Article In Press : Article / Volume 3, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2833-0943/023
Occurrence and Health Risk Assessment of Organochlorine Residues in Cowpea Grains Marketed in Abuja, Nigeria
- Chemistry Advanced Research Centre, Sheda Science and Technology Complex Abuja, Nigeria
- Faculty of Science, Chemistry Department, University of Abuja, Nigeria
- Department of Crop Protection, Faculty of Agriculture, University of Abuja, Nigeria
Fagbohun Adebisi Akinyemi & Anjorin Samuel Toba
A. Fagbohun, M.S. Dauda, T.S. Anjorin, (2024), Occurrence and Health Risk Assessment of Organochlorine Residues in Cowpea Grains Marketed in Abuja, Nigeria. Pesticide Science and Pest Control. 3(1). DOI: 10.58489/2833-0943/023
© 2024 Adebisi Fagbohun, T.S. Anjorin, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
- Received Date: 18-05-2024
- Accepted Date: 11-06-2024
- Published Date: 18-07-2024
Gas chromatography, QuEChERS, contamination, population, food crop, pesticide residue.
Abstract
The main objective of this study was to determine the prevalence and concentration level of organochlorine residue in marketed cowpea grains in the Federal Capital Territory (FCT) -Abuja, Nigeria, and assessed the health risk to the consumers. The randomly collected 140 samples were bulked into 28 laboratory samples for analysis. The samples were extracted and cleanup using Quick Easy Cheap Efficient Rugged and Safe (QuEChERS) methods. Twenty organochlorine compounds comprising benzene hexachloride (BHC), heptachlor, aldrin, chlordane, endosulfan, dichlorodiphenyltrichloroethane, methoxychlor, heptachlor, endosulfan, and their metabolites were quantified using gas chromatography-mass spectrophotometer (GC-MS). The recovery percentage of OCs residues were in the range of 90.1 to 93.7% and the limit of detection ranged between 0.00003 and 0.00020 mg kg-1. The prevalence of the contaminated cowpeas samples with p,pâ-dichlorodiphenyltrichloethane (DDT), endosulfan sulphate, and endrin ketone was 89.29%. Aldrin, dieldrin ppâ- p,pâ-dichlorodiphenyldichloethylene (DDE), alpha BHC, Delta-BHC had an incidence of 75.00, 32.14 and 57.14 % respectively. None of the OC residues were detected in the cowpea grains obtained from Utako market (UW). Mean residual concentration of the OCs insecticides in the samples ranged between 0.000068 to 0.006633 mg kg-1. The mean concentration level of heptachlor epoxide (0.0550 mg kg-1), methoxychlor (0.0147 mg kg-1), and endrin ketone (0.0663 mg kg-1) were above the EU limits of 0.020, 0.010 and 0.050 mg kg-1 respectively.and the concentration level of all the other 17 compounds in all the samples were below the EU maximum limit. Human risk assessment indicated that all the detected OCs residues were within Acceptable Daily Intake (ADI). The mean value for Hazard index (HI) revealed that endrin ketone (27.2616) was > 1 while the combined health hazard of OCs was < 100 and consumption of the samples might poses health risk due to presence of the banned OCs. This study suggests that cowpea grains sold in the study area pose a potential health risk to the consumers.
Introduction
Organochlorines represent the first important class of insecticides developed by the burgeoning chemical industry in the first half of the 20th century (Rincón-Rubio et al., 2024, Van den, 2023). Some organochlorine compounds have been banned in the past several years but still define a substantial share of the insecticides market (Van den, 2023). Chemical insecticides including OCs are neurotoxicants, as they act by poisoning the nervous system of the target organisms including insects (Wahab et al., 2016; Shah, 2020; Namiki et al., 2020; Ruan et al., 2023). Organochlorine insecticides are known to be toxic to several insect pests, mammals including humans (Jayaraj et al., 2015; Lotti et al., 2015; Martín-Durán et al., 2021; Thakur et al., 2023).
Cowpea (Vigna unguiculata) - white and brown beans are common leguminous crops and the most important staple plant protein food crops in sub-Sahara Africa (Abebe & Alemayehu, 2022). According to the Food and Agriculture Organization of the United Nations (FAOSTAT) for 2020, the global production of cowpea was approximately 6.2 million metric tons where Nigeria produced around 3.1 million metric tons of cowpea, which accounted for more than 50% of Africa's cowpea production and approximately 50% of the global production (FAOSAT, 2020, FAOSAT, 2023, Nwagboso et al., 2024). Heavy losses in cowpea production are reported in Africa as a result of high incidence of insect pests and diseases (Kamara et al., 2018). Farmers who applied insecticides recorded a tenfold increase in yield as compared to uncontrolled plots. Insect pests inflict injury on cowpea plants in the course of their feeding on crop parts and these pests indirectly transmit diseases by acting as vectors of pathogens (Ezeaku 2015, Fadina et al., 2021). These therefore constitute an important constraint in cowpea production. The different parts of cowpea are prone to an adopted pest species (FAOSAT, 2020, FAOSAT, 2023).
Previous studies reported that cowpeas insect attack varies from pod-feeding bugs, flower bugs and legume pod borers were the major insect pests affecting cowpeas in the dry savannas of North Eastern Nigeria (Kamara et al., 2018). Abnormal pod and seed formation arises from activities of adults and nymphs of pod bugs while removing sap from green pods (Singh et al., 2015). Callosobruchus maculatus and the bruchid cause major losses in storage which could be as high as 90 percent loss of cowpea grains kept in the stores (Tripathi, 2018, Lastushkina et al., 2023). Feeding insect pests on stored cowpea inflicts damage resulting in their loss of value for planting, marketing, or consumption (Richard et al., 2023). Several OCs pesticides have been used as insecticides to prevent field and storage losses (Gabi 2022, Tadesse, 2020, Rolania et al., 2021).
There have been several reports on the danger and health hazards of insecticides usage in Nigeria (Amusat et al., 2019). Most of which were a result of the indiscriminate and inappropriate use of these chemicals. These have led to food poisoning, several health cases and even some have led to death. (Mohammad et al., 2018, Ogwo, 2021). Organochlorine pesticide residues have been reported in different food commodities in Nigeria (Sosan et al., 2020, Mohammad et al., 2018, et al., 2020, Oshatunberu, 2023, Idowu et al., 2022). Also, a study carried out in Nigeria showed contamination levels of DDT, endrin, dieldrin, and lindane in beans (P. vulgaris) collected from both field and storage facilities (Fadina et al., 2021). Adefemi et al. (2018) reported multi-residue OCs pesticide and health risk assessment in edible vegetables while Akande et al., (2020) also evaluated organophosphate insecticide in post–harvest cowpea in Gwagwalada Abuja. While Idowu et al. (2022) examined OCs residue in cocoa pods and beans. Similarly, Fagbohun et al. (2023b) and Fagbohun et al. (2024) assessed glyphosate residue in retailed cowpea and maize grains in FCT, Abuja markets respectively.
In Nigeria, to mitigate cowpea production and post-production losses, farmers and merchants apply insecticides such as OCs indiscriminately. This resulted into pesticide malpractices by many cowpea farmers and traders to preserve these legumes for future sale; therefore, most harvested cowpea has high pesticide residues (Anaduaka et al., 2023). Recently, “Nigeria was banned in 2015 by European Union (EU) for supplied of cowpea with dichlorvos pesticide residue at 0.3 mgkg-1 that exceeded the 0.1 mgkg-1 acceptable limits” (Idowu et al., 2022, Anaduaka et al., 2023, FAOSAT, 2023; Zu’amah et al., 2023). Nigeria-exported cowpea was found to contain other chemicals like Omethoate, Chlorpyrifos, Trichlorphon, Cyhalothrin, and Dimethoate at high, unacceptable levels. These indicate the use of the above pesticides either on the farm during cultivation or at storage (Salihu et al., 2023; Oshatunberu, 2023; FAOSAT, 2023). And as such, a lot of revenue was lost inform of FOREX.
