Research Article | DOI: https://doi.org/10.58489/2836-5836/006
Infrared Spectroscopy Analysis of Kidney Stones: Methodology of Identification
- Bioactive Molecules and Chiral Separation Laboratory, Faculty of Exact Sciences, Tahri Mohamed University, Béchar, 08000, Istiklal street, PO417, Algeria.
- Al-Ihsan clinic for kidney diseases and surgery, Accredited by the Directorate of Health and Population Bechar, City Belkhdim Sliman, Bechar 08000, Algeria
*Corresponding Author: Malika BENZINE
Citation: Malika BENZINE, Nasser BELBOUKHARI, Khaled SEKKOUM, Abdelouahed OULED DJAAFRI, (2023). Infrared Spectroscopy Analysis of Kidney Stones: Methodology of Identification. Journal of Clinical Trials and Bioavailability Research. 2(1). DOI: 10.58489/2836-5836/006
Copyright: © 2023 Malika BENZINE, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 14 January 2023 | Accepted: 23 January 2023 | Published: 31 January 2023
Keywords: urolithiasis, kidney stone, infrared spectroscopy, recurrence, vibration, band.
Abstract
The urinary stone analysis is the first step in looking for the causes of urolithiasis, in order to avoid the problems of recurrence. This analysis sometimes makes it possible to propose a diagnosis, and most often helps to direct the exploration towards the most appropriate examinations. To be reliable, the identification of the components of urinary stones must be carried out by infrared spectroscopy. This study made it possible to make a profile approach on a case of a 67-year-old male adult patient, hospitalized in the urology department of the Bechar hospital (Algeria). Morpho-constitutional analysis of urinary stones showed that the main component was calcium oxalate monohydrate (whewellite) with a percentage of (70%), weddellite (20%), carbapatite (10%), and trace protein. According to the results obtained we notice that the periphery of the stone is Wheddellite, and the nucleus formed essentially of: Whewellite + CA + trace of Protein. According to the superficial and internal morphological type and the morphological association, we can distinguish the main causes that go into the formation of stones: intermittent hypercalciuria (nutritional or absorptive) + intermittent hyperoxaluria (normal or slightly increased oxaluria), these results allow more specific medical management of lithiasis and better prevention of recurrent problems.
Introduction
Urolithiasis is a complex multifactorial pathology which requires an understanding of the mechanisms involved in lithogenesis. The analysis of the urinary stones is an essential element of this diagnosis, carried out according to adequate methods, it provides exclusive information not only on the current situation, but on the history of the lithiasic disease and on the conditions which determined the genesis of the stones [1].
Analysis of the composition of kidney stones helps to establish the etiology of their formation, and in some cases, to make a diagnosis. In addition, the identification of the constituents is important in order to identify patients at risk of recurrence and to define a strategy for the prevention or treatment of the underlying etiology. To this end, preventive treatment algorithms exist for each major type of kidney stone [2-4].
The risk of kidney stone recurrence is approximately 50% at 5 years and 70% at 20 years and is influenced mainly by the sex, the amount of stones, their location, the presence of residual fragments and the presence of anatomical abnormalities or functional of the urinary system [2,5,6]. Certain types of stones are associated with a high risk of recurrence (eg cystine and PAM [struvite] stones) [7,3] and the composition of kidney stones may vary during recurrence [7,8]. The analysis of kidney stones must be repeated for a new episode of urolithiasis since the composition of the stone’s changes in nearly a third of patients [8].
The chemical analysis of the urinary stones was replaced by a morpho-constitutional analysis based on a precise description at the macroscopic scale supplemented by a characterization technique by vibrational spectroscopy and more precisely by infrared spectroscopy with Fourier transform [8,9].
At the macroscopic scale, in the identification of urinary stones, it is a question of specifying the shape, the size, the aspect of the surface, the texture, the shape and the aspect of the crystals, the color, the hardness, the organization and the main characteristics of the section.
