Archive : Article / Volume 2, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-2322/014
Nephroprotective Effect of Ethanolic Leaf Extract of Clausena anisata Against Chlorpromazine-Induced Nephrotoxicity in Wistar Rats
1Department of Biochemistry and Molecular Biology,Faculty of Science,Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
2Biology Unit, Facultyof Science, Air Force Instituteof Technology, P.M.B.2104, Kaduna, Nigeria
3Departmentof Biochemistry, Facultyof Life Sciences, University of Benin, Benin-City, Nigeria
4Department of Biochemistry, Faculty of Life Science, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria
O. Oluwagbenga J
Apata, Joseph T., Ogunbiyi, Oluwagbenga J., Animashaun, Fatimoh A., Babalola, Olusegun O., Igharo Eghosa, Jegede, Rotimi J. & Akinola, Funke T., (2023). Nephroprotective Effect of Ethanolic Leaf Extract of Clausena anisata Against Chlorpromazine-Induced Nephrotoxicity in Wistar Rats. Journal of Pharmacy and Drug Development. 2(1). DOI: 10.58489/2836-2322/014.
© 2023 O. Oluwagbenga J, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 26-12-2022
- Accepted Date: 16-01-2023
- Published Date: 02-02-2023
Clausena anisata; chlorpromazine; nephroprotective; kidney; leaf extract
Abstract
Nephroprotective drugs used in the management and treatment of kidney ailments are most times associated with numerous side effects. The desire to search for alternative safer drugs become imperative, hence, the need to explore nephroprotective drugs from the leaf of indigenous plants. This study evaluates the ameliorative effects of ethanolic leaf extract of Clausena anisata on chlorpromazine-induced renal toxicity in Wistar rats. Twenty-five male Wistar rats were grouped into five groups of five rats each. Group 1 rats served as control and received distilled water; Group 2 rats were induced with Chlorpromazine only every other day for 30 days; Group 3, 4 and 5 rats were intraperitoneally administered with Chlorpromazine for 1h and subsequently followed with 50mg/kg bwt, 100mg/kg body weight and 150mg/kg body weight ethanol extract orally every other day for 30 days respectively. The food was withdrawn from the rats and the rats were fasted overnight. The rats were sacrificed, dissected and blood samples were collected through ocular puncture. Nephroprotective effects of the extracts were evaluated by estimating the concentration levels of renal biomarkers such as albumin, creatinine and total protein concentration. Renal histopathological study of the rats was also investigated to validate the findings obtained from the in vivo study. The results concluded that Clausena anisata ethanol leave extract is safe at a dose of 50mg/kg body weight and 100mg/kg body weight; hence, it could be employed at appropriate concentration as an alternative synthetic pharmaceutical with promising effect in the management and treatment of kidney damage.
Introduction
Chlorpromazine is an organochlorine compound regarded as a substituted phenothiazine because the ring nitrogen at position 10 is connected to C-3 of an N, N-dimethylpropanamine moiety (Figure 1). It works as a phenothiazine antipsychotic, an antiemetic, a dopaminergic antagonist, a prolyl oligopeptidase inhibitor, and an anticoronaviral agent (Pubchem NIH, National Library of Medicine, National Center for Biotechnology Information).
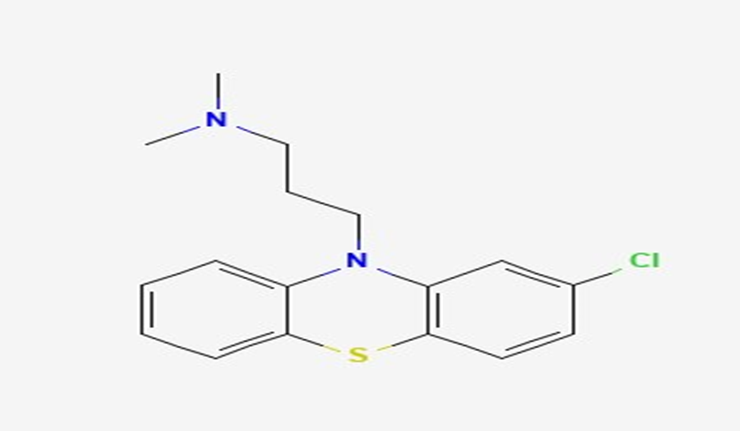
Chlorpromazine (CPZ) has long been used as an in vitro model of intrahepatic cholestasis (IHC), as there is evidence that it causes impaired biliary function, though the mechanisms behind this are not fully understood and side effects are usually idiosyncratic (Anthérieu et al., 2013). Cholestasis is a condition characterized by impaired ability of the bile ducts to secrete bile acids, bilirubin and cholesterol (Anthérieu et al., 2012).
Clausena anisata (Willd.) Hook.f. ex Benth. is a deciduous shrub which is of 20ft. high. It has odorous leaves (Figure 2), cream-white flowers and shining black drupes. It belongs to the Rutaceae or Citrus family (Suksaeree et al., 2021), and widely spread within the Afrotropical realm or Sub-Saharan Africa, but absent from the drier regions (Tropical Plants Database, 2022). It is also found in tropical and South-East Asia, growing in India and Sri Lanka (Omara et al., 2022). The leaves, which are foetid when bruised, produce the common name 'Horsewood' or the more descriptive Afrikaans common name 'Perdepis', meaning 'horse urine' (Agyepong et al., 2014; Lunyera et al., 2016; Omara et al., 2022). It is commonly called Iperepesi in Xhosa language. In Nigeria it is called Mbietekpene among the Ibibios and it is also known as Atabari Obuko, Agbasa among the Yorubas (Omara et al., 2022; Kadiri et al., 2015).
Different chemical compounds are isolated from the varied morphological parts of the Clausena anisata plant, including many terpenoid hydrocarbons, sesquiterpenoids and fatty acids; and methychavicol, myrcene, umbelliferone, scopoletin, xanthotoxin, pimpenellin, xanthotoxol, bergapten; the alkaloids clausanitine and mupamine (Hutchings et al., 1996).

Previously, Clausena anisata (Willd) hook methanolic root extract has been shown to possess hypoglycemic effect in normal (normoglycaemic) and in streptozotocin-treated diabetic rats (Ojewole, 2002). Preliminary screening of ethanolic extracts of Clausena anisata for anticonvulsant activity revealed the root bark to exhibit partial protection at a dose level of 800 mg/kg in convulsed mice induced with Pentylenetetrazole (Mbah and Kenechukwu, 2009). Recent investigation on the antihypertensive effects of an aqueous leaf extract of Clausena anisata in a spontaneously hypertensive rat model to determine whether blood pressure lowering effects could be attributed to diuresis, linked to the inhibition of the renin-angiotensin-aldosterone blood pressure control system and possible negative inotropic or chronotropic cardiac effects has revealed that Clausena anisata was effective in reducing aortic blood pressure at 400 mg/kg body weight. In addition, Clausena anisata was found to significantly reduce the blood pressure over 40 days. This observed activity was linked to mechanism that causes a reduction in plasma angiotensin II levels but not via diuresis or negative chronotropic effects (Lechaba et al., 2016).
The hydroethanolic leaf extract of Clausena anisata (Willd.) Hook.f. ex Benth. has been shown to possess antinociceptive activity and anti-arthritic properties.The mechanism of antinociceptive activity has been linked to occur through possible interaction with opioidergic, noradrenergic, L-arginine-nitric oxide and serotonergic pathways while anti-arthritic property has been linked to its ability to prevent the release of inflammatory mediators and oxidative stress (Ishola et al., 2015). Antiplasmodial and analgesic activities of the crude leaf extract and fractions of Clausena anisata have been evaluated for antimalarial and analgesic activities against chloroquine-sensitive Plasmodium berghei infections in mice using suppressive, prophylactic and curative models and analgesic activity against acetic acid, formalin and heat-induced pains (Okokon et al., 2012; Omosa et al., 2019).The activity of the extract and its fractions were reported to occur via dose-dependently reduced parasitaemia induced by chloroquine-sensitive P. berghei in prophylactic, suppressive and curative models in mice (Okokon et al., 2012).
Available reports show that nephroprotective drugs used in the management and treatment of kidney ailments are associated with numerous side effects which can result to kidney failure, internal injury, organ malfunctioning and mortality (Pazhayattil and Shirali, 2014). Hence, it is desirable to search for alternatives medicine with little or no side effect. There is therefore the need to explore nephroprotective pharmaceutics from the leaf of indigenous plants. However, the aim of this present study is to evaluate the ameliorative effects of ethanol leaf extract of Clausena anisata against chlorpromazine-induced renal toxicity in Wistar rats.