To mitigate the risk to human health, regulatory authorities such Food and Agricultural Organization (FAO), the World Health Organization (WHO) and the CODEX Alimentarius Commission (CODEX) have previously set Maximum Residue Limits (MRLs) and Acceptable Daily Intake (ADI) for insecticide and other pesticides on various food crops, including cowpea grains (WHO, 2022; Bhawan et al., 2022). These MRLs are based on scientific studies and aim to ensure that the levels of insecticide residues in food products remain below the threshold deemed safe for human consumption. Furtherance to this, the researcher observed that there is a paucity of current information on the level of insecticide residue in retailed cowpea grains in the FCT, Abuja.
Ingesting cowpeas with high insecticide residue levels may lead to acute or chronic health effects. Acute effects can manifest shortly after consumption and include symptoms like nausea, vomiting, headache, or dizziness (Amare et al., 2022, Lastushkina et al., 2023, Fagbohun et al., 2023a). Chronic exposure to low levels of insecticide residues over time can lead to more serious health issues, including developmental and neurological disorders, hormonal imbalances, and even certain types of cancers. This has engendered research regarding the risks associated with the consumption of food stuffs containing the aforementioned insecticidal contaminants (Riaz et al. 2018, Mohammad et al., 2018).
Accumulation of OCs insecticide in cowpea grains poses health risks, necessitating an assessment to safeguard public health of the consumers. The environmental impact of OCs underscores the need to assess their presence to mitigate ecological damage. The EU ban on Nigerian cowpea exports due to pesticide violations highlights economic losses, emphasizing the need for mitigation of pesticide residue in food commodities in order to ensure effective regulation. Cowpeas are crucial for food security, requiring protection from contamination. Monitoring pesticide residues aligns with international standards and fills a critical knowledge gap, informing the regulatory agents to effectively mitigate contamination risks.
The objectives are to determine the prevalence of OCs residues in the study area, determine their concentrations and assess the associated health risks in adherence to international safety standards. Report from this surveillance study will serve as a reference material for policy makers in ensuring public health and food safety in the FCT Nigeria.
Materials and Methods
The study location
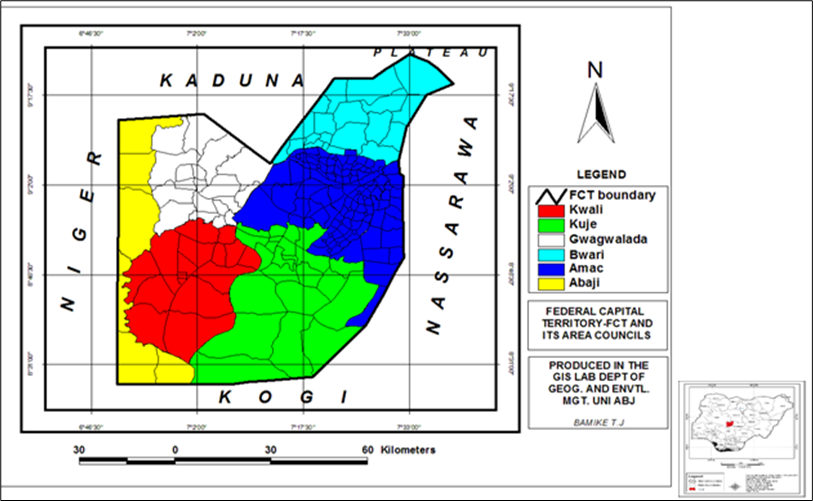
FCT - the administrative capital of Nigeria is one of Nigerian leading urbanized centers. Due to its centrality, the territory is well-connected and accessible from other states. Being in the Guinea Savanna zone and having favorable soil properties, cowpeas are commonly produced and sold in the FCT. The FCT is divided into six area councils; Kuje, Abaji, Bwari, Gwagwalada, Kwali, and Municipal Area Council (AMAC) (Fig.1.)
Sampling method
A total of 28 dried cowpea samples were purchased randomly from several traders from some selected markets in the six Area Councils of the FCT for the assessment of OCs residue and human health risk assessment as illustrated in the Table 1.
Table 1: Cowpea grain Samples, Location and Code
S/N | Location/Market | Cowpea Sample ID | Lab Code/ID |
1 | Abaji (A) | AW* | EISL/05/1360 |
2 | Gwagwalada (G)
| GI | EISL/05/1372 |
3 | GSW | EISL/05/1373 | |
4 | GB | EISL/05/1374 | |
5 | GW | EISL/05/1375 | |
6 | Kwali (KW) | KWW | EISL/05/1384 |
7 | T. Hospital (TH) | THB | EISL/05/1396 |
8 | Fish market (FM)
| FMW | EISL/05/1363 |
9 | FMI | EISL/05/1364 | |
10 | FMB | EISL/05/1365 | |
11 | Garki market (G’)
| G’D | EISL/05/1366 |
12 | G’O | EISL/05/1367 | |
13 | G’W | EISL/05/1369 | |
14 | Karimo market (K)
| KI | EISL/05/1379 |
15 | KW | EISL/05/1380 | |
16 | KWS | EISL/05/1382 | |
17 | Nyanya market (N)
| NI | EISL/05/1390 |
18 | NDB | EISL/05/1391 | |
19 | NBO | EISL/05/1392 | |
20 | NSW | EISL/05/1393 | |
21 | Utako market (U)
| UI | EISL/05/1398 |
22 | UDB | EISL/05/1399 | |
23 | UOB | EISL/05/1400 | |
24 | USW | EISL/05/1401 | |
25 | UW | EISL/05/1402 | |
26 | Wuse market (Wu)
| WI | EISL/05/1403 |
27 | WB | EISL/05/1404 | |
28 | WSW | EISL/05/1406 |
W=white bean, I= Iron beans, S = Small bean, B= Brown bean, D: Drum bean, O = Oloyin bean
In each market, five composite grains of the same variety were collected and bulked together. The collected samples were labeled, placed into sterile black polythene bags placed in ziplock bags under complete aseptic conditions and immediately transported to Chemistry Advanced Research Centre, Sheda Science and Technology Complex (SHESTCO) Abuja and kept in a -20 oC refrigerator pending analytical determination.
Reagents used and Sample Preparation in SHESTCO laboratory
The chemicals used were organochlorine pesticide (OCPs) standard, acetonenitrile, acetone, and methanol, all solvents are 99.90 % HPLC grade and purchased from Sigma-Aldrich USA. Besides, Sodium sulphate (Na₂SO4), Magnesium sulphate anhydrous fine powder (MgSO4), graphitized carbon black (GCB), primary secondary amine (PSA), disodium hydrogen citrate sesquihydrate (C6H6Na2O71.5H2O), trisodium citrate dehydrate (C6H2Na3O7.2H2O), sodium chloride (NaCl), Solid phase extraction tubes (SPE tubes), ceramic discs, all purchased from Bioccomma Limited, Hong Kong.
