Article In Press : Article / Volume 3, Issue 2
- Review Article | DOI:
- https://doi.org/10.58489/2836-3582/014
Legislative and Regulatory Systems for the Blood Supply. Is there any Progress?
- IQM Consulting, Zuidhorn and University of Groningen, Netherlands.
Cees Th. Smit Sibinga*
Cees Th. Smit Sibinga. (2024). Legislative and Regulatory Systems for the Blood Supply. Is there any Progress? Journal of Hematology and Disorders. 3(2); DOI: 10.58489/2836-3582/014
© 2024 Cees Th. Smit Sibinga, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 07-07-2024
- Accepted Date: 17-07-2024
- Published Date: 18-07-2024
GBT+Blood, Legislative and Regulatory Systems, Essential Medicines, LMIC/L-and M-HDI.
Abstract
In 2021, four years ago, an editorial was published 3 years after the WHO 2019 initiative to include blood, blood components, plasma and plasma derived medicinal products (PDMP) in the WHO Global Benchmarking Tool called GBT+Blood. The burning question raised was: ‘How about the effectiveness’. GBT+Blood was published and distributed some 6 years after the inclusion of labile blood products as special ‘medicines’ in the WHO List of Essential Medicines (2013), accommodated in 2017 by Guidelines on Management of Blood and Blood Components as Essential Medicines, to ease implementation focused on Low- and Middle-Income Countries (World Bank LMICs) or the Low- and Medium- Human Development Index countries (UNDP L- and M-HDI countries).
This article raises the question: ‘Is there any progress?’ and attempts to provide a realistic answer based on available literature.
Introduction
Based on data collected between 2013 and 2018 through the Global Database on Blood Safety (GDBS), the World Health Organization (WHO) published in 2021 the latest Global Status Report on Blood Safety and Availability 2021 [1]. The previous version was published five years earlier in 2016 [2].
This 2021 report presents new information on the processes for establishing governance and an appropriate organization inclusive existing legislative and regulatory systems for national blood supply and transfusion systems. The span of years data were collected includes the initial implementation and management years of the 2013 WHO List of Essential Medicines [3] and the 2017 Guidelines on Management of Blood and Blood Components as Essential Medicines (EM) [4]. It is acknowledged that the source of data shows limitations including varying amounts of missing data and the challenges of data verification. To an extent, these problems can be assessed by longitudinal changes in reports over time. This new report is accompanied by an additional trend analysis for a number of measures. A further gap highlighted by this WHO report is a lack of good descriptive data on contents of the existing legislative and regulatory systems. A simple ‘yes’ to the relevant question is in fact very limited in its interpretation; there is something but is it appropriate and effective?
As before in 2016, the GDBS requests and analyses data from ministries of health of WHO Member States. The terminologies used in the survey questionnaire were given standardized definitions to promote consistent reporting. Where possible, efforts were made to validate the data reported to WHO with WHO regional and country offices. Countries were contacted for clarification or correction when discrepancies or unusual patterns were observed. However, not all the data provided by every country could be systematically verified. In particular, answers to the questions on the existence of policies, legislative frameworks, programs or mechanisms could be affected by individual interpretation of the questions asked given the political sensitivities that exist.
Progress Noticeable
This 2021 Global Status Report is based on data that were reported by 171 of 194 WHO Member States to the WHO central GDBS in Geneva. Data included for analysis were primarily from 2018, as reported by 108 countries. To give a more complete overview of the global situation where 2018 data were not available, data for 2017 from 40 countries, and for 2015 from 23 countries, were used. These 171 countries accounted for a total population of 7.2 billion, representing 98.03% of the global population. The Global Status Report provides a number of detailed annexes. Annex 1 presents a list of the 171 responding countries to GDBS 2018 (Table 1):
Table 1: WHO Regions, number of responses per total countries in the Region
|
A total of 136 countries (80%) did have a ‘unit’ within the ministry of health (or other government department) with responsibility for governing all activities related to supply and transfusion of blood and blood products (Table 2). In addition, as reported 125 countries (73%) had a national blood policy, and 101 countries (59%) had a multiyear national strategic plan for blood safety. In 113 countries (66%), there was specific legislation or other legal instruments covering the safety and quality of blood and blood products for transfusion. However, none discloses the real contents of the reported policies, strategic plans or ‘specific’ legislation or legal instruments which casts a shadow on the transparency, trustworthiness and effectiveness of the information.
