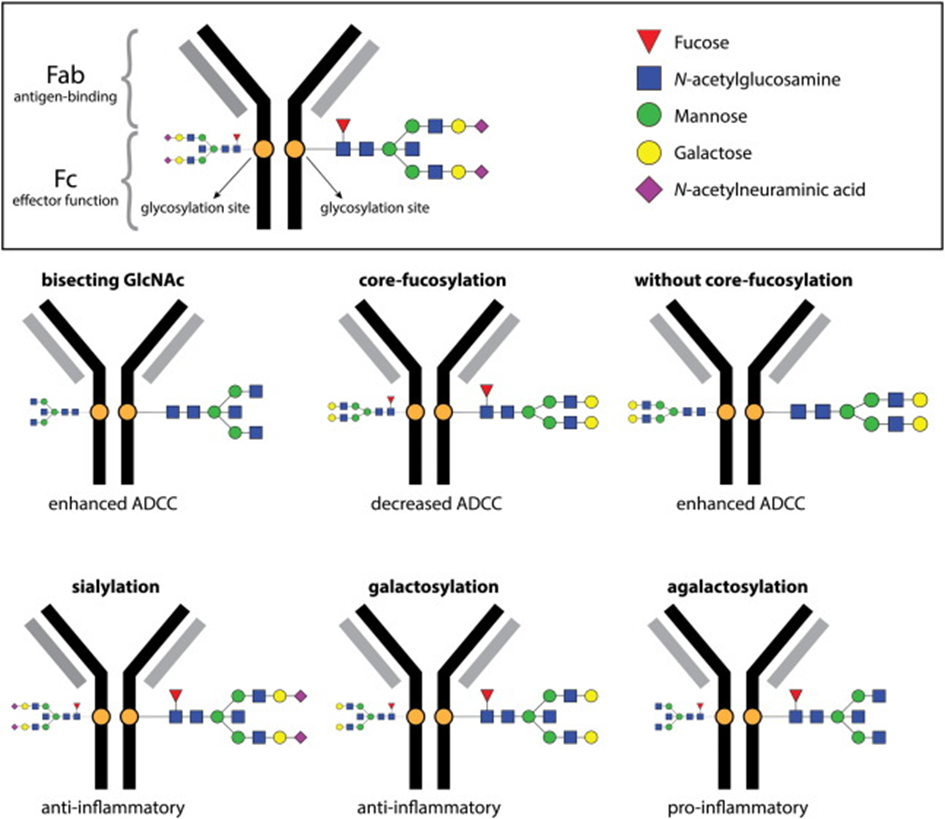
Since the effector functions of IgG are modulated by N-glycosylation of the Fc region, the structure and possible function of the IgG N-glycome in relation to divergent COVID-19 disease courses was investigated [24, 34-38].
Although immune responses in humans generally produce fully fucosylated IgG [38-42], some antigen-specific IgG responses may be predominantly afucosylated. The consequences of afucosylated reactions vary depending on the setting. Afucosylated antigen-specific IgG leads to immunopathology in SARS-CoV-2 [43-46] and dengue virus infections:
Changes in galactosylation, fucosylation and sialysation are now well-established factors driving differential IgG function, ranging from inhibitory/anti-inflammatory to activating complement and promotion of antibody-dependent cellular cytotoxicity (ADCC).
Fc receptors (FcR) are expressed on immune cells and bind the Fc portion of immunoglobulin. Fcγ receptors (FcγR), the largest group of FcR, bind IgG and include several subtypes [48,49].
Antibody effector functions such as the ADCC, ADC and ADCD play a crucial role in immunity against several pathogens, especially in the absence of neutralizing activity. Two modifications of the IgG constant domain (Fc domain) regulate the effector function of immunoglobulins: changes in the antibody subclass and changes in a single N-linked glycan located in the CH2 domain of IgG Fc (Fig.6).