Foreign matters such as stone and admixtures were sorted out by handpicking. The samples were later pulverized with a laboratory blender (MasterChef) and then extracted and analyzed for the presence of OCPs in cowpea samples. Quick, Easy, Cheap, Efficient, rapid and safe (QuEChERS) method and dispersive liquid-liquid micro-extraction (DLLME) were used for sample extraction.
A QuEChERS-DLLME method previously described by Anastassiades et al., 2003; AOAC Official method, 2007-01) was used for extraction of the samples. Ten grams of finely pulverized sub-sample was transferred into a polypropylene centrifuge tube (50 mL) and 10 mL water was added. Followed by the addition of 15mL acetonitrile and the mixture vortex vigorously for 5 mins. Further, 0.5 g disodium hydrogencitrate sesquihydrate, 1g trisodium citrate dihydrate, 4 g anhydrous magnesium sulphate, and 1 g sodium chloride were added, and the mixture was immediately vortex for another five minutes, then centrifuged at 4500rpm for 5 min. At this stage, an optional low-temperature clean step was performed before dispersive-SPE for the most complex matrices such as beans. For this, an aliquot of the supernatant was transferred into a glass test tube and stored for at least 2 hours in a freezer (−20 oC). The extract was then separated from the precipitates by simple decantation. An aliquot of the extract was transferred into a solid-phase extraction tube containing 100mg anhydrous magnesium sulphate, 75 mg graphitized carbon black (GCB), and 20mg PSA per mL acetonitrile extract. The extract was eluted into a GC vial by gravity and acidified by adding 15µL of 5 % (v/v) formic acid in acetonitrile per mL of extract and analysed for OCs using GC-MS.
Instruments and equipment for GC-MS determination
The concentrations of OCPs in the sample extracts were determined using an Agilent HP-5-60 to 325 oC GC column (30 m × 320 m x 0.25 m film thickness) attached to a gas chromatograph (6890N Agilent technologies) and a mass selective detector (Agilent 5975B) (GC-MS). The volume of the sample injected in the splitless mode was 1µL. The initial oven temperature was maintained at 100 oC for 2 minutes, then increased to 180 oC at a rate of 15 o C/minute, ramped up to 300 oC at a rate of 3 oC/minute, and held for 9 minutes. The carrier gas was helium with a flow rate of 0.8 mL/min. The operation mode of the mass spectrometer was electron impact ionization with the use of automatic gain control. The storage window was programmed at full scan mode in the range of m/z 200–500, and the selected ion monitoring (SIM) mode was employed in acquiring data by Agilent Chemstation software.
Analytical Method Validation
Quality control: The glassware used was washed and rinsed with Milli-Q water and further with acetone before use. The effectiveness of the analytical procedure for the extraction of the OCs was evaluated by employing method blanks and matrix-spiked samples. Known standards of the OCs compounds were added to selected sample aliquots that have been previously analyzed and reanalyzed. The concentrations of OCs in the samples were quantified using the external calibration method.
Linearity of the Standard Curves: Calibration curves have been produced for quantification. Linearity has been observed all along the area of concentration studied depending on the target pesticide chemicals. These ranges of concentrations were selected in function of the sensitivity of the gas chromatography towards each pesticide from the correlation coefficient (r2) of the linear regression. The calibration curves were obtained by injecting eight different concentrations of the pesticide standards in a range of 4-300 ng/ml. The r2 values obtained from the plot of known concentrations of OCs against their peak areas ranged between 0.9997 and 0.9999.
Limits of Detection and Limits of Quantification: The limit of determination (LODs) of the OCs compound ranged from 0.03 to 0.20 ng g-1. Limits of detection (LOD) and limits of quantification (LOQ) of the method were measured by spiked serial dilution of working standards prepared for calibration curves and calculated by considering a value 3 and 10 times of background noise, respectively. LOD was determined considering it as 3 times the signal-to-noise ratio, while LOQ was determined as 10 times the signal-to-noise ratio. This means that LOD and LOQ were determined as the lowest concentrations yielding a signal-to-noise (S/N) ratio of 3 and 10, respectively.
Recovery Studies: The cowpea sample was spiked with a solution containing a mixture of the 20 OCs pesticide standards. A pesticide standard was spiked into a laboratory blank sample to give 0.25 mg/g, and recovery was based on 4 replicates. The spiked samples were left for 1 hour before extraction to allow the insecticide residue to partition into the matrices and the percentage recovery was obtained according to the following formula:
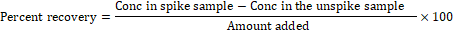
(Barriga- Vélez et al., 2023, Liao et al., 2018)
Human health risk assessment
The US EPA (2000) highlighted a range of standards and instructions on calculating the health risks associated with the consumption of grains contaminated with environmental pollutants. An assessment of health risks can be performed by comparing mean levels of contaminants with international guidelines. However, these assessments do not consider variables such as consumption rates and eating habits.
The estimated daily intake (EDI) of the 20 OCs pesticides (alpha-BHC, beta-BHC, gamma-BHC, heptachlor, delta-BHC, aldrin, heptachlor epoxide, gamma chlordane, alpha-chlordane, endosulfan I, P,p'-DDE , dieldrin, endrin, P,P'-DDD, endsulfan II, P,P'-DDT, endrin aldehyde, endosulfan sulfate methoxychlor, endrin ketone) were determined based on their mean concentration in each cowpea varieties and the daily intake in grams. The food supply value of cowpea was 18 kg/capita/year according to the Food and Agriculture Organization (FAO), 2000, the food supply value was divided by the number of days in the year (365 days). The result obtained was an intake of 0.04932, approximately 0.049 kg/capita/day, which is the food ingestion rate (FIR) of cowpeas in Nigeria.
Equation 1 was used for the calculation of the EDI (Antoine et al., 2017)
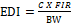
where C is the dry weight concentration of the organochlorine pesticides in the cowpea variety in mgkg-1, FIR is the daily FIR in kg/day and BW is the reference body weight of 60 kg for an adult human.
Hazard quotient
The hazard quotient (HQ) was regarded as the probable risk of undesirable health effects from pesticide mixtures to specify the long-term assessment of risk and was computed by dividing the EDI by the pertinent acceptable daily intake (ADI) and multiplying by 100, Equation 2 (Elgueta et al., 2017; Silipunyo et al., 2017)
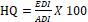
The ADI is defined as the quantity of a definite chemical that can be ingested daily for the life span of a human without substantial health risks (Verger, 2013).
The combined hazard index (CHI) indicates the interactive and/or additive effects upon the exposure of two or more pollutants. The US EPA has been used to estimate the risk posed by a group of pesticides that act by a common mechanism or that are toxicologically similar [26]. The combined hazard index is determined using Equation
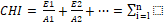
Where E1 E2 En and Ei are the estimated daily intakes of each individual pesticide in a mixture of n pesticides in the food sample, whereas A1 A2 An and Ai are the acceptable daily intakes (ADIs) for each pesticide.
Statistical analysis
The results obtained from the matrices were statistically analysed through MS Excel and SPSS version 21. Elements of descriptive statistics of samples generated included mean, range, minimum, maximum and standard deviations. The concentration of OC pesticide residues in cowpea samples was compared with the MRLs recommended by the European Union (2011). MRL of a pesticide is the maximum concentration of its residue that is legally permitted to remain in food after it has been treated with the pesticide (FAO, 2002).