Infrared spectrophotometry has become the reference method because of its versatility, speed, easy implementation and its ability to simultaneously identify crystalline and non-crystallized species, mineral and organic components, metabolic and medicinal species. In contrast, infrared spectrophotometry cannot identify a new substance not yet described [10].
Urinary stones can be gathered in a classification comprising 6 types and 21 subtypes, which allows to classify more than 95% of urinary tree stones:
-Calcium oxalates [11,12] constitute class I for whewellite and class II for weddellite.
-Uric acid stones and urates [13,14] form class III
-Calcium and magnesium phosphates, ie carbapatite, struvite, brushite, whitlockite [15,16] are grouped in class IV.
-Cystine stones (Class V) are linked to cystinuria. This is a genetic defect that causes the kidneys to excrete excessive amounts of cystine. This type of calculation can occur from childhood [17,18].
The stones made up of proteins are gathered in Class VI. Note the existence of other types of urinary tree stones such as stones of drug origin [19,20] or stones made of rare purines linked to genetic diseases [21].
Material and methods
Case presentation:
Patient: the study concerns a lithiasic subject, aged 67 years of sex Male, operated on Urology service of Bechar hospital (south of Algeria). the stones located in the left side of the kidney with a mass of 14.26g, 0.10g.
Method of recovering from stones:
Analysis of the different methods of extracting stones shows that conventional surgery remains the most frequent since it represents 79.7% of cases against 17.7% of spontaneous expulsion, 2.3% mixed (extra-corporeal lithotripsy + conventional secondary surgery due to poor fragmentation of stones or obstructive complications) and 0.2% of treatment by extra-corporeal lithotripsy alone.
Apparatus and methods
The kidney stone was dried in air and at room temperature for 24 hours, then subjected to a morpho-constitutional analysis according to the protocol described previously [22].
kidney stone analysis was carried out sequentially, from the nucleus to the surface, using an infrared spectrophotometer with Fourier transform (Spectrum One model, Perkin-Elmer). The spectral range is from 4000 to 650 cm-1 (or even 550 cm-1), in order to determine the composition of the nucleus and that of subsequent layers, the two can be very different and provide information on particular lithogenic processes [23].
The proportions of the various constituents were determined from an overall stone powder. The results are compared with different references spectra [24-26].
Results and discussion
kidney stones recovered by the surgical extraction method have a dark yellow-brown crystalline form, spicule, amorphous peripheral deposit and a radial section with two very different successive structures: a central concentric zone with light brown-yellow radial crystallization (Ia) of whewellite, covered with a diffuse dark brown radial structure of Wheddellite type (IIa) (Figure 1).
Composition of stones: The majority component of a stone is essential to know since it is a reflection of the urinary environment and therefore of the pathology or abnormalities responsible for the activity of the lithogenic process 24. The morpho-constitutional analysis of calculation showed that the main component was calcium oxalate monohydrate or whewellite with a percentage of (70%), weddellite (20%), carbapatite (10%), trace of protein. The periphery of the calculation: Wheddellite and the Composition of the nucleus: Whewellite + CA + trace of Protein. (Figure 2)
Infrared spectroscopic analysis:
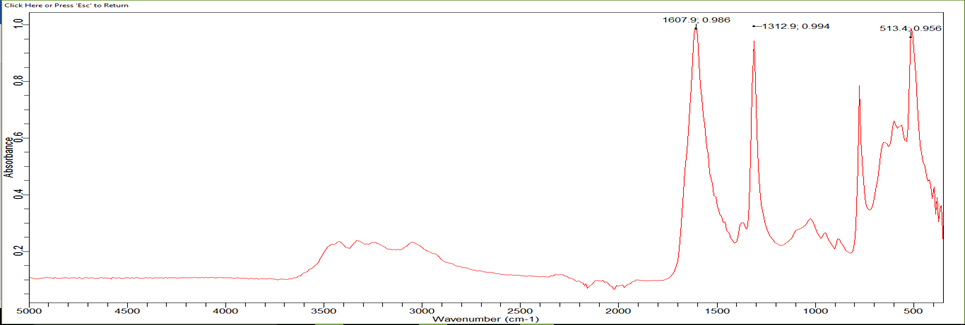
The infrared spectrum of this kidney stone shows 12 characteristic absorption bands (Table 1):
A broad band of medium intensity located at 3492 cm-1 corresponds to the stretching vibrations of the OH bonds of H2O, another band towards the same zone located at 3334 cm-1 attributed to the stretching vibration of the intermolecular hydrogen bonds, this is confirmed by the presence of bands around 1460 cm-1 attributed to the deformation vibration of the OH bonds of H2O.