Materials And Methods
Reagents and Chemicals
All reagents were procured from reputable sources and of analytical grade. These reagents include n-Hexane, ethanol, dichloromethane (DCM), sodium hydroxide, monopotassium dihydrogen phosphate, and dipotassium mono-hydrogen phosphate. Diagnostic kits of total protein, albumin, bilirubin, urea and creatinine were product of Randox Laboratories Limited, United Kingdom. All solutions, buffers and reagents were prepared with distilled water and store in the refrigerator at -4°C until required.
Experimental Animals
Twenty-five male Wistar rats weighing between 100 – 150 g used in this study were obtained from the Animal House in College of Health Sciences, Ladoke Akintola University of Technology (LAUTECH), Osogbo, Osun State, Nigeria. The animals were housed in a clean and well-ventilated cage at the Animal House Department of Biochemistry and Molecular Biology, Obafemi Awolowo University, Ile-Ife, Nigeria and maintained under ambient conditions. The animals were fed with standard rat chow and drinking water ad libitum. The animals were acclimatized to laboratory conditions for two weeks prior to experimentation. United States National Institute of Health (NIH, 1985) guidelines for care and use of animals for experimental studies were strictly adhered.
Plant Collection and Identification
Fresh Clausena anisata leaves were collected from swampy area in Oyo town, Modakeke, Osun
State, Nigeria. The study plant was identified and authenticated at Ife Herbarium Department of Botany, Obafemi Awolowo University, lle-Ife, Nigeria.
Preparation of Ethanol extract of Clausena anisata Leaves
The leaves of Clausena anisata were chopped into smaller pieces, thoroughly washed with clean distilled water and air-dried for a period of four weeks. The dried leaves were crushed into fine powder with a mechanical grinding machine. The powdered plant material (357 g) was soaked in ethanol for 72 h at room temperature. The ethanol extract was concentrated using rotary evaporator at a 35℃. The crude ethanol extract was stored in the desiccator until required for further processing.
Determination of Median Lethal Dose (LD50) of Acute Toxicity Study
Acute toxicity study was carried out according to the method of Lorke (1983) using eighteen mice weighing between 18 – 25 g. In the first phase, nine mice were separated into three groups of three mice each and given orally 10, 100 and 1000 mg/kg body weight of the ethanol leaf extract of Clausena anisata. They were monitored for indicators of toxicity for 24 h. In the second phase, another set of nine mice were separated into three groups of three mice each and given orally 1600, 2900 and 5000 mg/kg body weight of ethanol leaf extract of Clausena anisata. The LD50 was estimatedby using the formula: LD50 = √D0 X D100 = √1600 X 1000 = 1264.91mg/kg body weight (Karber, 1931).
Grouping and Treatment of Experimental Animals
Twenty-five male Wistar rats were divided into five groups of five animals per group. The experimental groups were designated as follows: Group 1 rats served as control and received distilled water. Group 2 rats were treated with Chlorpromazine every other day for 30 days; Group 3 rats were given Chlorpromazine and after 1 h, 50 mg/kg body weight of Clausena anisata extract was administered; Group 4 rats were given Chlorpromazine and after 1 h, 100 mg/kg body weight of Clausena anisata extract was administered and Group 5 rats were given Chlorpromazine and after 1 h, 150 mg/kg body weight of Clausena anisata extract was administered. The administration lasted for 30 days. After 30 days of administration, the animals were then allowed to fast overnight on the last day and sacrificed the next day by cervical dislocation. The kidney was removed after blood collection, washed in physiological saline, subsequently weighed and kept in phosphate buffer until required for analysis.
Collection and Preparation of Blood Plasma
The blood sample was collected by ocular puncture into heparinized bottles and centrifuged at 4000 rpm for 10 minutes. The supernatants obtained was collected, stored in sterile vials and kept in the freezer at -8℃ until required for biochemical assays.
Preparation of Kidney Homogenate
The kidney homogenates were prepared using one-gram (1 g) of the tissue which was homogenized with 100 mM Phosphate buffer, pH 7.4, to produce 10 % (W/V) homogenates using pestle and mortar. The homogenates were carefully transferred into centrifuge tubes and volumes adjusted to 10 ml. This was centrifuged at 4000 rpm for 30 minutes. The supernatants were collected into clean bottles, labelled and kept in freezer at -8℃until required for biochemical assays.
Evaluation Of Biochemical Parameters
Estimation of Plasma and Kidney Homogenate Albumin Concentration
The activity of Albumin was estimated using colorimetric method of Reitman and Frankel (1957) as described in a commercially available Randox kit (UK).
Estimation of Plasma Total Protein Concentration
The total protein concentration of plasma was assayed using Biuret method as described by Henry et al. (1974).
Estimation of Bilirubin Concentration
Bilirubin concentration in the plasma was determined according to the method of Jendrassik and Grof (1938). Total bilirubin was determined in the presence of caffeine, which releases albumin bound bilirubin, by the reaction with diazotized sulphanilic acid.
Estimation of Creatinine Concentration
The concentration of creatinine was determined according to the method described by Bones et al. (1945) and Toro et al. (1975). Creatinine in alkaline solution reacts with picric acid to form an orange coloured complex with the alkaline picrate. The amount of the complex formed is directly proportional to the creatinine concentration in the sample.
Hematological Determination
Blood samples were collected by ocular puncture into ethylenediamine tetraacetic acid (EDTA) tubes. The haematological parameters of Red Blood Cell (RBC) count, White Blood Cell (WBC) count, Haemoglobin Concentration (HGB), Mean Corpuscular Haemoglobin Concentration (MCHC), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Platelet (PLT) count, Percentage Lymphocyte (% LYM), Percentage Monocyte (% MON) were analyzed using standard techniques reported by Cheesbrough (2000).
Histological Examination of Kidney
Histological examinations of the kidney obtained from the representative animals from each group were carried out in line with standard procedures involving Hematoxylin and Eosin staining techniques as described by Feldman and Wolfe (2014).
Statistical Analysis
The results were expressed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) was used for data collected, followed by a Tukey multiple range comparisons test using the software GraphPad Prism 6.0. Statistical significance was set at p<0>
Results Discussion
The yield of Crude Ethanol Leaf Extract of Clausena anisata
The extract yielded 37.674gwhich represents 10.54% of the starting material.
Effect of Clausena anisata Ethanol Leaf Extract on the Weight of Rats
Table 1 shows the summary of the effect of the leaf of Clausena anisata on the weight of Wistar rats. The results of the study showed a significant percentage decrease in the body weight of the rats induced with Chlorpromazine only compared with the control (group 1). Similarly, rats repeatedly treated with 50, 10 and 50 mg/kg body weight (bwt) for 30 days demonstrated a significant percentage decrease in body weight in a dose-dependent manner compared to the control. Additionally, the rats repeatedly administered with 50, 100, and 150 mg/kg bwt of the extract exhibited a significant dose-dependent ameliorative effect compared with the group induced with the toxicant (group 2) (Table 1).
Table 1: Effect of C. anisata Ethanol Leaf Extract on Body Weight of Chlorpromazine-Induced Rats.
| Treatment Group | Day 1 Body Weight (g)
| Day 30 Body Weight (g) | Percentage Changes |
| Group 1 (Control) | 95.5 ± 1.5 | 131.78± 9.45 | 27.07 ± 6.37 |
| Group 2 (CPZ only) | 118.5± 6.5 | 138.53 ± 8.73 | 14.41 ± 0.70a |
| Group 3 (CPZ+50mg/kg bwt) | 124.5± 1.5 | 139.62± 5.25 | 10.75± 2.29a |
| Group 4 (CPZ+100mg/kg bwt) | 137.5 ± 2.5 | 159.92± 7.28 | 13.77 ± 5.49a |
| Group 5 (CPZ+150mg/kg bwt) | 143.5 ± 3.5 | 166.7± 1.23 | 13.91 ± 2.74a |
CPZ: Chlorpromazine (Toxicant)
Effect of Clausena anisata Ethanol Leaf Extract on the Relative Kidney Weight of Rats
Table 2 shows the summary of the effect of the leaf of Clausena anisata on the relative kidney weight of rats induced with Chlorpromazine to cause kidney damage. The findings of the study showed a significant reduction in the relative kidney weight of rats compared with the control. This indicated that Chlorpromazine can cause severe damage to the kidney after 30 days of administration. Rats administered with 50mg/kg bwt from day 1 to day 30 demonstrated a significant marginal increase (p < 0> 0.05) difference in the relative kidney weight of the rats. This suggests that the leaf of C. anisata could possibly restore the kidney damage at 50mg/kg bwt dose (Table 2).