Results and Discussion
From the recovery experiment, OCs insecticides were recovered in the range of 90.1 to 93.7%, and none of the OCs compounds was found in the blank sample. This was similar to the work done by Oyekunle et al., 2017 who recorded the percentage recovery of OCs residue in cocoa beans in Western Nigeria to be between 80.13 to 109.21%. These results show that the methods used in this study have a suitable range with good reproducibility. The limits of detection (LOD) determined as 1:3 noise to signal varied for the different organochlorine insecticides. They were generally in the range of 0.00003 to 0.00020 mg kg-1 (dry weight).
Cowpea samples were analysed for residues of 20 OCs compounds (Alpha-BHC, Beta-BHC, Gamma-BHC, Heptachlor, Delta-BHC, Aldrin, Heptachlor Epoxide, Gamma Chlordane, Alpha-Chlordane, Endosulfan I, P,p'-DDE, Dieldrin, Endrin,P,P'-DDD, Endsulfan II, P,P'-DDT, Endrin aldehyde, Endosulfan sulfate Methoxychlor, Endrin ketone) as displayed in the Table and figures 2,3a and 3b.
In terms of occurrence of these compounds, 89.29% of the cowpea samples were contaminated with P,P’-DDT, Endosulfan sulphate, Endrin ketone and, while aldrin, dieldrin and pp’- DDE residue contaminants occurred in 75% of all the samples analysed. Alpha BHC (32.14%) and Delta-BHC (57.14%) were among the OCs residues contaminated analysed samples. All the OCs contaminants were detected in the following samples from FMB, G’D, G’O, G’W, GI, GSW, GB and GW. The samples USWB NIB and NDB were contaminated with about 50 % and 35 % respectively of OCs insecticides. While none of the OCs residues were detected in the grains obtained from the Utako market (UW).
The mean residual concentration and the maximum values of the various OCs insecticides in the cowpea grain samples are presented in Table 2. It was further observed that endrin ketone had the highest mean value of 0.006633 mg kg-1 in the grains above the EU MRL of 0.005 mg kg-1. While endrin aldehyde0 had the lowest mean value of 0.000068 mg kg-1. The Isomers of BHC namely, Alpha-BHC, Beta-BHC, Gamma-BHC and Delta BHC were detected at a respective mean level of 0.00045, 0.00127, 0.00089 and 0.00014 mg kg-1 in the cowpea grains below the EU limit. Heptachlor epoxide (0.05014 mg kg -1) and Methoxychlor (0.01468 mg kg-1) slightly violated EU MRL at 0.020 mg kg -1. While aldrin and its metabolites: endrin, dieldrin, endrin aldehyde, and endrin ketone were detected below MRL at mean concentrations of 0.00093, 0.00048, 0.00046, 0.000068 and 0.06633 mg kg-1 were detected below the EU limit respectively. The mean levels of Alpha and Gamma Chlordane were 0.00067 and 0.00354 mg kg-1 respectively in the cowpea grains. Endosulfan I, endosulfan II, and endosulfan sulfate an organochlorine insecticide of the cyclodiene subgroup were detected at mean levels of 0.00037, 0.00921, and 0.00089 mg kg-1 respectively in the cowpea grain. Also, the mean concentration of the DDT and its metabolite namely, p,p 0-DDE and p,p 0- DDD in the samples were 0.00232, 0.00578 and 0.00074 mg kg-1 respectively. The total mean value mean concentration of OC residue in all the samples was 0.160558 mg kg-1.
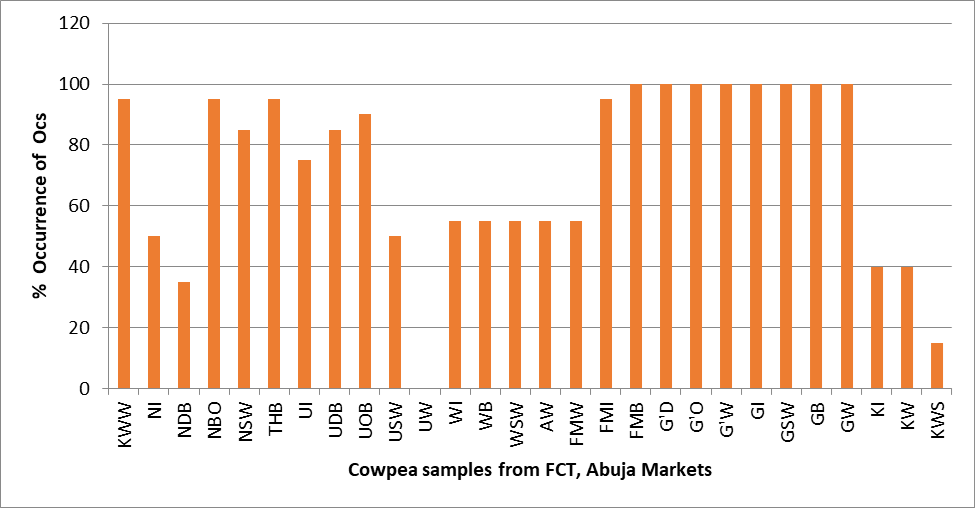
Figures 3a and 3b depict a 3D graphical illustration of the 20 OCs contamination profiles of cowpea grains together with their various limit in some markets in FCT, Abuja. In Figure 3a, the OCs concentration levels in first 14 samples were shown. Endrin ketone had high concentration in NDRB and NIB in order ranking while heptachlor epoxide residue level was highest UIB.
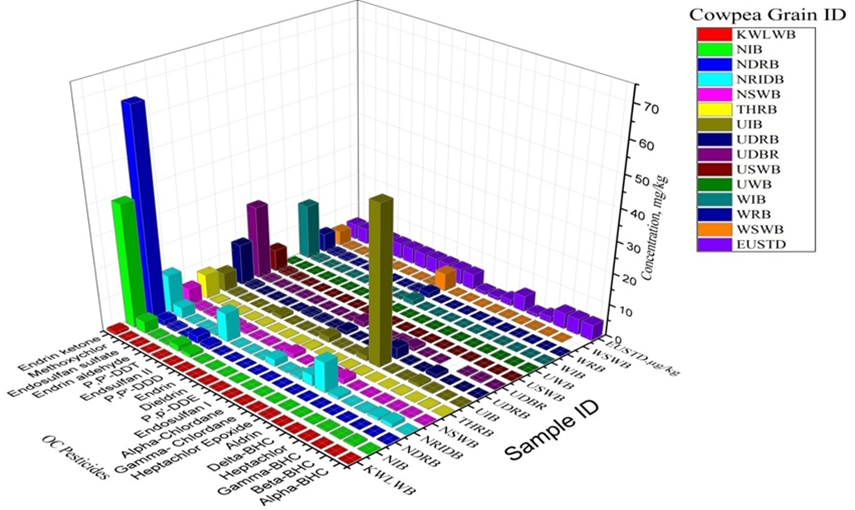
In Figure 3b, the OCs concentration levels in the other 14 samples were shown. Also, endrin ketone had high concentrations in the samples from KWB, GOB and GDB.