In 100 of the reporting countries (58%), the ministry of health was assisted by a national blood committee (NBC) or equivalent in formulating policy and plans, setting standards and advising on (be it not specified) key issues. In 93 countries (54%), an annual report of activities of the national blood program was published largely by advanced countries spread over the WHO Regions with a high number in the Western cluster of the European Region. The overall results as reported are listed in Table 2:
Table 2: Policy and governance 2018
| Provision | Yes | No | No information |
| Responsible blood supply and transfusion Unit within the ministry of health | 136 | 28 | 7 (4%) |
| National Blood Policy | 125 | 39 | 7 (4%) |
| Multiyear strategic plan for blood safety | 101 | 57 | 13 (7.6%) |
| Specific legislation covering safety and quality of blood and blood products | 113 | 45 | 13 (7.6%) |
| National Blood Committee | 100 | 62 | 9 (5.3%) |
| Published Annual Report | 93 | 58 | 20 (11.7%) |
NB: of each of these provisions contextual details and specificities are dominantly missing. No information on updates and changes.
A number of responding countries did not provide answers to these fundamental policy and governance questions.
Countries that provided a ‘No’ answer may be those with technically effective blood transfusion services but with different policy and governance arrangements in place, or those where there was a lack of effective policy and governance, which could have an adverse impact on patient transfusion capacity and safety. It is important to distinguish between these two scenarios.
Follow-up of these responses may be appropriate to determine if questions or definitions should be modified in future questionnaires to ensure that all effective policy and governance models are accommodated and reported accordingly and hopefully more transparent, trustworthy and effective. The lack of specific legislation covering the safety and quality of blood and blood products for transfusion in many (34%) countries is of major concern. Registration, licensing, regulation and inspection or audit of blood services, all of which are essential to ensure safety, quality and availability of blood, require an appropriate legislative framework to operate effectively. Even within countries e.g., Pakistan, there are observable differences per Province and Territory.
In total 113 countries (66%) reported the existence of specific legislation ‘covering’ the safety and quality of blood transfusion, compared with 92 countries (56%) in 2008, an 22.8% improvement.
Across the 6 WHO regions (ranked by percentage), 22 (51%) countries in Africa, 17 (52%) in the Americas, 14 (56%) in the Western Pacific, seven (70%) in South-East Asia, 13 (72%) in the Eastern Mediterranean, and 40 (95%) in Europe reported having such legislation. However, a survey conducted in 2018 and 2019 in the Eastern Mediterranean Region and published in 2020 [5] unveiled a different picture. The response to the survey was only 9/20 countries. These legislations largely provide detailed descriptions of the regulatory authority, and detailed technical and specific requirements for operational establishments. However, none of these legislative documents complies with the WHO recommended format and contents (WHO 2012 Assessment Criteria for National Blood Regulatory Systems) [6] and the recent Review on ‘Existing and recommended legislative framework for a national blood transfusion policy’ [7]) and do not seem to be based on an established National Blood Policy (WHO Aide Mémoire for National Health Policy Makers on Global Policy Process for Blood Safety and Availability) [8].
A legislative framework consists of a Legislation or Law based on the National Blood Policy and anchoring the moral-ethical principles and the framework necessary requirements such as a quality system, standards etc., a regulatory system providing in a flexible way the details and a National Regulatory Authority (NRA) responsible for the oversight, inspections and audits of the blood supply establishments and transfusion Institutes, and licensing (Figure 1).
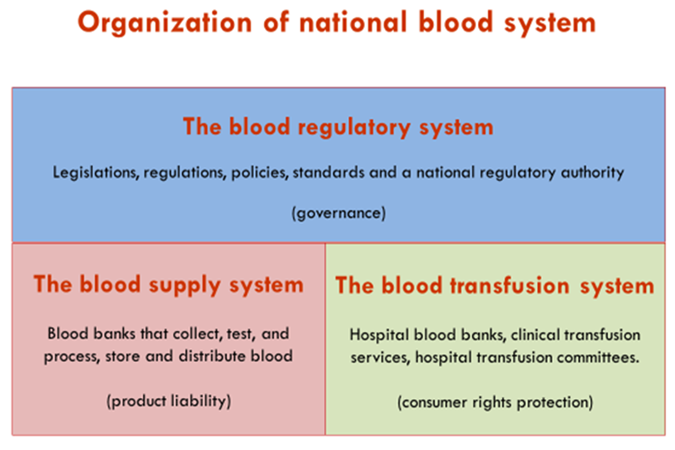
Fig 1: Organizational structure of a national blood system
The Global Status Report 2021 provides a total of 101 countries (59%) that had a system of regular inspection of blood transfusion services by the national regulatory authority or another entity. Similarly, 101 countries (59%) had a system of licensing of the national blood transfusion service (NBTS) or other blood transfusion services/establishments by the NRA or another entity. Fifty-seven countries (33%) had an accreditation system for NBTS or other blood transfusion services/establishments (Table 3).
Table 3: Responding countries: inspection/audit and licensing
| Yes | No | No information | |
| Is there a system of regular inspections of the NBTS/blood transfusion service(s)/ establishments by the National Regulatory Authority or another entity? | 101 | 61 | 9 |
| INs there a system of licensing of the NBTS/ blood transfusion services/establishments by the National Blood Authority or another entity? | 101 | 63 | 7 |
| Are NBTS/blood transfusion service(s)/ establishments accredited? | 57 | 105 | 5 |
NB: there are no details provided of these actions. No periods of validity of the accreditation. No accrediting entity mentioned.