Table 2: Effect of C. anisata Ethanol Leaf Extract on Relative Kidney Weight of Chlorpromazine- Induced Rats
| Group 1 (Control) | 0.740 ± 0.19 |
| Group 2 (CPZ only) | 0.665 ± 0.015a |
| Group 3 (CPZ+50mg/kg bwt) | 0.705 ± 0.065b |
| Group 4 (CPZ+100mg/kg bwt) | 0.610 ± 0.08 |
| Group 5 (CPZ+150mg/kg bwt) | 0.655 ± 0.025 |
CPZ: Chlorpromazine
Acute Toxicity of Oral Administration of Ethanol Leaf Extract of C. anisata
Table 3 shows the summary of the acute toxicity effect of ethanol leaf extract of C. anisata. There was no record of mortality at 10 and 100mg/kg bwt of the tract while at high concentration (1000 to 5000mg/kg bwt), there was record of mortality. The mean median lethal dose (LD50) was estimated to be less than 5000mg/kg bwt. This suggests that the ethanol leaf extract of C. anisata was toxic at high concentrations (Table 3).
Table 3: Acute Toxicological Effect of Ethanol Leaf Extract of C. anisata
Dose (mg/kg) Mortality Survival Observation after 72 h
|
First Phase 10 0/3 3 No mortality 100 0/3 3 No mortality 1000 1/3 2 Mortality |
Second Phase 1600 2/3 1 Mortality 2900 3/3 0 Mortality 5000 3/3 0 Mortality |
Effect of C. anisata Ethanol Leaf Extract on Plasma Creatinine, Urea, Albumin and Total Protein in Chlorpromazine Induced Wistar Rats
Table 4 shows the summary of the effect of ethanol leaf extract of C. anisata on plasma creatinine, urea, albumin and total protein concentration in Chlorpromazine-induced wistar rats. It was found that rats induced with only Chlorpromazine (group 2) have a higher creatine concentration level than the control rats (group 1). Rats administered 50, 100 and 150 mg/kg bwt of the ethanol leaf extract of C. anisata demonstrated a significant (p < 0>C. anisata after 30 days at a dose of 50, 100 and 150 mg/kg bwt, there was significant decrease in the plasma urea and albumin concentration when compared with the normal rats. The results of the study as well revealed that rats induced chlorpromazine toxicity exhibited a significant reduction in the plasma total protein concentration compared with the normal rats. While animals treated with 50, 100 and 150 mg/kg bwt of C. anisata ethanol leaf extract demonstrated a significant increase in the plasma total protein concentration compared with the normal rats. Rats administered with 150 mg/kg bwt after 30 days showed excellent performance as it was able to restore the depleted plasma total protein concentration (Table 4).
Table 4: Effect of Clausena anisata Extract on Plasma Creatinine, Urea, Albumin and Total Protein Concentration
| Treatment | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | ||
| Creatinine | 11.18±0.0075 | 39.88±0.0075a | 18.36±0.080b | 19.055±0.044b | 13.664±0.226b | ||
| Urea | 6.695±0.0150 | 10.262±0.0470a | 8.43±0.006b | 6.7195±0.00849b | 9.224±0.0234b | ||
| Albumin | 5.42 ± 0.0045 | 8.33 ± 0.004 a | 5.46 ±0.0045b | 5.479 ± 0.004 b | 5.37 ± 0.0045b | ||
| Total Protein | 27.32±0.0350 | 8.51 ± 0.035a | 9.54 ± 0.04b | 8.82 ± 0.029b | 26.28±0.0650ab | ||
Values without any superscripts along the same roll are not significantly different from each other; values carrying superscript ‘a’ along the same roll are significantly different from group 1 while values carrying superscript ‘b’ along the same roll are significantly different from group 2. (n=5). Group 1(control), Group 2 (Chlorpromazine), Group 3 (50mg/kg bwt), Group 4 (100mg/kg bwt) and Group 5 (150mg/kg bwt)
There was significant increase (p<0>anisata. Contrastingly, a decrease in total protein concentration was observed in plasma of Chlorpromazine treated group when compared to control. This observed decrease was ameliorated by the extract at 150mg/kg bwt but did not show any effect at lower dosages at which the extract was administered.
Effect of C. anisata Ethanol Leaf Extract on Kidney Homogenate in Chlorpromazine-Induced Wistar Rats
The effect of C. anisata ethanol leaf extract on kidney homogenate in Chlorpromazine-induced rats’ toxicity was presented in Table 5. The results of the study showed that rats administered with chlorpromazine (group 2) only for a period of 30 days have significantly higher kidney creatine, urea, albumin, and bilirubin concentrations compared with the normal rats. On the other hand, rats treated with 50, 100 and 150 mg/kg bwt showed a significant decrease in the kidney creatine, urea, albumin and bilirubin concentration compared to the normal rats except rats treated with 100 mg/kg wt of the extract. There was no any significant alteration in the kidney albumin concentration when the toxicant was administered (Table 5).
Table 5: Effect of Clausena anisata ethanol leaf extract on Kidney Albumin, Urea, Bilirubin, and Creatinine Concentration
| Treatment | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| Creatinine | 4.775 ± 0.025 | 6.08 ± 0.0099a | 3.21 ± 0.029b | 2.59 ± 0.040b | 1.535 ± 0.055 b |
| Urea | 2.8295±0.0055 | 7.4605±0.0014a | 2.735±0.0034 b | 3.684±0.0044b | 0.607±0.0080b |
| Albumin | 26.09 ± 0.330 | 29.585±0.495a | 28.2995±0.210b | 29.876±0.003a | 27.073±0.006b |
| Bilirubin | 7.141 ± 0.0069 | 23.611±0.0049a | 9.596 ± 0.0025b | 6.662±0.0199b | 11.803±0.0044b |
Values without any superscripts along the same roll are not significantly different from each other; values carrying superscript ‘a’ along the same roll are significantly different from group 1 while values carrying superscript ‘b’ along the same roll are significantly different from group 2. (n=5). Group 1(control), Group 2 (Chlorpromazine), Group 3 (50mg/kg bwt), Group 4 (100mg/kg bwt) and Group 5 (150mg/kg bwt)
There was significant increase (p<0>p<0>Clausena anisata extract in the kidney bilirubin was more pronounced in rats treated with 100 mg/kg body weight Clausena anisata extract and to a certain extent at 50 mg/kg body weight.
Effect of Ethanolic Extract of C. anisata on the Haematological Parameters of Chlorpromazine-Induced Toxicity in Rats
Table 6 summarizes the effect of C. anisata ethanol leaf extract on haematological parameters in rats with chlorpromazine toxicity. The study's findings revealed a significant (p < 0 xss=removed>The MCV concentration in rats treated with 100 and 150 mg/kg bwt C. anisata extract decreased significantly. Furthermore, animals exposed to the toxicant (group 2) showed a substantial drop in mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) as compared to control rats (group 1). When compared to chlorpromazine-induced toxicity in rats, rats treated with 50, 100, and 150 mg/kg bwt showed no significant difference (p > 0.05) in MCH and MCHC. Furthermore, platelet concentrations in rats induced with chlorpromazine toxicity and treated rats at all dosages were considerably higher than in normal rats (Table 6).