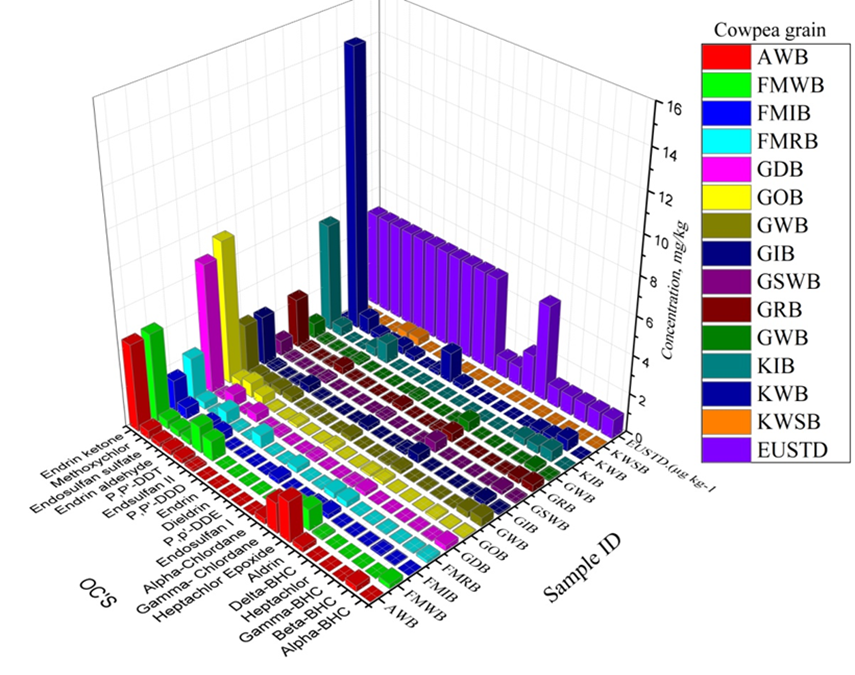
It was revealed that KWLWB and UWB were far below EU standard while samples NIB, NDRB, and UIB had the highest contamination of endrin ketone and heptachlor epoxide respectively. Figure 3b showed that some samples (KWSB, KWB, GWB and GOB) contaminated endrin ketone above the MRL.
Table 2: Mean Concentration of organochlorine pesticide residues detected in cowpea compared with EU MRL
| Pesticide | % contamination | Mean value (mg kg-1) | Max Conc. (mg kg-1) | EU MRLs (mg kg-1) |
| Alpha-BHC | 32.14 | 0.00045 | 0.0047 | 0.010 |
| Beta-BHC | 64.29 | 0.00127 | 0.00047 | 0.010 |
| Gamma-BHC | 64.29 | 0.00089 | 0.00036 | 0.010 |
| Heptachlor | 78.57 | 0.0012 | 0.00033 | 0.020 |
| Delta-BHC | 57.14 | 0.00014 | 0.00003 | 0.010 |
| Aldrin | 75 | 0.00093 | 0.00021 | 0.050 |
| Heptachlor Epoxide | 64.29 | 0.05014 | 0.0554 | 0.020 |
| Gamma- Chlordane | 64.29 | 0.00354 | 0.00128 | 0.010 |
| Alpha-Chlordane | 64.29 | 0.00067 | 0.00024 | 0.010 |
| Endosulfan I | 60.71 | 0.00037 | 0.0003 | 0.050 |
| P,p'-DDE | 75 | 0.00578 | 0.00578 | 0.050 |
| Dieldrin | 75 | 0.00046 | 0.00046 | 0.050 |
| Endrin | 64.29 | 0.00048 | 0.00697 | 0.050 |
| P,P'-DDD | 64.85 | 0.00074 | 0.00181 | 0.050 |
| Endsulfan II | 85.71 | 0.00921 | 0.00084 | 0.050 |
| P,P'-DDT | 89.29 | 0.00232 | 0.00441 | 0.050 |
| Endrin aldehyde | 85.71 | 0.000068 | 0.00958 | 0.050 |
| Endosulfan sulfate | 89.29 | 0.00089 | 0.00867 | 0.050 |
| Methoxychlor | 92.86 | 0.01468 | 0.00555 | 0.010 |
| Endrin ketone | 89.29 | 0.06633 | 0.1577 | 0.050 |
| Total OCs | 0.160558 |
Table 3: Health Risk Assessment of 20 organochlorine residue in Cowpea grains
| Pesticide | EDI | ADI | HI/CHI | Health Risk |
| Alpha-BHC | 0.0003699 | 0.005 | 0.007398 | No |
| Beta-BHC | 0.00000104394 | 0.0030 | 0.034798 | No |
| Gamma-BHC | 0.00000073158 | 0.0003 | 0.024386 | No |
| Heptachlor | 0.0009864 | 0.0001 | 0.9864 | No |
| Delta-BHC | 0.00000011508 | 0.005 | 0.0023016 | No |
| Aldrin | 0.00000076446 | 0.0001 | 0.76446 | No |
| Heptachlor Epoxide | 0.00004121508 | 0.0001 | 0.4121508 | No |
| Gamma- Chlordane | 0.00000290988 | 0.0005 | 0.581976 | No |
| Alpha-Chlordane | 0.00000055074 | 0.0005 | 0.110148 | No |
| Endosulfan I | 0.00000030414 | 0.0050 | 0.0060828 | No |
| P,p'-DDE | 0.00000475116 | 0.0200 | 0.0237558 | No |
| Dieldrin | 0.00000037812 | 0.0001 | 0.37812 | No |
| Endrin | 0.00000039456 | 0.0002 | 0.19728 | No |
| P,P'-DDD | 0.00000060828 | 0.0200 | 0.0030414 | No |
| Endsulfan II | 0.0000075707735 | 0.0080 | 0.09463275 | No |
| P,P'-DDT | 0.001000190704 | 0.0200 | 0.0095352 | No |
| Endrin aldehyde | 0.00000055896 | 0.0002 | 0.27948 | No |
| Endosulfan sulfate | 0.00000073158 | 0.006 | 0.00012193 | No |
| Methoxychlor | 0.00001206696 | 0.0050 | 0.2413392 | No |
| Endrin ketone | 0.00005452326 | 0.0002 | 27.26163 | Yes |
| Total | 31.42 |
With a view to understanding the health implications of consuming the cowpea samples contaminated with organochlorine insecticidal residue, a Health risk assessment was computed. The mean values for Health Risk Assessment and Health hazard indices systemic effects associated with insecticidal residues encountered in cowpeas are summarized in Table 3. The mean EDIs and ADIs of respective 28 cowpea samples showed that they were all within their respective ADIs. The ADI refers to the quantity of a definite chemical that can be ingested daily for a life span without substantial health risks. To understand the human health risk factor of contamination in food commodities, Joint Food and Agriculture Organization of the United Nations FAO/WHO Codex Alimentarius Commission has set the Acceptable daily intake (ADI) for different food grains and other food crops (Acquaah et al., 2007; Crépet, et al., 2021).
The mean value recorded for HI revealed that hazard indices of all the pesticides analysed were less than 1 except endrin ketone which was 27.26163. The hazard index of aldrin ketone in the cowpea samples >1, indicating a great potential for systemic toxicity with the consumption of cowpea. Endrin ketone is a highly toxic organic compound that is derived from endrin, a pesticide that was widely used in the past for the control of various agricultural pests (Chandra et al., 2021). It is a white crystalline solid and belongs to a class of chemicals known as cyclodiene organochlorine insecticides. Endrin itself is a cyclodiene organochlorine, and endrin ketone is one of its degradation products (Chandra et al., 2021). The total non-carcinogenic effect from the consumption of these cowpea samples is the sum of all the OCPs detected in the samples. From the result displayed in Table 3, it was observed that the combined hazard index is 31.42. A CHI exceeding 100 indicates that the cowpea varieties pose a risk to consumers. However, a CHI lower than 100 indicates that the cowpea types are fit for consumption (Elgueta et al., 2017, Alla et al., 2015).