The addition of different glycans can significantly alter the conformation of the Fc, with dramatic consequences for the IgG effector functions as Figure 7 shows [50]:
This astonishing phenomenon that both SARS-Cov-2 and the immune cells involved use post-translational glycosylation, on the one hand to penetrate the cells more easily and thereby "mask" themselves or to make the humoral response to the infection more effective, speaks for the evolutionary interdependence. It is therefore not surprising that immunoglobulin G glycans have been measured as an early indicator of the severity of COVID-19[36, 37, 51] and can be used to monitor the disease [52]. The decoding of glycosylation processes by high-throughput glycomics and proteomics open up future perspectives that explain pandemics from zoonotic origins to the mechanisms of infectious diseases in a well- founded way. [53-57].
References
- Zhou, P., et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020. 579(7798): p. 270-273.
View at Publisher | View at Google Scholar - Dong, E., H. Du, and L. Gardner, An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases, 2020. 20(5): p. 533-534.
View at Publisher | View at Google Scholar - Moriyama, M., W.J. Hugentobler, and A. Iwasaki, Seasonality of Respiratory Viral Infections. Annual Review of Virology, 2020. 7(1): p. 83-101.
View at Publisher | View at Google Scholar - Corman, V.M., et al., Chapter Eight - Hosts and Sources of Endemic Human Coronaviruses, in Advances in Virus Research, M. Kielian, T.C. Mettenleiter, and M.J. Roossinck, Editors. 2018, Academic Press. p. 163-188
View at Publisher | View at Google Scholar - Zhou, P., et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020. 579(7798): p. 270-273.
View at Publisher | View at Google Scholar - Dong, E., H. Du, and L. Gardner, An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases, 2020. 20(5): p. 533-534.
View at Publisher | View at Google Scholar - Moriyama, M., W.J. Hugentobler, and A. Iwasaki, Seasonality of Respiratory Viral Infections. Annual Review of Virology, 2020. 7(1): p. 83-101.
View at Publisher | View at Google Scholar - Corman, V.M., et al., Chapter Eight - Hosts and Sources of Endemic Human Coronaviruses, in Advances in Virus Research, M. Kielian, T.C. Mettenleiter, and M.J. Roossinck, Editors. 2018, Academic Press. p. 163-188.
View at Publisher | View at Google Scholar - Kung, Y.-A., et al., Molecular Virology of SARS-CoV-2 and Related Coronaviruses. Microbiology and Molecular Biology Reviews, 2022. 86(2): p. e00026-21.
View at Publisher | View at Google Scholar - Gorbalenya, A.E., et al., The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology, 2020. 5(4): p. 536-544.
View at Publisher | View at Google Scholar - Chow, E.J., T.M. Uyeki, and H.Y. Chu, The effects of the COVID-19 pandemic on community respiratory virus activity. Nature Reviews Microbiology, 2022.
View at Publisher | View at Google Scholar - Musuuza, J.S., et al., Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLOS ONE, 2021. 16(5): p. e0251170.
View at Publisher | View at Google Scholar - Nickbakhsh, S., et al., Virus–virus interactions impact the population dynamics of influenza and the common cold. Proceedings of the National Academy of Sciences, 2019. 116(52): p. 27142-27150.
View at Publisher | View at Google Scholar - Piret, J. and G. Boivin, Viral Interference between Respiratory Viruses. Emerg Infect Dis, 2022. 28(2): p. 273-281.
View at Publisher | View at Google Scholar - Tang, J.W., et al., Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. Journal of Medical Virology, 2021. 93(7): p. 4099-4101.
View at Publisher | View at Google Scholar - V’kovski, P., et al., Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology, 2021. 19(3): p. 155-170.
View at Publisher | View at Google Scholar - Hartenian, E., et al., The molecular virology of coronaviruses. Journal of Biological Chemistry, 2020. 295(37): p. 12910-12934.
View at Publisher | View at Google Scholar - Magazine, N., et al., Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses, 2022. 14(3): p. 640.
View at Publisher | View at Google Scholar - Chawla, H., E. Fadda, and M. Crispin, Principles of SARS-CoV-2 glycosylation. Curr. Opin. Struct. Biol., 2022. 75: p. 102402.
View at Publisher | View at Google Scholar - Reis, C.A., R. Tauber, and V. Blanchard, Glycosylation is a key in SARS-CoV-2 infection. Journal of Molecular Medicine, 2021. 99(8): p. 1023-1031.