Effect of Ethanolic Extract of C. anisata on the Renal Histopathological Parameters of Chlorpromazine-Induced Toxicity in Rats
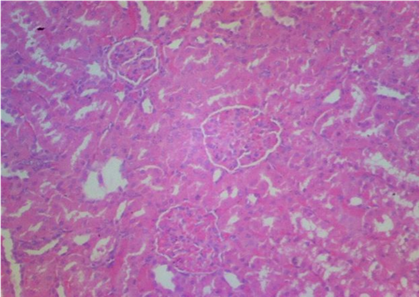
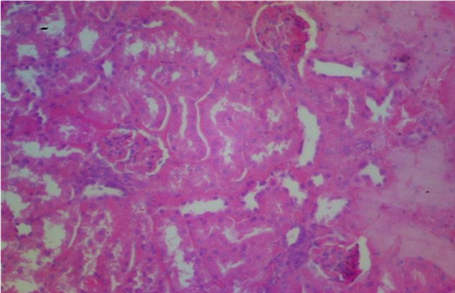
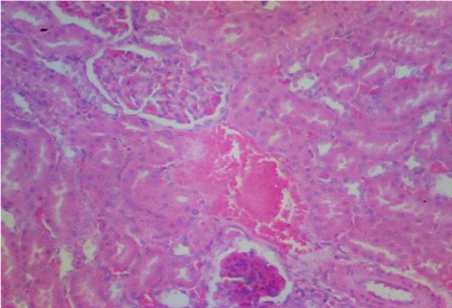
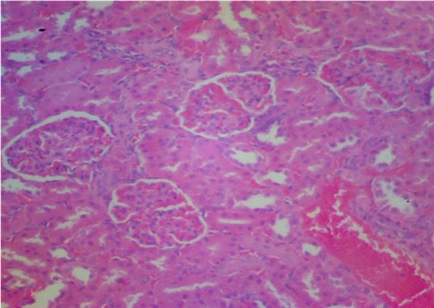
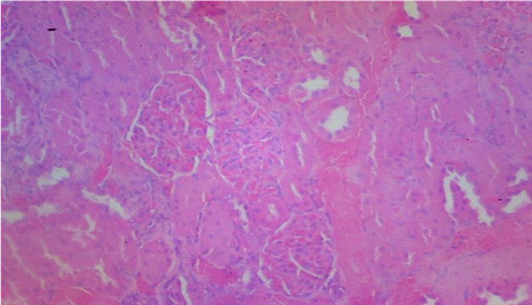
Plates 1–5 illustrate the histological effects of C. anisata ethanol leaf extract on renal tissue. The study's findings revealed that the kidney histoarchitecture of group 1 rats was normal, with no abnormalities in the tissue such as abortive glomeruli or the presence of hemorrhagic cells or blood (Plate 1). A photomicrograph investigation of the renal histoarchitecture of rats induced by chlorpromazine toxicity revealed the presence of localized necrosis of the epithelial lining in renal tubules (tubulorrhexis) as well as a high number of abortive glomeruli. The appearance of mid hemorrhage was also found in the kidney architecture of the rats induced with the toxicant (Plate 2). Rats treated with 50 mg/kg bwt ethanol leaf extract of C. anisata show the sign of mild tissue hemorrhage, few aborting glomeruli which mostly appeared normal, mild signs of tubulorrhexis and interstitum was adequate in the renal tissue of the rats induced with chlorpromazine toxicity (Plate 3).In addition, rats administered with 100 mg/kg bwt of the ethanol leaf extract of C. anisata show the appearance of the diffuse tissue hemorrhage which extends into the glomeruli. Except for the hemorrhagic symptoms, the glomeruli were normal. Few glomeruli are abortive, and the cuboidal epithelia of the tubules seem inflamed, lowering luminal diameter and indicating widespread edema. In rats induced with chlorpromazine toxicity, photomicrographs revealed severe tissue bleeding, sparse glomeruli exhibiting glomerulonephritis, tubular epithelium, and the formation of oedematous (Plate 5).
Table 6: Effect of C. anisata on Haematological Parameters of Chlorpromazine-Induced Toxicity in Rats
| Parameter/ Group | WBC (10x3/µL) | LYM (%) | MON (%) | RBC (10x6/µL) | HGB (g/dL) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (10x3/µL) |
| Group 1 | 8.2±2.11 | 68.75±2.55 | 13.65±0.75 | 7.83±0.06 | 16.4±0.01 | 42.40±21.8 | 20.55±0.05 | 27.65±8.35 | 330.40±3.15 |
| Group 2 | 6.40±1.61* | 71.40±4.10* | 10.10±3.50* | 7.38±0.00* | 14.95±0.04* | 67.40±0.80* | 20.25±0.05* | 35.35±0.55* | 426.50±2.08* |
| Group 3 | 12.35± 1.35 | 66.55±0.54 | 11.80±1.00 | 6.88±0.24 | 14.35±2.05 | 68.4±1.50 | 20.90±2.10 | 35.95±2.65 | 507.00±1.40 |
| Group 4 | 10.35±0.95 | 71.55±0.45 | 10.75±1.05 | 8.01±0.51 | 15.95±1.15 | 59.45±5.05 | 19.85±0.15 | 36.55±0.34 | 468.50±1.92 |
| Group 5 | 8.65±0.75 | 51.95±9.05 | 13.85±2.75 | 7.24±0.53 | 13.9±1.3 | 56.20±0.09 | 19.15±0.35 | 34.15±0.75 | 485.50±3.95 |
| Limit | 2.5-10.5 | 20.0-40.0 | 1.0-15.0 | 3.5-5.5 | 11.0-16.0 | 80.0-99.0 | 26.0-32.0 | 32.0-36.0 | 90-400 |
Data are expressed as Mean ± SEM; *Significant difference at p < 0>
Reagents and Chemicals
All reagents were procured from reputable sources and of analytical grade. These reagents include n-Hexane, ethanol, dichloromethane (DCM), sodium hydroxide, monopotassium dihydrogen phosphate, and dipotassium mono-hydrogen phosphate. Diagnostic kits of total protein, albumin, bilirubin, urea and creatinine were product of Randox Laboratories Limited, United Kingdom. All solutions, buffers and reagents were prepared with distilled water and store in the refrigerator at -4°C until required.
Experimental Animals
Twenty-five male Wistar rats weighing between 100 – 150 g used in this study were obtained from the Animal House in College of Health Sciences, Ladoke Akintola University of Technology (LAUTECH), Osogbo, Osun State, Nigeria. The animals were housed in a clean and well-ventilated cage at the Animal House Department of Biochemistry and Molecular Biology, Obafemi Awolowo University, Ile-Ife, Nigeria and maintained under ambient conditions. The animals were fed with standard rat chow and drinking water ad libitum. The animals were acclimatized to laboratory conditions for two weeks prior to experimentation. United States National Institute of Health (NIH, 1985) guidelines for care and use of animals for experimental studies were strictly adhered.
Plant Collection and Identification
Fresh Clausena anisata leaves were collected from swampy area in Oyo town, Modakeke, Osun
State, Nigeria. The study plant was identified and authenticated at Ife Herbarium Department of Botany, Obafemi Awolowo University, lle-Ife, Nigeria.
Preparation of Ethanol extract of Clausena anisata Leaves
The leaves of Clausena anisata were chopped into smaller pieces, thoroughly washed with clean distilled water and air-dried for a period of four weeks. The dried leaves were crushed into fine powder with a mechanical grinding machine. The powdered plant material (357 g) was soaked in ethanol for 72 h at room temperature. The ethanol extract was concentrated using rotary evaporator at a 35℃. The crude ethanol extract was stored in the desiccator until required for further processing.
Determination of Median Lethal Dose (LD50) of Acute Toxicity Study
Acute toxicity study was carried out according to the method of Lorke (1983) using eighteen mice weighing between 18 – 25 g. In the first phase, nine mice were separated into three groups of three mice each and given orally 10, 100 and 1000 mg/kg body weight of the ethanol leaf extract of Clausena anisata. They were monitored for indicators of toxicity for 24 h. In the second phase, another set of nine mice were separated into three groups of three mice each and given orally 1600, 2900 and 5000 mg/kg body weight of ethanol leaf extract of Clausena anisata. The LD50 was estimatedby using the formula: LD50 = √D0 X D100 = √1600 X 1000 = 1264.91mg/kg body weight (Karber, 1931).
Grouping and Treatment of Experimental Animals
Twenty-five male Wistar rats were divided into five groups of five animals per group. The experimental groups were designated as follows: Group 1 rats served as control and received distilled water. Group 2 rats were treated with Chlorpromazine every other day for 30 days; Group 3 rats were given Chlorpromazine and after 1 h, 50 mg/kg body weight of Clausena anisata extract was administered; Group 4 rats were given Chlorpromazine and after 1 h, 100 mg/kg body weight of Clausena anisata extract was administered and Group 5 rats were given Chlorpromazine and after 1 h, 150 mg/kg body weight of Clausena anisata extract was administered. The administration lasted for 30 days. After 30 days of administration, the animals were then allowed to fast overnight on the last day and sacrificed the next day by cervical dislocation. The kidney was removed after blood collection, washed in physiological saline, subsequently weighed and kept in phosphate buffer until required for analysis.
Collection and Preparation of Blood Plasma
The blood sample was collected by ocular puncture into heparinized bottles and centrifuged at 4000 rpm for 10 minutes. The supernatants obtained was collected, stored in sterile vials and kept in the freezer at -8℃ until required for biochemical assays.
Preparation of Kidney Homogenate
The kidney homogenates were prepared using one-gram (1 g) of the tissue which was homogenized with 100 mM Phosphate buffer, pH 7.4, to produce 10 % (W/V) homogenates using pestle and mortar. The homogenates were carefully transferred into centrifuge tubes and volumes adjusted to 10 ml. This was centrifuged at 4000 rpm for 30 minutes. The supernatants were collected into clean bottles, labelled and kept in freezer at -8℃until required for biochemical assays.