Discussion
Cowpea grains suffer from insect damage, prompting insecticide use such as organochlorines (OCs). However, insecticide residues persist in grains, posing health risks to the consumers in Nigeria. In this work, mean OCs concentration residues were found in the cowpea at various concentrations. Mean values for endrin ketone, methoxychlor and heptachlor epoxide were found to violate EU standards at 0.06633, 0.01468 and 0.05014 mg kg-1 and the number of OCs detected ranged from 0.06633-0.000068 mg kg-1 while others were detected within the limit of regulatory body (EU). Organochlorine insecticide residues have been reported in different food commodities in Nigeria. Omokpariola et al. (2024) recorded between 0.048- 0.298 mg kg-1 for beans, 0.018- 0.337 mg kg-1for maize grains and 0.045-0.442 mg kg-1 for rice. Besides, Odion et al. (2023) also detected γ-HCH ranged between 0- 0.064 mg kg-1 and 0-11.42 mg kg-1 for DDT in cowpea grains obtained from some markets in southwest Nigeria. A study carried out in northeastern Nigeria showed different contamination levels of DDT, endrin, dieldrin and lindane in beans (Phaseolus vulgaris) collected from both field and storage facilities were above regulatory limits (Gwary et al., 2011). Adefemi et al. (2018) reported multi-residue organochlorine pesticide and health risk assessment in edible vegetables. In this study, Heptachlor Epoxide, Methoxychlor and Endrin ketone which are among the banned insecticides were detected even above the FAO/WHO MRLs. Also, Akande et al. (2020) reported the occurrence of organophosphate insecticide in cowpea grains obtained in Gwagwalada Abuja market which includes malathion (033, 0.38 mg kg-1), parathion (0.82, 0.6 mg kg-1), ethion (0.06, 0.09 mg kg-1) and carbophenothion (0.48, 0.37 mg kg-1) in both brown and white beans grain respectively and they were all above EU/FAO/WHO MRLs .
In this present study, the concentration of BHC metabolites ranged between 0.00014 - 0.0012 mg kg-1. This residual level was lower than the one reported by Fadina et al. (2021) in cowpea grain obtained from three markets located in Ibadan, Oyo State Nigeria. From the study, β and γ BCH were detected at 0.0038, 0.00029 and 0.0007 mg kg-1. In this current study, heptachlor epoxide, methoxychlor and endrin ketone were detected at 0.05014, 0.01468 and 0.06633 mg kg-1 respectively above the MRLs of regulatory bodies. While Fadina et al. (2021) detected lower values for heptachlor epoxide at 0.0462mg kg-1 and methoxychlor at 0.0005 mg kg-1 and higher values for endrin at 0.0165 mg kg-1 and endrin aldehyde at 0.0347 mg kg-1. The mean concentration values recorded for DDT, DDE and DDD in this work were lower than those of Fadina et al. (2021) who reported 0.005, 0.005 and 0.004 mg kg-1 respectively for the same OCs insecticides respectively. Oyekunle et al. (2017) also recorded higher mean residual concentration of 0.08217 mg kg-1 for p,p’ DDT and 0.37 mg/kg for Endrin in cocoa beans samples from Ile-Ife western Nigeria.
According to Diarra, (2021), OCs insecticides were also discovered in a pre-cooked Fonio sold in the store of Bamako, out of 36 samples tested, 29 (75%) were contaminated with different level OCs pesticides and the researcher obtained a similar value for DDT with a mean concentration range of 0.037 to 1.874 mg kg-1 when compared with value obtained in this study range from 0 to 2.32 mg kg-1. Adefemi et al. (2018) also reported some level of residual contamination of OCs pesticides in an edible vegetable with 83.3 % of the samples were contaminated with p,p’-DDT followed by Aldrin 75 % and this results was comparable with mean concentration values recorded for p,p’- DDT (0-89.29%) and Aldrin (0-75%) in this study. Akoto et al. (2013) conducted an analysis on post-harvest cowpea and maize grains in Ghana, identifying 15 organochlorine (OC) insecticides, including b-HCH, c-HCH, d-HCH, heptachlor, methoxychlor, aldrin, dieldrin, endrin, c-chlordane, a-endosulfan, b-endosulfan, endosulfan sulfate, p,p-DDT, p,p-DDE, and p,p-DDD, in both cowpea and maize grains and the values obtained were similar. The concentrations of OCs insecticides found in maize ranged from 0.001 to 0.103 mg/kg, while in cowpea, they ranged from 0.001 to 0.118 mg kg-1, indicating higher levels compared to those detected in the current study. Also, and specifically, Akoto et al. (2013) detected BHC and its isomers at concentrations ranging from 0.001 to 0.045 mg/kg in maize grains and from 0.002 to 0.025 mg/kg in cowpea. The mean concentration of heptachlor epoxide detected in this study (0.05014 mg/kg) was comparable to the findings of Akoto et al. (2013), who reported 0.005 mg/kg in maize and 0.010 mg/kg in cowpea. Methoxychlor concentrations in maize and cowpea detected by Akoto et al. (2013) were 0.002 mg/kg and 0.003 mg/kg, respectively, slightly higher than those detected in the present study. Akoto et al. (2013) also reported detection of p,p-DDE and its metabolites at a similar concentration of 0.002 mg kg-1. Additionally, a study conducted in Nigeria revealed contamination levels of DDT, endrin, dieldrin, and lindane in cowpea collected from both field and storage facilities (Gwary et al., 2011). Okoye et al. (2021) detected DDT and its metabolites in a range of 0-0.02 mg/kg, similar to the values obtained in this study for certain cowpea varieties sourced from Ogige markets in Nsukka, eastern Nigeria.
The National Agency for Food and Drug Administration and Control (NAFDAC), responsible for regulating food standards in Nigeria, has banned several pesticides detected in this study for agricultural and industrial uses. Among them, Hexachlorocyclohexane (HCH) or benzene hexachloride (BHC) and its isomers, historically employed to combat various pests, are now prohibited. Similarly, Heptachlor and its isomer, heptachlor epoxide, belonging to the cyclodiene group, along with Alpha and Gamma Chlordane, once used extensively for pest control, are now banned by NAFDAC. Endosulfan and its metabolites, which act as insect poisons and acaricides. Environmental concerns and risks to human health led to the banning of aldrin and dieldrin by the Environmental Protection Agency (EPA) in 1974, except for termite control, with a complete ban enforced in 1987, a measure mirrored in Nigeria.
Furthermore, despite DDT, DDE, and DDD concentrations in this study not exceeding the EU standard of 0.05 mg kg-1, NAFDAC has banned their use as agrochemicals in Nigeria. DDT, which degrades slowly into dichlorodiphenyldichloroethylene (p,p0-DDE) and dichlorodiphenyldichloroethane (p,p 0-DDD) under aerobic conditions, is banned for agricultural use by NAFDAC. The global effort to control or ban DDT usage, spearheaded by the United Nations Environment Program since the 1970s, led to the creation of the Stockholm Convention on Persistent Organic Pollutants (POPs), recognizing the widespread presence of DDT worldwide and its adverse environmental and health impacts.
Conclusion
This study revealed occurrence of banned OCs insecticide prevalence ranged between 89.20-32.14% in cowpea samples. Methoxychlor and endrin ketone were found to violate MRLs specifications of CODEX Alimentarius Commission and EU. Besides, some banned OCs insecticides such as aldrin, dieldrin, endrin, p,p'-DDT, p,p'-DDE, p,p'-DDD BHC, endosulfan, chlordane, heptachlor, and heptachlor epoxide were found in some of the samples at different concentrations. Although, the mean values for respective EDIs did not exceeded their ADIs and study of Hazard index indicated that there was no health Risk for all the OCs insecticide except for aldrin ketene that has value greater one. Nonetheless, the combined health index (CHI) is not up to 100 in value. However, consumers of cowpea grains contaminated with OCs insecticide in the research area might be at risk surpassing one in a million individuals which could potentially induce cancer. The detection of these OCs insecticides residues in cowpea indicates a persistent threat to human health, particularly in terms of cancer risks.
