Review Article | DOI: https://doi.org/10.58489/2836-2322/002
Models And Pharmacological Approaches to The Study of Endothelioprotective Properties of Drugs Used to Treat Coronavirus Infection Covid-19 - Requirements for The Construction and Application of The Model
*Corresponding Author: Vasil Lyubenov Kanisov
Citation: Kanisov V.L, (2022). Models And Pharmacological Approaches to The Study of Endothelioprotective Properties of Drugs Used to Treat Coronavirus Infection Covid-19 - Requirements for The Construction and Application of The Model. Pharmacy and Drug Development. 1(1). DOI: 10.58489/2836-2322/002
Copyright: © 2022 Vasil Lyubenov Kanisov, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 23 July 2022 | Accepted: 05 August 2022 | Published: 15 August 2022
Keywords: Covid-19, Endothelioprotective, Drug.
Abstract
In addition to the appearance of a functionally multi-connected model of the object under study, we observe another feature: the SARS-CoV-2 virus penetrates into the cell for a short time, after replication, from the infected cell penetrates into a neighboring cell. And the peculiarity lies in the fact that the replication of the virus penetrates into a neighboring cell along the path of direct contact. Observation and detection of the SARS-CoV-2 virus in various environments and tissues of the body indicates that the virus is able to overcome cellular barriers, as mentioned above, without passing through the receptor channels known to us.
Introduction
In addition to the appearance of a functionally multi-connected model of the object under study, we observe another feature: the SARS-CoV-2 virus penetrates into the cell for a short time, after replication, from the infected cell penetrates into a neighboring cell. And the peculiarity lies in the fact that the replication of the virus penetrates into a neighboring cell along the path of direct contact [1]. Observation and detection of the SARS-CoV-2 virus in various environments and tissues of the body indicates that the virus is able to overcome cellular barriers, as mentioned above, without passing through the receptor channels known to us [2].
The usual route sequence of the virus in COVID-19 infection is as follows: 1. Adhesion of the virus on the mucous membranes of the upper respiratory tract; 2. Cells that usually have ACE2 receptors will cause the penetration of the virus; add a feature: 2.1. Penetration of the virus, bypassing cellular receptors 3. Reproduction of the virus in the cell; 4. Dropping and spreading the virus through the cell - or tissues and organs.
Another feature: in my opinion, a modest observation and opinion, the SARS-CoV-2 virus adheres "initially" not only to the cells of the mucous membranes of the upper respiratory tract of the respiratory system, but although unevenly across the cells of all other organs and systems, following the blood flow of the cardiovascular systems (CVS)."
Exhibition
The main damaging mechanism of the virus is the ability to cause inflammation and increase blood clotting. ACE2 expression is observed in the arterial and venous endothelium of the lungs and kidneys. As a result of viral infection, endothelial damage is observed. This damage is accompanied by a local increase in the level of von Willebrand factor, the amount of activation of neutrophils and macrophages, which leads to excessive thrombin production, suppression and fibrinolysis, activation of the attachment cascade and, ultimately, to the appearance of microthrombi and microcirculation disorders.
The simultaneous interaction of platelets with neutrophils and the activation of macrophages in this case contribute to the appearance of various pro-inflammatory conditions, and the release of cytokines. This condition threatens the normal function of all organs and systems and comes to a threat to life. At the same time, we observe the formation of extracellular neutrophil traps and the formation of fibrin, and microthrombi [3, 4].
Before considering the model and pharmacological approaches for studying the endothelioprotective properties of drugs used to treat coronavirus infection COVID-19, we consider:
Model pharmacological approaches and mechanisms of action, drugsin the treatment of COVID-19:
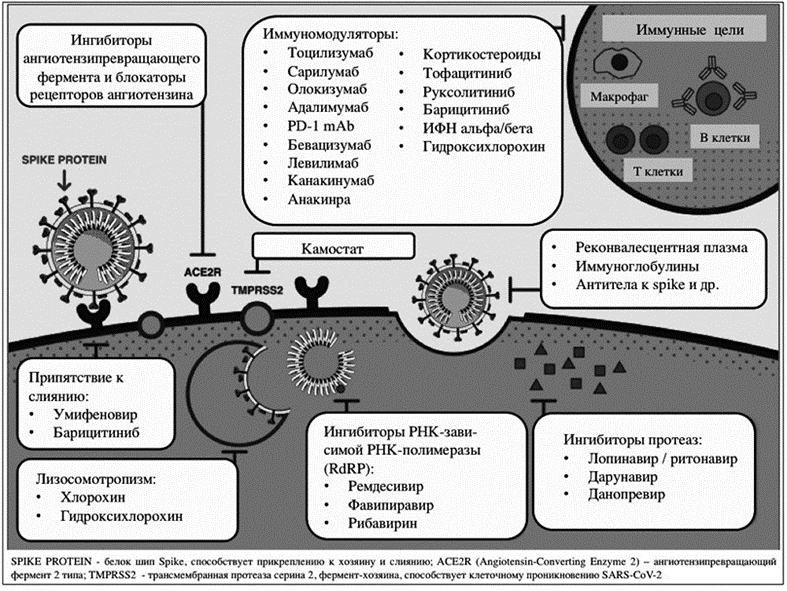
Pharmacological approaches and mechanisms of action of drugs in the treatment of COVID-19 can be divided, depending on the pharmacodynamics of the process, into several categories (Figure 1.1.):
Medicines - Mechanisms of action and pharmacological approaches to the treatment of COVID-19:
1. Lysosomotropism: hydroxychloroquine and chloroquine
The model study of the pharmacological activity of hydroxychloroquine and chloroquine can be attributed to in vivo modeling.