Evaluation Of Biochemical Parameters
Estimation of Plasma and Kidney Homogenate Albumin Concentration
The activity of Albumin was estimated using colorimetric method of Reitman and Frankel (1957) as described in a commercially available Randox kit (UK).
Estimation of Plasma Total Protein Concentration
The total protein concentration of plasma was assayed using Biuret method as described by Henry et al. (1974).
Estimation of Bilirubin Concentration
Bilirubin concentration in the plasma was determined according to the method of Jendrassik and Grof (1938). Total bilirubin was determined in the presence of caffeine, which releases albumin bound bilirubin, by the reaction with diazotized sulphanilic acid.
Estimation of Creatinine Concentration
The concentration of creatinine was determined according to the method described by Bones et al. (1945) and Toro et al. (1975). Creatinine in alkaline solution reacts with picric acid to form an orange coloured complex with the alkaline picrate. The amount of the complex formed is directly proportional to the creatinine concentration in the sample.
Hematological Determination
Blood samples were collected by ocular puncture into ethylenediamine tetraacetic acid (EDTA) tubes. The haematological parameters of Red Blood Cell (RBC) count, White Blood Cell (WBC) count, Haemoglobin Concentration (HGB), Mean Corpuscular Haemoglobin Concentration (MCHC), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Platelet (PLT) count, Percentage Lymphocyte (% LYM), Percentage Monocyte (% MON) were analyzed using standard techniques reported by Cheesbrough (2000).
Histological Examination of Kidney
Histological examinations of the kidney obtained from the representative animals from each group were carried out in line with standard procedures involving Hematoxylin and Eosin staining techniques as described by Feldman and Wolfe (2014).
Statistical Analysis
The results were expressed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) was used for data collected, followed by a Tukey multiple range comparisons test using the software GraphPad Prism 6.0. Statistical significance was set at p<0>
Results Discussion
The yield of Crude Ethanol Leaf Extract of Clausena anisata
The extract yielded 37.674gwhich represents 10.54% of the starting material.
Effect of Clausena anisata Ethanol Leaf Extract on the Weight of Rats
Table 1 shows the summary of the effect of the leaf of Clausena anisata on the weight of Wistar rats. The results of the study showed a significant percentage decrease in the body weight of the rats induced with Chlorpromazine only compared with the control (group 1). Similarly, rats repeatedly treated with 50, 10 and 50 mg/kg body weight (bwt) for 30 days demonstrated a significant percentage decrease in body weight in a dose-dependent manner compared to the control. Additionally, the rats repeatedly administered with 50, 100, and 150 mg/kg bwt of the extract exhibited a significant dose-dependent ameliorative effect compared with the group induced with the toxicant (group 2) (Table 1).
Table 1: Effect of C. anisata Ethanol Leaf Extract on Body Weight of Chlorpromazine-Induced Rats.
| Treatment Group | Day 1 Body Weight (g)
| Day 30 Body Weight (g) | Percentage Changes |
| Group 1 (Control) | 95.5 ± 1.5 | 131.78± 9.45 | 27.07 ± 6.37 |
| Group 2 (CPZ only) | 118.5± 6.5 | 138.53 ± 8.73 | 14.41 ± 0.70a |
| Group 3 (CPZ+50mg/kg bwt) | 124.5± 1.5 | 139.62± 5.25 | 10.75± 2.29a |
| Group 4 (CPZ+100mg/kg bwt) | 137.5 ± 2.5 | 159.92± 7.28 | 13.77 ± 5.49a |
| Group 5 (CPZ+150mg/kg bwt) | 143.5 ± 3.5 | 166.7± 1.23 | 13.91 ± 2.74a |
CPZ: Chlorpromazine (Toxicant)
Effect of Clausena anisata Ethanol Leaf Extract on the Relative Kidney Weight of Rats
Table 2 shows the summary of the effect of the leaf of Clausena anisata on the relative kidney weight of rats induced with Chlorpromazine to cause kidney damage. The findings of the study showed a significant reduction in the relative kidney weight of rats compared with the control. This indicated that Chlorpromazine can cause severe damage to the kidney after 30 days of administration. Rats administered with 50mg/kg bwt from day 1 to day 30 demonstrated a significant marginal increase (p < 0> 0.05) difference in the relative kidney weight of the rats. This suggests that the leaf of C. anisata could possibly restore the kidney damage at 50mg/kg bwt dose (Table 2).
Table 2: Effect of C. anisata Ethanol Leaf Extract on Relative Kidney Weight of Chlorpromazine- Induced Rats
| Group 1 (Control) | 0.740 ± 0.19 |
| Group 2 (CPZ only) | 0.665 ± 0.015a |
| Group 3 (CPZ+50mg/kg bwt) | 0.705 ± 0.065b |
| Group 4 (CPZ+100mg/kg bwt) | 0.610 ± 0.08 |
| Group 5 (CPZ+150mg/kg bwt) | 0.655 ± 0.025 |
CPZ: Chlorpromazine
Acute Toxicity of Oral Administration of Ethanol Leaf Extract of C. anisata
Table 3 shows the summary of the acute toxicity effect of ethanol leaf extract of C. anisata. There was no record of mortality at 10 and 100mg/kg bwt of the tract while at high concentration (1000 to 5000mg/kg bwt), there was record of mortality. The mean median lethal dose (LD50) was estimated to be less than 5000mg/kg bwt. This suggests that the ethanol leaf extract of C. anisata was toxic at high concentrations (Table 3).
Table 3: Acute Toxicological Effect of Ethanol Leaf Extract of C. anisata
Dose (mg/kg) Mortality Survival Observation after 72 h
|
First Phase 10 0/3 3 No mortality 100 0/3 3 No mortality 1000 1/3 2 Mortality |
Second Phase 1600 2/3 1 Mortality 2900 3/3 0 Mortality 5000 3/3 0 Mortality |
Effect of C. anisata Ethanol Leaf Extract on Plasma Creatinine, Urea, Albumin and Total Protein in Chlorpromazine Induced Wistar Rats
Table 4 shows the summary of the effect of ethanol leaf extract of C. anisata on plasma creatinine, urea, albumin and total protein concentration in Chlorpromazine-induced wistar rats. It was found that rats induced with only Chlorpromazine (group 2) have a higher creatine concentration level than the control rats (group 1). Rats administered 50, 100 and 150 mg/kg bwt of the ethanol leaf extract of C. anisata demonstrated a significant (p < 0>C. anisata after 30 days at a dose of 50, 100 and 150 mg/kg bwt, there was significant decrease in the plasma urea and albumin concentration when compared with the normal rats. The results of the study as well revealed that rats induced chlorpromazine toxicity exhibited a significant reduction in the plasma total protein concentration compared with the normal rats. While animals treated with 50, 100 and 150 mg/kg bwt of C. anisata ethanol leaf extract demonstrated a significant increase in the plasma total protein concentration compared with the normal rats. Rats administered with 150 mg/kg bwt after 30 days showed excellent performance as it was able to restore the depleted plasma total protein concentration (Table 4).
Table 4: Effect of Clausena anisata Extract on Plasma Creatinine, Urea, Albumin and Total Protein Concentration
| Treatment | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | ||
| Creatinine | 11.18±0.0075 | 39.88±0.0075a | 18.36±0.080b | 19.055±0.044b | 13.664±0.226b | ||
| Urea | 6.695±0.0150 | 10.262±0.0470a | 8.43±0.006b | 6.7195±0.00849b | 9.224±0.0234b | ||
| Albumin | 5.42 ± 0.0045 | 8.33 ± 0.004 a | 5.46 ±0.0045b | 5.479 ± 0.004 b | 5.37 ± 0.0045b | ||
| Total Protein | 27.32±0.0350 | 8.51 ± 0.035a | 9.54 ± 0.04b | 8.82 ± 0.029b | 26.28±0.0650ab | ||
Values without any superscripts along the same roll are not significantly different from each other; values carrying superscript ‘a’ along the same roll are significantly different from group 1 while values carrying superscript ‘b’ along the same roll are significantly different from group 2. (n=5). Group 1(control), Group 2 (Chlorpromazine), Group 3 (50mg/kg bwt), Group 4 (100mg/kg bwt) and Group 5 (150mg/kg bwt)
There was significant increase (p<0>anisata. Contrastingly, a decrease in total protein concentration was observed in plasma of Chlorpromazine treated group when compared to control. This observed decrease was ameliorated by the extract at 150mg/kg bwt but did not show any effect at lower dosages at which the extract was administered.