It is important that monitoring and regulation of organochlorine usage in food commodities in Nigeria should not be taken with levity. Regulatory agencies in Nigeria should implemented measures to monitor pesticide residues in food, enforce pesticide regulations, and promote the safe and responsible use of pesticides.
Acknowledgement
Authors are highly grateful to Tertiary Education Trust Fund, Ministry of Education, Nigeria, for sponsoring of the research through Institutional Based Research and the management of Chemistry Advanced Research Centre, Sheda Science and Technology Complex for providing necessary facilities to carry-out part of this work.
Conflict of Interest
Arthurs declare no conflicting interest
References
- Abebe, B. K., & Alemayehu, M. T. (2022). A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. Journal of Agriculture and Food Research, 10, 100383.
- Acquaah G.D. 2020. Level of organo-chlorine pesticides residues in meat,” International Journal of Environmental Science and Technology, vol. 4, no. 4, pp. 521–524, 2007.
- Adefemi, S. O., Asaolu, S. S., Ibigbami, O. A., Orege, J. I., Azeez, M. A., & Akinsola, A. F. (2018). Multi-residue levels of persistent organochlorine pesticides in edible vegetables: a human health risk assessment. Journal of Agricultural Chemistry and Environment, 7(4), 143-152.
- Akande, M. G., Sanni, F. S., & Enefe, N. G. (2021). Assessment of the concentrations and health risk of some heavy metals in cowpea (Vignus unguiculata) in Gwagwalada, Nigeria. Drug and Chemical Toxicology, 44(5), 518-523.
- Akoto, O., Andoh, H., Darko, G., Eshun, K., & Osei-Fosu, P. (2013). Health risk assessment of pesticides residue in maize and cowpea from Ejura, Ghana. Chemosphere, 92(1), 67-73.
- Alla, S. A. G., Loutfy, N. M., Shendy, A. H., & Ahmed, M. T. (2015). Hazard index, a tool for a long term risk assessment of pesticide residues in some commodities, a pilot study. Regulatory Toxicology and Pharmacology, 73(3), 985-991.
- Amare, C., Golibe, E., & Adelusi, A. (2022). Risks Associated with the Use of Insecticides in Cowpea Conservation. International Journal Papier Advance and Scientific Review, 3(1), 1-7.
- Amusat, A. I., Okewole, S. A., Rafiu, R. A., & Amusat, M. A 2019. Overview of Pesticide Usage, Misuse and Its Impact on Environmental Degradation in South-western States and Some Part of Northern States in Nigeria. Journal of Pesticide Science and Pest Control, 2(1), 2833-0943.
- Anaduaka, E. G., Uchendu, N. O., Asomadu, R. O., Ezugwu, A. L., Okeke, E. S., & Ezeorba, T. P. C. (2023). Widespread use of toxic agrochemicals and pesticides for agricultural products storage in Africa and developing countries: Possible panacea for ecotoxicology and health implications. Heliyon.
- Anastassiades, M., Lehotay, S. J., Štajnbaher, D., & Schenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC international, 86(2), 412-431.
- Antoine, J. M., Fung, L. A. H., & Grant, C. N. (2017). Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicology reports, 4, 181-187.
- Barriga-Vélez, M. A., Ramírez-Vargas, L. C., Lopez-Barrera, E. A., & Peña-Rincón, C. A. (2023). Potential ecological risk index for metals in a grazing area, Guasca, Cundinamarca. Revista Facultad de Ingeniería Universidad de Antioquia, (106), 103-112.
- Bhawan, F. (2022). Guidance document & standard operating procedures for fixation of maximum residue limits (MRLs) of pesticides in food commodities.
- Chandra, R., Sharpanabharathi, N., Prusty, B. A. K., Azeez, P. A., & Kurakalva, R. M. (2021). Organochlorine pesticide residues in plants and their possible ecotoxicological and agri food impacts. Scientific reports, 11(1), 17841.
- Crépet, A., Luong, T. M., Baines, J., Boon, P. E., Ennis, J., Kennedy, M., ... & Verger, P. (2021). An international probabilistic risk assessment of acute dietary exposure to pesticide residues in relation to codex maximum residue limits for pesticides in food. Food Control, 121, 107563.
- Diarra, N. (2021). Research and Evaluation of Organochlorine Pesticides in the Precooked Fonio Sold in the Food Stores of Bamako. Sch Acad J Biosci, 11, 333-338.
- Elgueta, S., Moyano, S., Sepúlveda, P., Quiroz, C., & Correa, A. (2017). Pesticide residues in leafy vegetables and human health risk assessment in North Central agricultural areas of Chile. Food Additives & Contaminants: Part B, 10(2), 105-112.
- Ezeaku, E. I. 2015. Productivity of grain cowpea (Vigna unguiculata (L.) Walp.) as influenced by season, genotype, insect pest management and cropping system in southeastern Nigeria (Doctoral dissertation).
- Fadina, O. O., Daodu, B. J., Fayinminnu, O. O., & Nwanguma, C. S. (2021). Determination of Organochlorine Residues in Cowpea (Vigna unguiculata L. WALP) From Selected Markets in Ibadan, Oyo State, Nigeria. Journal of Agricultural Studies, 9(4), 72-86.
- FAGBOHUN, A. A., Dauda, M. S., & Anjorin, T. S. (2023). A Review on Global Pesticide Use and Food Contamination: Africa perspective. Pollution.
- Fagbohun, A. A., Dauda, M. S., & Anjorin, T. S. (2023). Comparative profile of glyphosate residues in cowpea grains sold in the municipal and satellite towns of Abuja, Nigeria.
- Fagbohun, A. A., Dauda, M. S., & Anjorin, T. S. (2024). Assessing the Profile of Glyphosate Residues in Maize Grains Sold in the Federal Capital Territory Abuja, Nigeria. Journal of Science and Mathematics Letters, 12(2), 25-33.
- FAO, Rome & ICRISAT, Addis Ababa. 2020. World Food and Agriculture – FAO Statistical Yearbook 2020 176 pp. Rome.
- FAOSTAT 2023. Crops and Livestock Products. Available at https://www.fao.org/faostat/en/#data/QCL Accessed October 16, 2023.
- FAOSTAT, F. 2020. Food and agriculture data. 2019. Available at: http://www. fao.org/faostat/en/#data/EP/visualize (Accessed October 2022).
- Gabi, A. U., Salihu, I. M., Hamza, U. I., Yahaya, I., Muhammad, H. M., Aliyu, A. D., & Ndayako, H. H. 2022. Effects of Callosobruchus maculatus Infestation on the Proxi mg kg-1 mate Composition of Cowpea (Vigna unguiculata L.) Sold in Lapai Market. UMYU Scientifica, 1(1), 204-210.
- Gwary, O. M., Hati, S. S., Dimariand, G. A., & Ogugbuaja, V. O. (2011). Pesticide residues in bean samples from Northeastern Nigeria. significance, 1(2).
- Idowu, G. A., Aiyesanmi, A. F., & Oyegoke, F. O. (2022). Organochlorine pesticide residues in pods and beans of cocoa (Theobroma cacao L.) from Ondo State Central District, Nigeria. Environmental Advances, 7, 100162.
