Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-2322/001
Optimization and Validation of Glycerol-induced Acute Renal Failure Model in Rats in Predicting the Pharmacokinetics in Patients with Acute Renal Failure
Department of Pharmacology, A.U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, Andhra Pradesh, India.
Correspondng Author: Ramachandra Rao Sangana*
Citation: Ramachandra Rao Sangana, Mallik Samarla (2022). Optimization and Validation of Glycerol-induced Acute Renal Failure Model in Rats in Predicting the Pharmacokinetics in Patients with Acute Renal Failure. Pharmacy and Drug Development. 1(1). DOI: 10.58489/2836-2322/001
Copyright: 2022 Ramachandra Rao Sangana, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-06-30, Received Date: 2022-06-30, Published Date: 2022-07-18
Abstract Keywords: Acute renal failure, glycerol, exposure, half-life, clearance, dose adjustment
Abstract
The study standardized the glycerol induced (intra-peritoneal and intra-muscular) acute renal failure (ARF) models in Sprague-Dawley rats and validated its potential to predict the change in pharmacokinetics of the selected probe drugs in renal impairment. We assessed the induction of ARF by measuring the serum creatinine and blood-urea-nitrogen (BUN) levels at different time points (baseline, 6 h, 12 h, 24 h, 48 h and 72 h). Glycerol administrations via both intra-peritoneal and intra-muscular routes caused a significant increase in serum creatinine and BUN levels in rats at 6 h; however, the levels decreased within 24 h with intra-peritoneal administration. In contrast, with intra-muscular administration, a sustained increase in serum creatinine and BUN levels was observed up to 72 h post glycerol treatment demonstrating a sustained ARF like condition in rats that was suitable to evaluate the pharmacokinetics of drugs. Therefore, intra-muscular glycerol administration model was used to evaluate the pharmacokinetics of selected probe drugs including metformin, digoxin, atorvastatin and linezolid. The pharmacokinetics of metformin and digoxin were significantly altered in ARF versus control rats with increase in plasma exposure, prolonged elimination half-life and decreased systemic clearance. However, the pharmacokinetics of atorvastatin and linezolid did not show significant differences between ARF and control rats. These results demonstrated the utility of the ARF model in rats in predicting pharmacokinetic changes in patients with renal impairment. Such models may prove useful in early discovery work to screen and prioritize compounds requiring dose adjustment in patients with renal impairment.
1. Introduction
Drug attrition in late phase clinical development or post-marketing stage causes a serious economic problem to the pharmaceutical industries [1] and unfavorable pharmacokinetic properties [2] are one of the major reasons for this. Therefore, optimizing pharmacokinetic parameters during early phase of drug development and identifying potential challenges with absorption, distribution, metabolism, and excretion (ADME) are prerequisite. Further, in disease conditions, important alterations in drug disposition, metabolism, and/or absorption occur compared to the healthy condition. In hepatic and renal impairment, the intrinsic capacity of the organs to metabolize/excrete drugs are compromised, and thereby affect the pharmacokinetics. Therefore, pharmacokinetics of drugs under these pathological conditions should be assessed in clinical studies to provide alternative dosing recommendations [3].
As majority of the drugs are eliminated by metabolism (mainly in the liver) and/or excretion (mainly via kidney), prediction of hepatic and renal clearances (Cl) is important in predicting their pharmacokinetics. For prediction of hepatic Cl, in vitro models comprising of liver microsomal preparations, isolated hepatocytes, 9000g supernatant (S9) fractions, liver slices or in situ gastrointestinal/liver single-pass perfusion preparations are generally used [2]. Although renal Cl in humans can be predicted using allometric scaling [4], the practical value of this approach is limited by the requirement of pharmacokinetic data in four to five animal species [5]. Further, the ability of this technique for accurate prediction of the renal clearance of a diverse set of drugs that exhibit both active secretion and net reabsorption is not also well known [6]. In absence of an in vivo mass balance study in humans, information on the renal Cl of a candidate drug might not be reliable. In this context, validated animal models in early drug discovery which could predict the possibility of a change in human hepatic or renal Cl in disease conditions, and thus affecting the pharmacokinetic profile help screen and prioritize compounds based on their potential for dose adjustment.