Effect of C. anisata Ethanol Leaf Extract on Kidney Homogenate in Chlorpromazine-Induced Wistar Rats
The effect of C. anisata ethanol leaf extract on kidney homogenate in Chlorpromazine-induced rats’ toxicity was presented in Table 5. The results of the study showed that rats administered with chlorpromazine (group 2) only for a period of 30 days have significantly higher kidney creatine, urea, albumin, and bilirubin concentrations compared with the normal rats. On the other hand, rats treated with 50, 100 and 150 mg/kg bwt showed a significant decrease in the kidney creatine, urea, albumin and bilirubin concentration compared to the normal rats except rats treated with 100 mg/kg wt of the extract. There was no any significant alteration in the kidney albumin concentration when the toxicant was administered (Table 5).
Table 5: Effect of Clausena anisata ethanol leaf extract on Kidney Albumin, Urea, Bilirubin, and Creatinine Concentration
| Treatment | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| Creatinine | 4.775 ± 0.025 | 6.08 ± 0.0099a | 3.21 ± 0.029b | 2.59 ± 0.040b | 1.535 ± 0.055 b |
| Urea | 2.8295±0.0055 | 7.4605±0.0014a | 2.735±0.0034 b | 3.684±0.0044b | 0.607±0.0080b |
| Albumin | 26.09 ± 0.330 | 29.585±0.495a | 28.2995±0.210b | 29.876±0.003a | 27.073±0.006b |
| Bilirubin | 7.141 ± 0.0069 | 23.611±0.0049a | 9.596 ± 0.0025b | 6.662±0.0199b | 11.803±0.0044b |
Values without any superscripts along the same roll are not significantly different from each other; values carrying superscript ‘a’ along the same roll are significantly different from group 1 while values carrying superscript ‘b’ along the same roll are significantly different from group 2. (n=5). Group 1(control), Group 2 (Chlorpromazine), Group 3 (50mg/kg bwt), Group 4 (100mg/kg bwt) and Group 5 (150mg/kg bwt)
There was significant increase (p<0>p<0>Clausena anisata extract in the kidney bilirubin was more pronounced in rats treated with 100 mg/kg body weight Clausena anisata extract and to a certain extent at 50 mg/kg body weight.
Effect of Ethanolic Extract of C. anisata on the Haematological Parameters of Chlorpromazine-Induced Toxicity in Rats
Table 6 summarizes the effect of C. anisata ethanol leaf extract on haematological parameters in rats with chlorpromazine toxicity. The study's findings revealed a significant (p < 0 xss=removed>The MCV concentration in rats treated with 100 and 150 mg/kg bwt C. anisata extract decreased significantly. Furthermore, animals exposed to the toxicant (group 2) showed a substantial drop in mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) as compared to control rats (group 1). When compared to chlorpromazine-induced toxicity in rats, rats treated with 50, 100, and 150 mg/kg bwt showed no significant difference (p > 0.05) in MCH and MCHC. Furthermore, platelet concentrations in rats induced with chlorpromazine toxicity and treated rats at all dosages were considerably higher than in normal rats (Table 6).
Effect of Ethanolic Extract of C. anisata on the Renal Histopathological Parameters of Chlorpromazine-Induced Toxicity in Rats
Plates 1–5 illustrate the histological effects of C. anisata ethanol leaf extract on renal tissue. The study's findings revealed that the kidney histoarchitecture of group 1 rats was normal, with no abnormalities in the tissue such as abortive glomeruli or the presence of hemorrhagic cells or blood (Plate 1). A photomicrograph investigation of the renal histoarchitecture of rats induced by chlorpromazine toxicity revealed the presence of localized necrosis of the epithelial lining in renal tubules (tubulorrhexis) as well as a high number of abortive glomeruli. The appearance of mid hemorrhage was also found in the kidney architecture of the rats induced with the toxicant (Plate 2). Rats treated with 50 mg/kg bwt ethanol leaf extract of C. anisata show the sign of mild tissue hemorrhage, few aborting glomeruli which mostly appeared normal, mild signs of tubulorrhexis and interstitum was adequate in the renal tissue of the rats induced with chlorpromazine toxicity (Plate 3).In addition, rats administered with 100 mg/kg bwt of the ethanol leaf extract of C. anisata show the appearance of the diffuse tissue hemorrhage which extends into the glomeruli. Except for the hemorrhagic symptoms, the glomeruli were normal. Few glomeruli are abortive, and the cuboidal epithelia of the tubules seem inflamed, lowering luminal diameter and indicating widespread edema. In rats induced with chlorpromazine toxicity, photomicrographs revealed severe tissue bleeding, sparse glomeruli exhibiting glomerulonephritis, tubular epithelium, and the formation of oedematous (Plate 5).
Table 6: Effect of C. anisata on Haematological Parameters of Chlorpromazine-Induced Toxicity in Rats
| Parameter/ Group | WBC (10x3/µL) | LYM (%) | MON (%) | RBC (10x6/µL) | HGB (g/dL) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (10x3/µL) |
| Group 1 | 8.2±2.11 | 68.75±2.55 | 13.65±0.75 | 7.83±0.06 | 16.4±0.01 | 42.40±21.8 | 20.55±0.05 | 27.65±8.35 | 330.40±3.15 |
| Group 2 | 6.40±1.61* | 71.40±4.10* | 10.10±3.50* | 7.38±0.00* | 14.95±0.04* | 67.40±0.80* | 20.25±0.05* | 35.35±0.55* | 426.50±2.08* |
| Group 3 | 12.35± 1.35 | 66.55±0.54 | 11.80±1.00 | 6.88±0.24 | 14.35±2.05 | 68.4±1.50 | 20.90±2.10 | 35.95±2.65 | 507.00±1.40 |
| Group 4 | 10.35±0.95 | 71.55±0.45 | 10.75±1.05 | 8.01±0.51 | 15.95±1.15 | 59.45±5.05 | 19.85±0.15 | 36.55±0.34 | 468.50±1.92 |
| Group 5 | 8.65±0.75 | 51.95±9.05 | 13.85±2.75 | 7.24±0.53 | 13.9±1.3 | 56.20±0.09 | 19.15±0.35 | 34.15±0.75 | 485.50±3.95 |
| Limit | 2.5-10.5 | 20.0-40.0 | 1.0-15.0 | 3.5-5.5 | 11.0-16.0 | 80.0-99.0 | 26.0-32.0 | 32.0-36.0 | 90-400 |
Data are expressed as Mean ± SEM; *Significant difference at p < 0>
Discussion
The nontoxic effect of the extract at 50 mg/kg and 100 mg/kg doses investigated on the renal function indices, albumin and urea, may suggest that the normal functionality of the nephrons at the tubular and glomerular levels were not altered. However, a significant decrease in urea at 150 mg/kg dose may be as a result of water overload (Nasir et al., 2017). Relative to the control, there was a significance increase (p<0>Clausena anisata extract groups at 50 mg/kg, 100 mg/kg and 150 mg/kg body weight respectively. The creatinine level in the kidney of rats was significantly increased in Chlorpromazine treated group when compared to the control. The significant increase was lowered when the rats were treated with 50 mg/kg, 100 mg/kg and 150 mg/kg body weight of the Clausena anisata extract. It was noted that there was a significant decrease in the creatinine level of the Clausena anisata extract treated groups in a dose-dependent fashion when compared to control group. The observed decrease in the bilirubin level of rats treated with the extract following Chlorpromazine administration is an indication of the ameliorative effect of Clausena anisata extract in the kidney which was more pronounced in rats treated with 100 mg/kg body weight Clausena anisata extract and to a certain extent at 50 mg/kg body weight.
Several investigators have considered the effect of Clausena anisata leaf extract on different animal organs and blood samples in other to elucidate their ethnomedicinal uses and pharmacological activities (Omara et al., 2022; Thomford et al., 2021; Omosa et al., 2019; Ntamo et al., 2016; Arsia et al., 2016; Ishola et al., 2015; Murungi, 2013; Okokon et al., 2012; Mkhombo, 2010). However, this study evaluates the nephroprotective effect of ethanolic leaf extract of Clausena anisata against chlorpromazine-induced nephrotoxicity in Wistar rats.
Clausena anisata leaf extract has been shown to lower albumin level in kidney when the rats were administered for 30 days. Albumin is the most abundant protein in serum, accounting for roughly 60% of the total protein composition. Albumin is primarily synthesized in the liver and serves a variety of physiological activities, including osmotic pressure regulation, redox balance, fatty acid transport, bilirubin transport, medicine transfer, hormone transport, and vitamin transport (Levitt and Levitt, 2016). At the renal level, circulating albumin is filtered in small amounts. Albumin is found in urine in three physiological situations which are postural, febrile, and exercise.