- Jayaraj, R., Megha, P., & Sreedev, P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary toxicology, 9(3-4), 90-100.
- Kamara, A. Y., Omoigui, L. O., Kamai, N., Ewansiha, S. U., & Ajeigbe, H. A. (2018). Improving cultivation of cowpea in West Africa.
- Lastushkina, E., Telichko, O., Syrmolot, O., & Belova, T. (2023). Using insecticides for the protection of maize plants against the Asian corn borer. In BIO Web of Conferences (Vol. 71, p. 01101). EDP Sciences.
- Liao, Y., Berthion, J. M., Colet, I., Merlo, M., Nougadère, A., & Hu, R. (2018). Validation and application of analytical method for glyphosate and glufosinate in foods by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1549, 31-38.
- Lotti, M., & Bleecker, M. L. (2015). The neurotoxicity of organochlorine and pyrethroid pesticides. Occupational Neurology, 135.
- Martín-Durán, J. M., & Hejnol, A. (2021). A developmental perspective on the evolution of the nervous system. Developmental biology, 475, 181-192.
- Mohammad, A. M., Chowdhury, T., Biswas, B., & Absar, N. (2018). Food poisoning and intoxication: A global leading concern for human health. In Food safety and preservation (pp. 307-352). Academic Press.
- Namiki, S., Armstrong, J. D., Börner, J., Card, G., Costa, M., Dickinson, M., ... & Shepherd, D. (2020). A systematic nomenclature for the Drosophila ventral nerve cord. Neuron, 107(6), 1071-1079.
- Nwagboso, C., Andam, K. S., Amare, M., Bamiwuye, T., & Fasoranti, A. (2024). The economic importance of cowpea in Nigeria trends and Implications for achieving agri-food system transformation.
- Odion, E. E., Abolagba, J. O., Igene, J. O., & Folajole, S. (2020). Levels of Organochlorine in Cowpea from South-West Nigeria using Gas Chromatography-Mass Spectroscopy. South Asian Research Journal of Agriculture and Fisheries, 2, 3.
- Ogwo, E. I. (2021). Assessing Pesticide Use, Human Exposure and Environmental Fate in Nigeria. Lancaster University (United Kingdom).
- Omokpariola, P. L., Okoye, P. A., Okechukwu, V. U., & Omokpariola, D. O. (2024). Concentration levels and risk assessment of organochlorine and organophosphate pesticide residue in selected cereals and legumes sold in Anambra State, south-eastern Nigeria. Physical Sciences Reviews, 9(3), 1353-1373.
- Oshatunberu, M. A. (2023). Evaluation of Pesticide Residues in Grains Sold at Selected Markets of Southwest Nigeria (Doctoral dissertation, Kwara State University (Nigeria)).
- Oyekunle, J. A. O., Akindolani, O. A., Sosan, M. B., & Adekunle, A. S. (2017). Organochlorine pesticide residues in dried cocoa beans obtained from cocoa stores at Ondo and Ile-Ife, Southwestern Nigeria. Toxicology reports, 4, 151-159.
- Riaz, G., Tabinda, A. B., Kashif, M., Yasar, A., Mahmood, A., Rasheed, R., ... & Mahfooz, Y. (2018). Monitoring and spatiotemporal variations of pyrethroid insecticides in surface water, sediment, and fish of the river Chenab Pakistan. Environmental Science and Pollution Research, 25, 22584-22597.
- Richard, I. B., Goni, A., Usman, D., Sharifah, S. G., Gaya, H. I. M., & Veronica, N. N. (2023). Occurrence of deteriorating seed-borne pathogens and weevils (Callosobruchus maculatus (f.) Bruchidae coleoptera) and their economic impacts on marketing of stored cowpea grains in maiduguri, borno state. FUDMA JOURNAL OF SCIENCES, 7(3), 168-176.
- Rincón-Rubio, A., Mérida-Ortega, Á., Ugalde-Resano, R., Gamboa-Loira, B., Rothenberg, S. J., González, F. B., ... & López-Carrillo, L. (2024). Carcinogenic, non-carcinogenic risk, and attributable cases to organochlorine pesticide exposure in women from Northern Mexico. Environmental Monitoring and Assessment, 196(5), 421.
- Rolania, K., Yadav, S. S., Singh, B., Yadav, J. L., Kumar, N., & Pilania, S. (2021). Assessment of losses due to pulse beetle in chickpea under stored conditions in Southern Haryana. Journal of Agriculture and Ecology, 12, 98-105.
- Ruan, T., Li, P., Wang, H., Li, T., & Jiang, G. (2023). Identification and prioritization of environmental organic pollutants: from an analytical and toxicological perspective. Chemical Reviews, 123(17), 10584-10640.
- Salihu, A. M., Ndamitso, M. M., Mathew, J. T., Etsuyankpa, M. B., Gwadabe, N. K., & Ajai, A. I. (2023). Determination of Pesticide Residues in Selected Cereals Crops Sold in Some Markets in Gbako Local Government, Niger State.
- Shah, R. (2020). Pesticides and human health. Emerging contaminants, 57-78.
- Silipunyo, T., Hongsibsong, S., Phalaraksh, C., Laoyang, S., Kerdnoi, T., Patarasiriwong, V., & Prapamontol, T. (2017). Determination of organophosphate pesticides residues in fruits, vegetables and health risk assessment among consumers in Chiang Mai Province, Northern Thailand. Res. J. Environ. Toxicol, 11(1), 20-27.
- Singh, S., Gupta, R., Kumari, M., & Sharma, S. (2015). Nontarget effects of chemical pesticides and biological pesticide on rhizospheric microbial community structure and function in Vigna radiata. Environmental Science and Pollution Research, 22, 11290-11300.
- Sosan, M. B., Adeleye, A. O., Oyekunle, J. A. O., Udah, O., Oloruntunbi, P. M., Daramola, M. O., & Saka, W. T. (2020). Dietary risk assessment of organochlorine pesticide residues in maize-based complementary breakfast food products in Nigeria. Heliyon, 6(12).
- Tadesse, M. (2020). Post-harvest loss of stored grain, its causes and reduction strategies. Food Science and Quality Management, 96, 26-35.
- Thakur, N., Nigam, M., Mann, N. A., Gupta, S., Hussain, C. M., Shukla, S. K., ... & Khan, S. A. (2023). Host-mediated gene engineering and microbiome-based technology optimization for sustainable agriculture and environment. Functional & Integrative Genomics, 23(1), 57.
- Tripathi, A. K. (2018). Pests of stored grains. Pests and their management, 311-359.
- Van den Bosch, R. (2023). The pesticide conspiracy. Univ of California Press.
- Verger, P. J. P. (2013). Risk analysis paradigm and total diet studies. In Total diet studies (pp. 19-25). New York, NY: Springer New York.
- Wahab, A., Hod, R., Ismail, N. H., & Omar, N. (2016). The effect of pesticide exposure on cardiovascular system: a systematic review. Int J Community Med Public Health, 3(1), 1-10.
- World Health Organization. (2022). Pesticide residues in food: 2021: toxicological evaluations: Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, virtual meeting, 6–17 September, 4 and 7 October 2021.
- Zu’amah, H., Harsanti, E. S., Wihardjaka, A., & Ardiwinata, A. N. (2023, September). Distribution of chlorpyrifos residue in maize (Zea mays). In IOP Conference Series: Earth and Environmental Science (Vol. 1230, No. 1, p. 012075). IOP Publishing.