The most studied to date are the antimalarial drugs hydroxychloroquine and chloroquine.
The mechanisms of possible action of the antimalarial drug (Fig. 1) occurs according to the scheme: - interaction, - impaired maturation of lysosomes, - inhibition of glycosylation of cell membranes by glycoproteins and - suppression of the production of tumor necrosis factor alpha [5,6].
Most patients with confirmed COVID-19 disease have been randomized trials (in vivo model) to investigate the efficacy of hydroxychloroquine and chloroquine. To date, randomized controlled trials have been the basis of evidence-based medicine.
The constructed models of the effect of hydroxychloroquine and chloroquine (on the intracellular organ field - lysosomes), and the many facts that indicate that the mechanisms of action of antimalarial agents on the SARS-CoV-2 virus remain unclear [7, p.16] - indicate the imperfections of this type of model.
2. Protease inhibitors: lopinavir / ritonavir, darunavir and danoprevir.
After the antimalarial drugs hydroxychloroquine and chloroquine According to the number of clinical studies conducted, protease inhibitors follow - lopinavir / ritonavir [7].
The model for the study of the pharmacological activity of lopinavir / ritonavir, darunavir and danoprevir can be attributed to in vitro modeling. However, in practice they are carried out mainly on an in vivo model. - And this is understandable, it is impossible to build an adequate biological model of the intracellular process.
"Protease inhibitor: lopinavir/ritonavir is one of the first drugs to be recommended for the treatment of COVID-19" [7, p. 16-17] Protease inhibitors include darunavir, which has at least been studied in only one (2020 data) clinical trial on an in vivo model. [6, 8].
3. Immunomodulators: tocilizumab, sarilumab, olokizumab, olokizumab, adalimumab, PD-1mAb, bevacizumab, levilimab, canakinumab, anakinra, corticosteroids, tofacitinib, baricitinib, IFN alpha / beta, hydroxychlorin (detected as an antimalarial drug), etc.
Note: Tocilizumab is at this point the only drug for the treatment of cytokine release syndrome, which is registered in clinical practice.
The model of the study of the pharmacological activity of immunomodulators can be attributed to in vitro modeling, but they are also carried out mainly on the in vivo model.
This group of drugs includes:
1. Immunomodulators with anti-inflammatory action,
2. Glucocorticosteroids (Immunoregulatory effect, anti-inflammatory activity, etc.),
3. "Monoclonal antibodies - block the action of interleukin IL-6 (tocilizumab, sarilumab, olokizumab) and IL-1 (anakinra, canakinumab) and
4. Janus kinase inhibitors (baricitinib, tofacitinib, ruxolitinib) » [7, p. 17-28].
The main purpose of the use of these groups of drugs is to suppress the syndrome of cytokine release.
It is known that the leading role in the pathogenesis of the cytokine release syndrome is played by IL-6. [9]
Clinical trials (by June 2020) of tocilizumab have been limited. Glucocorticoids are also being studied relatively limitedly, mainly as inhibitors of interleukin (IL) production. Of the numerous clinical studies on the effects of glucocorticoids, only 5 studies (relevant 2020) have published results published in scientific journals [1 0.11].
The most actively studied medicinal substances in the fourth group are:
4. "Inhibitors of RNA-dependent RNA polymerase: favipiravir and remdesivir" [7].
The model for the study of pharmacological activity can be attributed with confidence to in vitro modeling.
The mechanism of action of favipiravir and remdesivir is theoretically accepted as widely spectral. It is also accepted that these drugs have a high degree of activity to a wide range of RNA viruses [12].
Representatives of other groups of drugs have been identified as inhibitors of RNA-dependent RNA polymerase are studied in single studies. The results from these studies at this point (2021) are not published.
In the treatment of COVID-19, there is a high interest in several groups of medicinal substances, such as:
5. Angiotensive enzyme ACE2 (ACE2) inhibitors and Angiotensin II receptor blockers [13]:
Mention should be made here of the bait protein (recombinant angiotensin-converting human enzyme APN01), the study of which began after the outbreak of the SARS-CoV virus in 2003 [14].