Figure 3 illustrates the infrared spectrum obtained for the main constituents of kidney stone analyzed.
Table 1: Main IR spectrum bands of the urinary calculus analyzed [27-29].
Exp.band (cm-1) | Intensity | Assignment | Composition |
3492 | average | Elongation Vibration of OH (H2O). |
Calcium Oxalate monohydrate (Whewellite) COM +Calcium Oxalate dihydrate (Wedellite) +Calcium Carbonate anhydride (calcite) Carbapatite (CA) |
3334 | average | Intermolecular hydrogen bond Elongation Vibration | |
1607 | strong | Elongation Vibration of carbonyle (C=O), δ(HOH) + νa(CO)perpendicular to BC plane | |
1460 | low | Deformation vibration in the same plane δ de OH (H2O). | |
1312 | strong | Elongation Vibration of C-O | |
1037 | average | Elongation Vibration of C-O | |
946 | Low | [ νs(CC) + νs(CO)] on BC plane+ L (HOH) | |
881 | low | [ νs(CC) + δ(OCO)] on BC plane+ L- W(HOH) | |
761 | strong | Deformation vibration outside the plane of OH (H2O). | |
668 | average | Deformation vibration outside the plane of (O-C-O) | |
570 | average | L-R(OCO) perpend to the BC plane | |
513 | strong | ν(Ca-O) + νs(CC) perpendicular to BC plane |
Five bands towards: 570-946 cm-1 corresponds to the deformation vibration outside the plane of the ester and OH bonds which form the hydrated calcium oxalate ring. An intense band located at 513 cm-1 which characterizes the presence of the Ca-O bonds.
The analysis of urinary stones by physical methods such as infrared spectrophotometry, allows to determine by precision with very little samples. By the knowledge of the molecular and crystalline structures entering into the composition of the nucleus, and different layers of a stone. Our results, and according to the surface and internal morphological type and the morphological association, can distinguish the main causes of this calculation: intermittent hypercalciuria (nutritional or absorptive) + intermittent hyperoxaluria (normal or slightly increased oxaluria), according to Y. Berland et al [29-31] hypercalciuria is a metabolic disorder, described a group of patients suffering from recurrent calcium lithiasis, regroups three categories (hypercalciuria of absorption, renal hypercaciuria, hypercalciuria of resorption). hyperoxaluria according to Baggio et al [31], a disorder of the transmembrane transfer of oxalate has been found in the erythrocytes of patients with oxalocalcic lithiasis. the presence of an abnormality in the membrane transfer of oxalate has been correlated with the existence of a distal tubular acidification defect in a third of the cases. Our research in the literature shows that blood acidosis and metabolic acidosis and the major metabolic disorder of lithiasis disease [32].
The morpho constitutional analysis of stones and the identification of risk factors, through the analysis of stones, allows more targeted medical management of lithiasis and ultimately better prevention of recurrences.
Conclusion
To distinguish the main types of lithiasis and their etiologies, by emphasizing the importance of an exact identification of the molecular and crystalline species constituting the calculation by infrared spectrophotometry, it contributes to the diagnosis by directing the clinician towards additional examinations and avoided recidivism.