View at Publisher | View at Google Scholar - Watanabe, Y., et al., Site-specific glycan analysis of the SARS-CoV-2 spike. Science, 2020. 369: p. 330.
View at Publisher | View at Google Scholar - Zhao, P., et al., Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host & Microbe, 2020. 28(4): p. 586-601.e6.
View at Publisher | View at Google Scholar - Aloor, A., et al., Glycosylation in SARS-CoV-2 variants: A path to infection and recovery. Biochemical Pharmacology, 2022. 206: p. 115335.
View at Publisher | View at Google Scholar - Casalino, L., et al., Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci., 2020. 6: p. 1722.
View at Publisher | View at Google Scholar - Sanda, M., L. Morrison, and R. Goldman, N- and O-Glycosylation of the SARS-CoV-2 Spike Protein. Analytical Chemistry, 2021. 93(4): p. 2003-2009.
View at Publisher | View at Google Scholar - Watanabe, Y., et al., Exploitation of glycosylation in enveloped virus pathobiology. Biochimica et Biophysica Acta (BBA) - General Subjects, 2019. 1863(10): p. 1480-1497.
View at Publisher | View at Google Scholar - Trbojević-Akmačić, I., et al., High-Throughput Glycomic Methods. Chemical Reviews, 2022. 122(20): p. 15865-15913.
View at Publisher | View at Google Scholar - Lauc, G., et al., Mechanisms of disease: The human N-glycome. Biochimica et Biophysica Acta (BBA) - General Subjects, 2016. 1860(8): p. 1574-1582.
View at Publisher | View at Google Scholar - Maverakis, E., et al., Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. Journal of Autoimmunity, 2015. 57: p. 1-13.
View at Publisher | View at Google Scholar - Reily, C., et al., Glycosylation in health and disease. Nature Reviews Nephrology, 2019. 15(6): p. 346-366.
View at Publisher | View at Google Scholar - Rudd, P.M., et al., Glycosylation and the Immune System. Science, 2001. 291(5512): p. 2370-2376.
View at Publisher | View at Google Scholar - Varki, A., Biological roles of glycans. Glycobiology, 2016. 27(1): p. 3-49.
View at Publisher | View at Google Scholar - Zhou, J.Y. and B.A. Cobb, Glycans in Immunologic Health and Disease. Annual Review of Immunology, 2021. 39(1): p. 511-536.
View at Publisher | View at Google Scholar - Wolfert, M.A. and G.-J. Boons, Adaptive immune activation: glycosylation does matter. Nature Chemical Biology, 2013. 9(12): p. 776-784.
View at Publisher | View at Google Scholar - Flynn, R.A., et al., Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell, 2021. 184(12): p. 3109-3124.e22.
View at Publisher | View at Google Scholar - Mimura, Y. and R. Jefferis, 5.12 - Human IgG Glycosylation in Inflammation and Inflammatory Disease☆, in Comprehensive Glycoscience (Second Edition), J.J. Barchi, Editor. 2021, Elsevier: Oxford. p. 215-232.
View at Publisher | View at Google Scholar - van de Bovenkamp, F.S., et al., The Emerging Importance of IgG Fab Glycosylation in Immunity. The Journal of Immunology, 2016. 196(4): p. 1435-1441.
View at Publisher | View at Google Scholar - Golay, J., A.E. Andrea, and I. Cattaneo, Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front Immunol, 2022. 13: p. 929895.
View at Publisher | View at Google Scholar - Grunst, M.W. and P.D. Uchil, Fc effector cross-reactivity: A hidden arsenal against SARS-CoV-2’s evasive maneuvering. Cell Reports Medicine, 2022. 3(2).
View at Publisher | View at Google Scholar - Petrović, T., G. Lauc, and I. Trbojević-Akmačić, The Importance of Glycosylation in COVID-19 Infection, in The Role of Glycosylation in Health and Disease, G. Lauc and I. Trbojević-Akmačić, Editors. 2021, Springer International Publishing: Cham. p. 239-264.