APN01 mimics the human enzyme ACE2, which is used by the SARS-CoV virus to enter cells. Thus, SARS-CoV binds to soluble ACE2/APN01 instead of ACE2 on the cell surface.
A model for the study of pharmacological activity of angiotensive enzyme inhibitors and angiotensin II receptor blockers can be attributed to in vitro modeling.
5.1. Chlorprothixen is a neuroleptic, used in the treatment of the mentally ill: highly active, but relatively mild.
It is proved that the SARS-CoV-2 virus penetrates into the cell through the mediation of the ACE2 receptor and the participation of serine protease TMPRSS2 located on the cell membrane. It is believed that by drug blockade of the connection of the ACE2 receptor and the "spike"-protein of the SARS-CoV-2 virus, we can re-establish the development of COVID-19 infection.
Today, the isolation of about 10 drugs is low molecular weight ligands that cause conformational changes in ACE2 - which can and do activate the blockade of the interaction between ACE2 and the spike-protein of the virus [159]. Research in this direction continues.
5.2. camostat and naphamostat - have properties on potent inhibitors of TMPRSS2 [16].
The SARS-CoV-2 virus is known to "... with the participation of the serine protease, TMPRSS2 uses the human ACE2 receptor to access human cells. Thus, blocking the interaction between ACE2 and the spike protein of the virus may prevent the development of viral infection." [7, p. 14-24]
5.3. meplasumab - Monoclonal antibodies (к Spike Protein)
To effectively control the SARS-CoV-2 virus for COVID-19 - Monoclonal antibodies to Spike Protein are prepared from the blood of patients who have had an infectious disease
Monoclonal antibodies (to Spike Protein) have the potential for therapeutic, prophylactic use in the fight against the virus. Monoclonal antibodies are also involved in the development of vaccines against the SARS-CoV-2 virus. [17,18].
Requirements for the construction and application of a model for studying the endothelioprotective properties of drugs used to treat coronavirus infection COVID-19
Potential drugs for the treatment of coronavirus infection COVID-19 should have endothelial protective properties, they should manifest as:
1. Dilators - causing the expansion and relaxation of the muscular layer of the vascular wall (Factors synthesized in the endothelium and regulating its function):
1.1. Оксид азота (NO) (I - очередь)
2.1. Large endothelin (BET)
3.1. Простациклин (PGI2) (II- очередь)
4.1. Endothelin depolarization factor (EDHF) (stage III)
5.1. Ангиотензин I (АТ I)
6.1. Adrenomedulin
Note: Endothelial cells synthesize and release vasoactive mediators in response to various neurohumoral substances (e.g., bradykinin or acetylcholine) and physical stimuli (e.g., cyclic stretching or fluid shift stress). The most well-characterized endothelial-derived relaxing factors are nitric oxide and prostacyclin. The most well-characterized relaxing factors derived from the endothelium are nitric oxide and prostacyclin. However, there is also an additional pathway of relaxation associated with hyperpolarization of smooth muscles. This hyperpolarization was initially associated with the release of endothelial derivative hyperpolarizing factor (EDHF), which diffuses into and activates smooth muscle K (+) channels. More recent evidence suggests that activation of endothelial cell receptors by these neurohumoral substances opens K (+) channels of endothelial cells.
2. Antithrombogenic- anticoagulation (Factors synthesized in the endothelium and regulating its function):
1.2. Nitric oxide (NO)
2.2. Tissue plasminogen activator (TAP)
3.2. Prostacyclin (PGI2)
4.2. Thrombomodulin
3. Inhibitors - affecting the growth of blood vessels and smooth muscle cells (Factors synthesized in the endothelium and regulating its function):
1.3. Nitric oxide (NO)
2.3. Prostacyclin (PGI2)
3.3. Natriuretic peptide C
4.3. Heparin-like growth inhibitors
4. Anti-inflammatory. - anti-inflammatory (Factors synthesized in the endothelium and regulating its function):
1.4. Nitric oxide (NO)
In addition, drugs (potential drugs) for the treatment of coronavirus infection COVID-19 should have endothelialprotective properties, not only in the direction of improving endothelial function, but also in the following areas of action (Table 1.1.):
Table 1.1. - Actions and directions of manifestation of endothelioprotective properties potential drugs for the treatment of coronavirus infection COVID-19.