Effective and sustainable governance depends upon mechanisms to identify and control the number of organizations legally permitted to act as blood transfusion services/establishments, and appropriate oversight of these organizations by an independent body reporting to the ministry of health. The WHO Aide-mémoire for Ministries of Health on ‘Developing a National Blood System’ [9] states: ‘Regulatory mechanisms should be established for the control, inspection and licensing of blood transfusion services to enforce blood product standards and monitor product safety’.
The number of countries that lack systems to license and inspect blood transfusion services continues to be a serious concern. However, there is progress. Various LMICs are involved in e.g., the Philippines and India but also the member states of the Africa Society of Blood Transfusion (AfSBT) are involved in or managed to reach the status of independent accreditation of the Association for the Advancement of Blood and Biotherapies (AABB) or ISO 2001, e.g., Mongolia. That means a competent quality system and quality system management as well as a quality culture has been demonstrably developed.
In the 6 WHO regions (ranked by percentage), a system of inspection was reported in 16 (37%) countries in Africa, 12 (48%) in the Western Pacific, 18 (55%) in the Americas, 12 (67%) in the Eastern Mediterranean, seven (70%) in South-East Asia, and 36 (86%) in Europe. A system of licensing was reported in 17 (40%) countries in Africa, four (40%) in South-East Asia, 10 (40%) in the Western Pacific, nine (50%) in the Eastern Mediterranean, 25 (76%) in the Americas, and 36 (86%) in Europe. Also, here we lack the details to justify the effectiveness of these reported systems.
How about 2016?
In the Global Status Report 2016 [2], 122 countries (68% of the total reporting countries) had a national blood policy. Ninety-six countries (53%) had a multiyear national strategic plan for blood safety in 2013 (year of reporting). In 127 countries (71%), a ‘unit’ within the ministry of health (or other government department) had responsibility for governing all activities related to provision and transfusion of blood and blood products.
No more than 105 countries (58%) reported the existence of specific legislation covering the safety and quality of blood transfusion, compared to 92 countries (56%) in 2008. In the WHO regions (ranked by percentage), 19 (41%) countries in Africa, 11 (44%) in the Western Pacific, 17 (49%) in the Americas, 11 (55%) in the Eastern Mediterranean, 7 (64%) in South-East Asia and 40 (93%) in Europe reported having such legislation.
That shows some movement towards effective development but the progress is not substantial. However, every inch counts!
Conclusion
Both Status Reports focus on numerical data largely of the primary operational functions and do not provide any relevant contextual details reflecting an understanding and motivation to develop. The question of how a reported system works or what a legal and regulatory system really contributes to safety and availability and the development of stewardship remains unanswered. Nevertheless, some incidental progress is noticeable.
Acknowledgement
This reflection is based on the data collected by the WHO GDBS and made public in the WHO Global Status Reports on Blood Safety and Availability of 2016 and 2021.
Conflict of Interest
The author declares to have no conflicting interests of any kind.
References
- World Health Organization. (2022). Global status report on blood safety and availability 2021. World Health Organization.
- Global Status Report on Blood Safety and Availability 2016. Geneva. World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO
- WHO Model List of Essential Medicines. 18th list (April 2013). [Available at: hhtp://www.who.int/mdedicines/publicatiions/essentalmedicines/en/index.html]
- WHO Expert Committee on Biological Standardization. Sixty-seventh Report. Geneva. World Health Organization; 2017. (WHO Technical Report Series; no. 1004). Licence: CC BY-NC-SA 3.0 IGO. Annex 3 – Guidelines on management of blood and blood components as essential medicines.
- WHO Global Benchmarking Tool + Blood (GBT + blood) for evaluation of national regulatory systems of blood products including whole blood, blood components and plasma derived products. [Available at: https://www.who.int/medicines/regulation/ benchmarking_tool_plus_blood/en/]
- Smit Sibinga CTh, Abdella YE, Konings FAJ. Legislative Instruments for Regulating Blood Systems in the WHO Eastern Mediterranean Region. AABB Annual Meeting, Baltimore, MD, USA. Transfusion 2020;60(S5):203A-204A.
- World Health Organization. Assessment Criteria for NATIONAL Blood Regulatory Systems. Geneva 2012
- Sibinga, C. T., Abdella, Y. E., & Konings, F. (2019). Review of existing legislative instruments for blood systems of countries in the WHO Eastern Mediterranean Region. Global Journal of Transfusion Medicine, 4(1), 6-15.
- World Health Organization. (2008). Aide-mémoire for national health policy makers: good policy process for blood safety and availability (No. WHO/EHT/08.02). World Health Organization.
- World Health Organization. Aide Mémoire for Ministries of Health, Developing a National Blood System. 2011, Blood Transfusion Safety, Dept. of Essential Health Technologies, Geneva, Switzerland