Acknowledgments
This work is supported by the General Direction of Scientific Research of Algeria (DGRSDT) and the authors are grateful to Dr A. Ouled Djaafri, Head of Al-Ihsan clinic for kidney diseases and surgery, Bechar, Algeria for collaboration in this field
References
- Daudon M (1989) Mécanismes de la lithogenèse. In: Jungers P, Daudon M, Leduc A (eds) Lithiase urinaire. Flammarion Médecine–Sciences, Paris, pp 114–57
View at Publisher | View at Google Scholar - Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. (2016), Kidney stones. Nat Rev Dis Primers; 2:16008.
View at Publisher | View at Google Scholar - Skolarikos A, Straub M, Knoll T, Sarica K, Seitz C, Petrik A, Turk C. (2015), Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol; 67(4):750-63.
View at Publisher | View at Google Scholar - Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. (2014), medical management of kidney stones: AUA guideline. J Urol; 192(2):316-24.
View at Publisher | View at Google Scholar - Kravdal G, Helgo D, Moe MK. (2015), Infrared spectroscopy is the gold standard for kidney stone analysis. Tidsskr Nor Laegeforen;135(4):313-4.
View at Publisher | View at Google Scholar - Abe T, Akakura K, Kawaguchi M, Ueda T, Ichikawa T, Ito H, et al. (2005), Outcomes of shockwave lithotripsy for upper urinary-tract stones: A large-scale study at a single institution. J Endourol;19(7):768-73.
View at Publisher | View at Google Scholar - Singh P, Enders FT, Vaughan LE, Bergstralh EJ, Knoedler JJ, Krambeck AE, et al. (2015), Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin Proc; 90(10):1356-65
View at Publisher | View at Google Scholar - Zeng G, Zhao Z, Wu W, Ou L, Liang Y, Yuan J. (2014), Interconversion of stone composition profiles from two recurrent stone episodes in stone formers. Clin Chem Lab Med; 52(7):1019-24.
View at Publisher | View at Google Scholar - M. Daudon, C.A. Bader, P. Jungers, (1993), Urinary calculi: review of classification methods and correlations with etiology, Scanning Microscopy; 7: 1081 - 1106.
View at Publisher | View at Google Scholar - M. Daudon, (1993), Comment analyser un calcul et comment interpréter le résultat, L’Eurobiologiste ; 27 : 35 - 46.
View at Publisher | View at Google Scholar - A. Le Bail, D. Bazin, M. Daudon et al., (2009), Racemic calcium tartrate tetrahydrate [form (II)] in rat urinary stones, Acta Cryst. B. 65: 350 - 354.
View at Publisher | View at Google Scholar - S.R. Khan, (1991), Pathogenesis of oxalate urolithiasis: lessons from experimental studies with Rats, Am. J. Kidney Dis. 17: 398 - 401.
View at Publisher | View at Google Scholar - M. Daudon, D. Bazin, P. Jungers, G. André, A. Cousson, P. Chevallier, E. Véron, G. Matzen, (2009), Opportunities offered by scanning electron microscopy, powder neutron diffraction in the study of whewellite kidney stones, J. App. Cryst. 42: 109 - 115.
View at Publisher | View at Google Scholar - R. Shirley, D.J. Sutor, (1967), Anhydrous uric acid: Nature and occurrence of a new form in urinary calculi, Science; 159: 544 - 550.
View at Publisher | View at Google Scholar - M. Normand, Le traitement médical de la lithiase urique, Progrès en Urologie - FMC, In Press.
View at Publisher | View at Google Scholar - L.W. Klee, C.G. Brito, J.E. Lingeman, (1991), The clinical implications of brushite calculi, J. Urol. 145: 715 - 718.
View at Publisher | View at Google Scholar - J.C. Williams, T. Hameed, M.E. Jackson, S. Aftab, A. Gambaro, Y.A. Pishchalnikov, H.E. Lingeman, J.A. McAteer, (2012), Fragility of Brushite stones in shock wave lithotripsy: Absence of correlation with computerized tomography visible Structure, Journal of Urology; 188: 996 - 1001.