Urea and albumin concentration are essential factors in the diagnosis of kidney problems. A sudden change in the concentration of urea and albumin has the tendency of creating severe damage to the kidney (Sridharan et al., 2015). In the present study, there was no significant change (p > 0.05) in the plasma concentration of urea and albumin of rats when ethanol leaf extract of C. anisata was treated with Chlorpromazine-induced rats. This may suggest that the extracts at the tested dose do not alter the concentration of plasma urea and albumin at the physiological state and thereby maintain the normal plasma urea and albumin content in the rats.
Chlorpromazine induces increase in the level of creatinine in the plasma of treated rat, however, this increase in plasma creatinine level was significantly lowered when Clausena anisata extract was administered at 50 mg/kg, 100 mg/kg and 150 mg/kg body weight respectively. This suggests the ability of the extract to offer protection against induced increase in creatinine level in kidney which may result to nephrotoxicity. Chlorpromazine induced increase in creatinine level in the kidney of rats when compared to the control. This increase was lowered and restored when the rats were treated with 50 mg/kg dose of the Clausena anisata extract. The observation on decrease in the creatinine level of the Clausena anisata extract treated groups in a dose-dependent fashion suggested that at higher dosage there will be less creatinine in the kidney for clearance in the blood. Creatinine is regarded as an essential marker of kidney dysfunction (Mukinda and Eagles, 2010). It had been reported that higher level of creatine concentration than the control is a symbol of kidney malfunction (Mukinda and Syce, 2007) in which if prompt care is not taken, it could lead to kidney death. In the current study, it was observed that rats administered with Chlorpromazine (group 2) have the highest plasma creatinine concentration compared with the control; this occurs after 30 days administration. This is an indication of a malfunctioning of kidney and it is dangerous for animal kidney health. Interestingly, after 30-day treatment with ethanol leaf extract of C. anisata at a dose of 50, 100 and 150mg/kg bwt, the plasma creatinine concentration was significantly restored to almost the normal rats compared with the control rats (group 1) especially at a dose of 150mg/kg bwt (Table 4). These findings suggest that the leaf of C. anisata plant could be used as a nephroprotective agent against any abnormal function of the kidney.
In this study, Clausena anisata extract has been shown to cause decrease in kidney urea level and increase in plasma urea of rats administered with 150 mg/kg body weight of the Clausena anisata extract suggested that Clausena anisata extract at 150 mk/kg body weight protects the kidney from urea toxicity hence, the need to migrate to the blood plasma where after ultrafiltration will be secreted from the kidney tubule into the bladder urine for excretion.
Chlorpromazine also resulted in increase in the bilirubin level in kidney of rats. This revealed the ability of Chlorpromazine to initiate impairment of hepatic excretion (Moyer and Balistreri, 2011). However, a significant decrease in the bilirubin level of rats treated the extract when compared to the Chlorpromazine treated group suggested that the Clausena anisata extract has the capacity to offer protection against Chlorpromazine induced increased in bilirubin level. The ameliorative effect of the Clausena anisata extract in the kidney bilirubin was more pronounced in rats treated with 100 mg/kg body weight Clausena anisata extract and to a certain extent at 50 mg/kg body weight.
It had been previously reported in the literature that increased total protein excretion could be implicated in renal diseases. Diet containing protein can modulate renal function and thus, consumption of dietary protein in excess of recommended amounts promotes chronic renal disease through increased glomerular pressure and hyperfiltration (Metges and Barth, 2000). When kidneys are not functioning properly, protein may escape from the blood into the urine. The high concentration level of total protein excreted is accompanied by simultaneous reduction in plasma total protein concentration (Good et al., 2010). The current study showed a significant (p < 0>
The results demonstrated an increase in White Blood Cells (WBC) in treated groups i.e.Group 3 and Group 4. It reveals that Clausena anisata extract, at a dosage of 50 mg/kg and 100 mg/kg body weight protects the rat against the alteration produced by Chlorpromazine toxicant in the plasma. The number of Lymphocytes (LYM#), Monocytes (MON), and Granulocytes (GRAN) grows as the immune system battles the poisons. At greater C. anisata concentrations, the LYM#, MON, and GRAN levels increase, demonstrating the influence of C. anisata extract on Groups 3, 4, and 5, respectively. It works better at 50 mg/kg dose but it appeared to work best at 150mg/kg dose.
The haematological effect of Clausena anisata leaf extract against Chlorpromazine-induced kidney injury on the plasma of the treated rats revealed increase in WBC, LYM#, HCT, MCV, PLT and decrease in MCH, MPV and PCT in Clausena anisata extract treated groups when compared to control. This suggest that the Clausena anisata extract which was administered at those varying doses appears to offer protection against xenobiotics in rat body as the immune system of the animal is perturb or altered. Also, data obtained from haematological parameters revealed that the ethanolic leaf extract of Clausena anisata did not alter the RBC, HGB, PDW and its related indices. The non-toxic effect on these parameters suggests that Clausena anisata neither modulates the incorporation of haemoglobin into red cells, nor alter the morphology and osmotic fragility of the red blood cells (Adebayo et al., 2005; Nasir et al., 2017). However, increase in MCV at 50 mg/kg dose may be associated with folic acid deficiency reported by Nasir et al. (2017) but not due to the extract. In addition, the observed elevation of WBC at both 50 mg/kg and 100 mg/kg doses may be either due to enhancement of white blood cell production and its transportation to the blood or due to a reduction in its removal from the blood circulation (Yakubu and Afolayan, 2009; Nasir et al., 2017). The increased in lymphocyte (LYM#) observed in this study is an indication of the ability of the extract to boost the immune system of the induced rats (Nasir et al., 2017). The elevated platelets count (PLT) observed in all the treated groups at varying doses may be unconnected with the extract administered.
The histopathology results also corroborate the findings made in this study showing mild tissue hemorrhage with few aborting glomeruli which mostly appeared normal at 50mg/kg Clausena anisata extract. In addition, at 100mg/kg dosage, Clausena anisata extract ameliorate the Chlorpromazine effect as evidenced in diffuse tissue hemorrhage which extends into the glomeruli which are many and appeared normal (see Plate 2- Plate 4).
Conclusion
In conclusion, ethanolic extract of Clausena anisata leaf exhibited good nephroprotective potential against chlorpromazine-induced nephrotoxicity in Wistar rats at lower doses of 50 and 100 mg/kg body weight, and showed nephrotoxic ability when administered at 150 mg/kg body weight, as evidenced by histopathology results. Administration at dosages of 50 mg/kg body weight and 100mg/kg body weight of the extract demonstrated a protective effect, but at 150 mg/kg body weight the protective effect was not observed. Clausena anisata extract at a dose of 150 mg/kg body weight should not be used or considered on a regular basis for treatment of kidney ailment. Clausena anisata is safe at doses of 50 mg/kg and 100 mg/kg body weight. Clausena anisata leaf extract holds much promise in protecting the kidney against xenobiotic, at the appropriate dosage; it may be employed as synthetic pharmaceuticals in the treatment of kidney disease.
Recommendation
It was evident from this study that the extract of Clausena anisata leaves has a protective effect at 50 mg/kg and 100 mg/kg doses whereas it does not at 150 mg/kg dose. It is therefore recommended that further research may be necessary to evaluate the Clausena anisata toxicity on animal organs at higher dosage before it could be administered as a promising novel drug.
References
- Adebayo, J.O., Adesokan, A.A., Olatunji, L.A., Buoro, D.O. and Soladoye, A.O. (2005). Effect of Ethanolic extract of Bougainvillea spectabilis leaves on haematological and serum lipid variables in rats. Biokemistri, 17: 4-49. https://doi.org/10.4314/biokem.v17i1.32588 2.
- Agyepong N., Agyare C., Adarkwa-Yiadom M. and Gbedema S.Y. (2014). Phytochemical investigation and anti-microbial activity of Clausena anisata (Willd), Hook. Afr J Tradit Complement Altern Med. 11(3):200–209. https://doi.org/10.4314/ajtcam.v11i3.28 3.
- Anthérieu S., Azzi PB-E., Dumont J., Abdel-Razzak Z., Guguen-Guil-louzo C., Fromenty B., Robin M-A. and Guillouzo A. (2013) Oxidativestress plays a major role in chlorpromazine-induced cholestasisin human HepaRG cells. Hepatology 57:1518–1529. https://doi.org/10.1002/hep.26160 4.
- Anthérieu, S., Chesné, C., Li, R., Guguen-Guillouzo, C., and Guillouzo, A. (2012). Optimization of the HepaRG Cell Model for Drug Metabolism and Toxicity Studies. Toxicol. Vitr. 26, 1278–1285. https://doi.org/10.1016/j.tiv.2012.05.008 5.