View at Publisher | View at Google Scholar - Petrović, T., et al., IgG N-glycome changes during the course of severe COVID-19: An observational study. eBioMedicine, 2022. 81: p. 104101.
View at Publisher | View at Google Scholar - Lofano, G., et al., Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Science Immunology, 2018. 3(26): p. eaat7796.
View at Publisher | View at Google Scholar - Arnold, J.N., et al., The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual review of immunology, 2007. 25(1): p. 21-50.
View at Publisher | View at Google Scholar - Burton, D.R. and R.A. Dwek, Sugar Determines Antibody Activity. Science, 2006. 313(5787): p. 627-628.
View at Publisher | View at Google Scholar - Golay, J., A. E. Andrea, and I. Cattaneo, Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Frontiers in Immunology, 2022. 13: p. 929895.
View at Publisher | View at Google Scholar - Li, J., et al., Unmasking Fucosylation: from Cell Adhesion to Immune System Regulation and Diseases. Cell Chemical Biology, 2018. 25(5): p. 499-512.
View at Publisher | View at Google Scholar - Chakraborty, S., et al., Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Science Translational Medicine, 2022. 14(635): p. eabm7853.
View at Publisher | View at Google Scholar - Larsen, M.D., et al., Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science, 2021. 371(6532): p. eabc8378.
View at Publisher | View at Google Scholar - Oosterhoff, J.J., et al., Afucosylated IgG responses in humans – structural clues to the regulation of humoral immunity. Trends in Immunology, 2022. 43(10): p. 800-814.
View at Publisher | View at Google Scholar - Wang, T.T. and J.V. Ravetch, Functional diversification of IgGs through Fc glycosylation. Journal of Clinical Investigation, 2019. 129(9): p. 3492-3498.
View at Publisher | View at Google Scholar - Oosterhoff, J.J., et al., Afucosylated IgG responses in humans – structural clues to the regulation of humoral immunity. Trends in Immunology, 2022. 43(10): p. 800-814.
View at Publisher | View at Google Scholar - Hayes, J.M., et al., Fc gamma receptors: glycobiology and therapeutic prospects. J Inflamm Res, 2016. 9: p. 209-219.
View at Publisher | View at Google Scholar - Nimmerjahn, F. and J.V. Ravetch, Fcγ receptors as regulators of immune responses. Nature Reviews Immunology, 2008. 8(1): p. 34-47.
View at Publisher | View at Google Scholar - Schwab, I. and F. Nimmerjahn, Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature Reviews Immunology, 2013. 13(3): p. 176-189.
View at Publisher | View at Google Scholar - Pongracz, T., et al., Immunoglobulin G1 Fc glycosylation as an early hallmark of severe COVID-19. eBioMedicine, 2022. 78: p. 103957.
View at Publisher | View at Google Scholar - de Haan, N., D. Falck, and M. Wuhrer, Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology, 2019. 30(4): p. 226-240.
View at Publisher | View at Google Scholar - Albóniga, O.E., et al., Metabolic Snapshot of Plasma Samples Reveals New Pathways Implicated in SARS-CoV-2 Pathogenesis. Journal of Proteome Research, 2022. 21(3): p. 623-634.
View at Publisher | View at Google Scholar - Chakravarty, S., COVID-19: The Effect of Host Genetic Variations on Host–Virus Interactions. Journal of Proteome Research, 2021. 20(1): p. 139-153.
View at Publisher | View at Google Scholar - McArdle, A., et al., Discovery Proteomics for COVID-19: Where We Are Now. Journal of Proteome Research, 2021. 20(10): p. 4627-4639.
View at Publisher | View at Google Scholar - Mohammed, Y., et al., Longitudinal Plasma Proteomics Analysis Reveals Novel Candidate Biomarkers in Acute COVID-19. Journal of Proteome Research, 2022. 21(4): p. 975-992.
View at Publisher | View at Google Scholar - Whetton, A.D., et al., Proteomics and Informatics for Understanding Phases and Identifying Biomarkers in COVID-19 Disease. Journal of Proteome Research, 2020. 19(11): p. 4219-4232.
View at Publisher | View at Google Scholar