View at Publisher | View at Google Scholar - F. Barbey, D. Joly, P. Rieu et al., (2000), Medical treatment of cystinuria: Critical reappraisal of long-term results, J. Urol. 163: 1419 - 1423.
View at Publisher | View at Google Scholar - R. Ragone, (2000), Medical treatment of cystinuria with vitamin C, Am. J. Kidney Disease; 35: 1020 - 1030.
View at Publisher | View at Google Scholar - E. Letavernier, O. Traxer, J.P. Haymann, D. Bazin, M. Daudon, Cystinurie, (2012), Prog. Urol. – FMC; 22: F119 - F123.
View at Publisher | View at Google Scholar - G. Zanetta, L. Maurice-Estepa, Ch. Mousson, E. Justrabo, M. Daudon, G. Rifle, Y. Tanter, (1999), Foscarnet induced crystalline glomerulonephritis with nephrotic syndrome and acute renal failure after kidney transplantation, Transplantation; 67: 1376 - 1378.
View at Publisher | View at Google Scholar - N.C. Bush, K. Twombley, J. Ahn, C. Oliveira, S. Arnold, N.M. Maalouf, K. Sakhaee, Prevalence and spot urine risk factors for renal stones in children taking topiramate, J. of Pediatric Urology, In Press.
View at Publisher | View at Google Scholar - M.Daudon, M.F. Protat, R.J. Reveillaud (1978), : Analyse des calculs par spectrophotométrie infrarouge : avantages et limites de la méthode. Ann. Biol. Clin., 36: 475-489.
View at Publisher | View at Google Scholar - G. Bollée, C. Dollinger, L. Boutaud, D. Guillemot, A. Bensman, J. Harambat, P. Deteix, M. Daudon, B. Knebelmann, I. (2010), Ceballos-Picot, Phenotype and genotype characterization of adenine phosphoribosyl transferase deficiency, J. Am. Soc. Nephrol. 21 : 679 - 688.
View at Publisher | View at Google Scholar - D.Harrache, Z.Mesri, A.Addou, A.Semmoud, LACOUR B.Lacour, M. Daudon (1997), Analyse des calculs urinaires de l’adulte dans l’ouest algérien par spectroscopie infrarouge à transformée de Fourrier. L’Eurobiologiste; 31 : 69-74.
View at Publisher | View at Google Scholar - D.Harrache, Z.Mesri, A.Addou, A.Semmoud, LACOUR B.Lacour,, M. Daudon (1997), La lithiase urinaire chez l’enfant dans l’ouest algérien. Ann. Urol., 31: 84-88.
View at Publisher | View at Google Scholar - M. Daudon and D. C. Bazin, (2012 ), “Application of Physical Methods to Kidney Stones and Randall’s Plaque Characterization,” in Urolithiasis, J. J. Talati, H.-G. Tiselius, D. M. Albala, and Z. Ye, Eds. London: Springer London, 683–707.
View at Publisher | View at Google Scholar - K. Lonsdale, (1968), “Epitaxy as a Growth Factor in Urinary Calculi and Gallstones,” Nature, 217, 56– 58.
View at Publisher | View at Google Scholar - M Daudon.
View at Publisher | View at Google Scholar - Daudon M, Donsimoni R, et al. (1995), Sex and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol Res; 23: 319-26.
View at Publisher | View at Google Scholar - Y.Berland et al : (1988), in vitro and clinical study of oxalate influence on calcium oxalate crystal formation-J.Crystal Growth. 87.494-506.
View at Publisher | View at Google Scholar - Baggio et al: (1983), Prevalence of hyperoxalurie in idiopathic calcium oxalate kidney stone disease. -Nnephron ,35,11-14.
View at Publisher | View at Google Scholar - R. Boistelle, Y. Berlind. (1992).
View at Publisher | View at Google Scholar