- Arsia, Tarnam Y., Nargis, Begum T., Muhammad, Ilyas M.H., Shilu Mathew, Archunan Govindaraju and Ishtiaq Qadri (2016). Green synthesis, antioxidant potential and hypoglycemic effect of silver nanoparticles using Ethanolic leaf extract of Clausena anisata (Willd.) Hook. F. Ex Benth. of Rutaceae. Pharmacognosy Journal 8 (6): 565-575
- Bones, R. W. etal. (1945). J. Biol. Chem. 158, 581. CHEBI:3647 – chlorpromazine.
- Cheesbrough, M. (2000). Microbiological Tests. In: Cheesbrough, M., Ed., District Laboratory Practice in Tropical Countries, Part II, Low Priced Edition, Cambridge University Press, Cambridge, pp105-130.
- Feldman, A.T. and Wolfe, D. (2014). Tissue Processing and Hematoxylin and Eosin Staining. In: Day, C. (eds) Histopathology. Methods in Molecular Biology, Vol 1180. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1050-2_3
- Good, D.M., Zurbig, P., Argiles, A., Bauer, H.W., Behrens, G., Coon, J.J., Dakna, M., Decramer, S., Delles, C., Dominiczak, A.F. and Ehrich, J.H. (2010). Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics.mcpM110.
- Henry, R.J., Cannon, D.C., Winkelman, J.W. (1974).
- Hutchings, A., Scott, A.H., Lewis, G. and Cunningham, A. (1996). Zulu Medicinal Plants: An Inventory. University of Natal Press, Pietermaritzburg.
- Ishola, I.O., Olayemi, S.O., Oreagba, I.A., Ifeanyi, C.I. and Popoola, T.O. (2015). Antinociceptive and anti-arthritic properties of hydroethanolic leaf extract of Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) in Rodents: possible mechanism of actions. Niger J Physiol Sci. 30(1-2):39-49.
- Jendrassik, L. and Grof, P. (1938). Simplified Photometric Methods for the Determination of Bilirubin. Biochemical Journal, 297, 81-89.
- Kadiri, M., Ojewumi, A.W. and Onatade, T.N. (2015). Indigenous Uses and Phytochemical Contents of Plants Used in The Treatment of Menstrual Disorders and After- Child Birth Problems in Abeokuta South Local Government Area of Ogun State, Nigeria. Journal of Drug Delivery and Therapeutics 5(3).
- Karber, G. (1931) BeitragzurkollecktivenBehandlungpharmakologischerReihenversuche. Arch. Exptl. Pathol. Pharmakol, 162, 480-483.
- Lechaba, N.M.T., Schutte, P.J., Hay, L., Böhmer, L. and Govender, M.M. (2016). The effects of an aqueous leaf extract of Clausena anisata (Willd.) Hook.f.exBenth. on blood pressure, urine output, angiotensin II levels and cardiac parameters in spontaneously hypertensive rats. Journal of Medicinal Plants Research, 10(28): 425-434.
- Levitt, D.G. and Levitt, M.D. (2016). Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurementsInt. J. Gen. Med. 2016; 9: 229–255.
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol. 54(4):275-287.
- Lunyera J., Wang D., Maro V., et al. (2016). Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: an ethnomedical survey. BMC Complement Altern Med. 16(1):282.
- Mbah, C.J. and Kenechukwu, F.C. (2009). Preliminary Screening of Ethanolic Extracts of Clausena anisata for Anticonvulsant Activity. Bio-Research, 7(2): 548 – 550.
- Metges, C.C. and Barth, C.A. (2000). Metabolic consequences of a high dietary protein intake in adulthood of available evidence. J Nutr.130:886-889.
- Mkhombo, M.H. (2010). The effects of Clausena anisata (WILLD) hook (RUTACEAE) leaf extracts on selected diabetic related carbohydrate metabolizing enzymes. A Thesis Submitted to University of Limpopo (Medunsa Campus) for the award of Master Degree (MSc Biochemistry).
- Moyer, K.D. and Balistreri, W.F. (2011). Liver Disease Associated with Systemic Disorders. In: Robert, M.; Stanton, B.F.; St. Geme, J.W.; Schor, N.F.; Richard, E.N. Textbook of Pediatrics. Saunders. P. 1405.
- 24. Mukinda, J. and Syce, J.A. (2007). Acute and chronic toxicity of aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology. 112, 138–144.
- 25. Mukinda, J.T. and Eagles, F.K. (2010). Acute and sub-chronic oral toxicity profile of the aqueous extract of Pohygalafruticosa in female mice and rats. Journal of Ethnopharmacology. 128, 236–240.
- 26. Murungi, J.M. (2013). Antimalarial activity and safety properties of Clausena anisata and Clutiar obusta in a mouse model. A Thesis Submitted in Partial Fulfillment for the Degree of Master of Science in Biotechnology in the Jomo Kenyatta University of Agriculture and Technology 2011.
- Nasir, A., Alhassan, A.J., Sule, M.S., Mannir, A.R., Yaradua, A.I., Muhammad, I.U. and Kanadi, A.M. (2017). Phytochemical Screening, LD50 Determination, and Sub-Chronic Toxicity Studies of Aqueous Leaf Extract of Ficus polita. Bayero Journal of Pure and Applied Sciences, 10(1): 295 – 298.
- National Institutes of Health, National Library of Medicine, National Center for Biotechnolog Information.
- Ntamo MacDonald, T.L., Paul, J.S., Leon, H., Linde, B. and Melvin, M.G. (2016). The effects of an aqueous leaf extract of Clausena anisata (Willd.) Hook. f. ex Benth. on blood pressure, urine output, angiotensin II levels and cardiac parameters in spontaneously hypertensive rats. Journal of Medicinal Plants Research 10 (28): 425-434.
- Ojewole, J.A.O. (2002). Hypoglycaemic effect of Clausena anisata (Willd) Hook methanolic root extract in rats. Journal of Ethnopharmacology, 81(2): 231-237.
- Okokon J.E., Etebong E.O., Udobang J.A., Essien, G.E. (2012). Antiplasmodial and analgesic activities of Clausena anisata. Asian Pacific Journal of Tropical Medicine 5(3): 214- 219.
- Omara, T., Kiprop, A.K., Kosgei, V.J. and Kagoya, S. (2022). Clausena anisata (Willd.) Hook. f. ex Benth. (Rutaceae): ethnomedicinal uses, phytochemistry, pharmacological activities, toxicity, and clinical application. Journal ofTraditional and Complementary Medicine 7(6)
- Omosa, L.K., Amugune, B., Mutai, P., Karumu, E., Mukungu, N., Induli, M., Kama-Kama, F., Kuete, V. (2019). Rapid Screening using GIBEX Screens-to-nature System of Ethnomedicinal Plants from Ngong Forest, Kenya for Potency against Infectious Diseases and Antioxidant Activities: A Qualitative Study. Pharmacogn. Commn. 9(2):59-74.
- Pazhayattil, G.S. and Shirali, A.C. (2014). Drug-induced impairment of renal function. International Journal of Nephrology and Renovascular Disease 7: 457-468.
- Reitman, S. and Frankel, S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28(1):56-63.
- Sridharan, B., Micheal, T.S., Ramachandran, A., Ganesh, N.R. and Pragasam, V. (2015). Citrus Bioflavonoids Ameliorate Hyperoxaluria Induced Renal Injury and Calcium Oxalate Crystal Deposition in Wistar Rats. Adv Pharm Bull. 5(3):419-427
- Suksaeree J., Chankana N., Luprasong C. and Monton C. (2021). Optimization of dynamic maceration of Clausena anisata (Willd.) Hook. f. ex Benth. leaves to maximize trans-anethole content. SN Appl Sci. 3(4):514.
- Thomford K.P., Yorke J., Thomford A.K. and Amponsah, I.K. (2021). A formulation of Clausena anisata (Willd.) Hook. f. Ex Benth and Cassia sieberiana DC. alleviates the symptoms associated with osteoarthritis: A single-blind, randomised controlled trial of a traditional Ghanaian remedy. Clinical Phytoscience 7 (1), 1-12.
- Toro, G. etal. (1975). Practical Clinical Chem. P.:154. Tropical Plants Database, Ken Fern. tropical. theferns. info.2022-11-16.
- Yakubu, M.T. and Afolayan, A.J. (2009). Effect of aqueous extract of Bulbinenatalensis Baker stem on haematological and serum lipid profile of male Wistar rats. Indian Journal of Experimental Biology, 47(4): 283-288.