Potential Medicines to Treat COVID-19 | Main properties | Directions of endothelioprotective actions |
|
| 1. Improved endothelial function - vasodilating - Antithrombotic - proliferative - anti-inflammatory |
Unknown | Endothelioprotector | 2. Inhibition of apoptosis endotheliocytes: - Reduction of initiator and effector caspase (Effector (−3, −6, −7), initiator (−2, −8, −9, −10, −12)) - Increased apoptosis regulator Bcl-2 (mitochondrial pathway regulators of apoptosis) |
|
| 3. Majesty e-NOS (Endothelial Nitric Oxide Synthase) and increase in reduced thiols (mercaptans, compounds with sh sulfhydryl groups) |
|
| 4. Decreased expression Endothelin 1 (ET I, EDN1), etc. |
Conclusion
It is becoming clear that the requirements for the creation of models for the study of the endothelioprotective properties of drugs used to treat coronavirus infection COVID-19 are, a difficult task at many levels.
It is clear that existing and still practical models in vitro, ex vivo and in vivo cannot reflect the full range of functions of the human endothelial system.
The targeted development of the principle of new models for the study of the endothelioprotective properties of drugs is becoming an urgent task of modern experimental pharmacology, since at present the functional state of the vascular endothelium plays a leading role in the development of cardiovascular, viral and neurological diseases.
Therefore, to study the effectiveness of drugs a that have endothelioprotective properties used to treat coronavirus infection COVID-19, it is necessary to use, not only methodological approaches, methods and models approved in pharmacological practice, but also weight apparatus interdisciplinary techniques and research models for complete descriptions of e of all processes observed in the clinic.
References
- Bagnenko S.F., Belyakov N.A., Rassokhin V.V., Trofimova T.N. (2020), et al. Beginning of the COVID-19 epidemic. [Text]/S.F. Bagnenko, N.A. Belyakov, V.V. Rassokhin, T.N. Trofimova et al.// SPb.: Baltic Medical Educational Center, 360 s.
View at Publisher | View at Google Scholar - Puelles V.G. (2020), et al. Multiorgan and renal tropism of SARS-CoV-2 [Text]/V.G. Puelles et al.//N. Engl. J. Med. doi: 10.1056/NEJMc2011400.
View at Publisher | View at Google Scholar - Varga A. (2020), et al. Endothelial cell infection and endothelilitis in COVID-19 [Text]/A. Varga et al. // Lancet. Vol. 395. Р. 1417–1418.
View at Publisher | View at Google Scholar - Hamming I. (2004), et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis [Text]/I. Hamming et al.//J. Pathol. Vol. 203. Р. 631–637.
View at Publisher | View at Google Scholar - Kolbin A.S. (2020), Treatment of COVID-19 with antimalarial agents from a clinical and pharmacological standpoint. [Text]/A.S. Kolbin//Wedge microbiol antimicrobe chemyoter;3 (in print).
View at Publisher | View at Google Scholar - Savarino A., Boelaert J., Cassone A., (2003), et al. Effects of chloroquine on viral infections: an old drug against today's diseases. [Text]/A. Savarino, J. Boelaert, A.Cassone, et al.//Lancet Infect Dis;3(11):722–7
View at Publisher | View at Google Scholar - Kolbin A.S. (2020), COVID-19 and Clinical Pharmacology [Text]/A.S. KolbinClinical Pharmacology and Therapy - Year:-Volume: 29 - No: 3 pp. 14-24
View at Publisher | View at Google Scholar - Kolbin A.S. (2020), Early evaluation of the effectiveness of drugs in the treatment of patients with COVID-19. [Text]/ A.S. Kolbin//Infection and Immunity;10(2):277–86.
View at Publisher | View at Google Scholar - Borrega J., Gödel P., Rüger M., (2019), et al. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. [Text]/J. Borrega, P. Gödel, M. Rüger, et al. //Hemasphere;3(2): e191.
View at Publisher | View at Google Scholar - Mahase E. Covid-19: Coronavirus was first described in The BMJ in 1965. [Text]/ E. Mahase//BMJ 2020;369:m1547.
View at Publisher | View at Google Scholar - Almeida J.D., Berry D., Cunningham C., еt al. (1968), Coronaviruses. [Text]/J.D. Almeida, D. Berry, C. Cunningham, еt al.//Nature ;220:16
View at Publisher | View at Google Scholar - Qingxian Cai, Minghui Yang, Dongjing Liu, et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, (2020).
View at Publisher | View at Google Scholar - Patel A., Verma А. (2020), COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence?
View at Publisher | View at Google Scholar - Safety and Tolerability Study of APN01 (Recombinant Human Angiotensin Converting Enzyme.
View at Publisher | View at Google Scholar - Brogi S., Calderone V. (2020), Off-target ACE2 ligands: possible therapeutic option for COVID-19?
View at Publisher | View at Google Scholar - Hoffmann M., Kleine-Weber H., Schroeder S., et al. (202), SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. [electronic resource].
View at Publisher | View at Google Scholar - Marovich M., Mascola J., Cohen M. (2020), Monoclonal antibodies for prevention and treatment of COVID-19.
View at Publisher | View at Google Scholar - Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. [electronic resource].
View at Publisher | View at Google Scholar