Acute renal failure (ARF), occurs in approximately 5% of hospitalized patients where glomerular filtration rate (GFR) decreases over days to weeks resulting in reduction in excretion of nitrogenous waste leading to fluid and electrolyte imbalances [7]. Patients with ARF are often asymptomatic, and the condition is diagnosed by observed elevations of blood urea nitrogen (BUN) and serum creatinine levels. Because of lack of consensus on ARF definition, comparison between different types is difficult in critically ill patients [8]. Therefore, we aimed to optimize a rat model for ARF to predict the possibility of pharmacokinetic changes in patients with renal impairment. Among the various models used for ARF, glycerol-induced hemoglobinuria in rats [9]has been widely used model for ARF. The pathogenic mechanisms involved in glycerol-induced renal failure include ischemic injury, tubular nephrotoxicity caused by myoglobin, and the renal actions of cytokines released after rhabdomyolysis [10,11]. In appearance, distribution, and reversibility, the histologic lesions produced during disease induction closely resemble the lesions seen in the human kidney with ARF [12].
In this study, we standardized the glycerol induced ARF model in rats and validated its potential to predict the change in pharmacokinetics of the selected probe drugs in renal impairment.
2. Materials and methods
2.1. Chemical and reagents
Glycerol, Metformin, Digoxin and Linezolid were procured from Sigma Aldrich, USA. Atorvastatin was obtained as a gift sample from Dr Reddy’s Labs, Hyderabad, India. All the other chemicals were of analytical grade and were purchased from commercial suppliers.
2.2. Experimental animals
Male Sprague–Dawley (SD) rats of weight range 200–250 g were procured from Piramal Enterprises Ltd, Goregaon, Mumbai and housed under ideal laboratory conditions (temperature 23–25°C, 12 h light/12 h darkness cycle, 45–55% relative humidity) and maintained on standard pellet diet and water libitum throughout the experimental period. The animals were deprived of water for at least 24 h before glycerol administration
2.3. Experimental design
The study was conducted in two phases.
Phase I: In the initial phase of the study, we standardized the glycerol induced ARF model in SD rats.
Phase II: In the second phase, we selected the suitable animal model of ARF for pharmacokinetic evaluation of drugs, and studied the pharmacokinetics of selected drugs.
2.3.1. Phase I: Development of animal model (glycerol induced ARF in SD rats)
Rats were deprived of water for 24 h prior and were divided into two groups (n=6 per group): Group 1 received 50% (v/v) glycerol in 0.9% saline via intra-peritoneal route (10 mL/kg), and Group 2 received 50% (v/v) glycerol in 0.9% saline at a dose volume of 10 mL/kg in divided doses via intra-muscular administration into the hind limbs under a light anesthesia with ether. These rats were studied up to 72 h post glycerol treatment. To assess the degree of nephrotoxicity, serum creatinine and blood urea nitrogen (BUN) levels were measured with the standard spectrophotometric assay kits. Blood samples were collected from both the groups at pre-glycerol treatment, 6, 12, 24, 48 and 72 h post glycerol treatment.
2.3.2. Phase II: Validation of the animal models
Metformin, digoxin, atorvastatin and linezolid were selected as probe drugs to validate the glycerol induced ARF models. These probe drugs were selected on the basis of clinical data indicating that metformin and digoxin require dose adjustment in renal impaired patients while atorvastatin and linezolid do not require dose adjustment.
2.3.3.1. Drug administration
Metformin, digoxin, linezolid and atorvastatin were formulated in a 2.5 µL/mL Tween 80 and 0.5% methyl cellulose suspension (q.s.). The drugs were administered orally (10 mL/kg) at 10 mg/kg dose, to control and ARF rats (n=4 per group). Blood samples were collected through retro orbital sinus at pre-dose, and 0.5, 1.0, 2.0, 4.0, 8.0, 24.0, 36.0 and 48.0 h post-dose. Plasma drug concentrations were analyzed by LCMS/MS.
2.3.3.2. Estimation of pharmacokinetic parameters
Pharmacokinetic analysis was performed by non-compartmental analysis using Winnonlin software (Version: 5.2.1) to determine plasma exposure (area under the plasma drug concentration-time curve [AUC]), half-life (t1/2), and Cl.
2.3.3.3. Ethical approval
The study was approved by Institutional Animal Ethics Committee (IAEC) and conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (1246/09/CPCSEA dated May 11, 2009).
2.4. Statistical analysis
Data was analyzed using parametric test of student paired ‘t’ test with the help of Graph Pad® Prism software (Trial version).
3. Results
3.1. Phase I: glycerol induced ARF in SD rats
Serum creatinine and BUN were used to assess the degree of ARF induced by glycerol administration. The values are shown in the Table 1.
Mean serum creatinine levels (mg/dL) significantly (p0.05) increased from 0.68 at baseline to 0.98 at 6 h after intra-peritoneal administration of glycerol. Similarly, a significant (p0.05) increase in mean BUN levels (mg/dL) from 19.39 at baseline to 37.56 at 6 h post intra-peritoneal glycerol administration was also observed. By the end of 24 h, both serum creatinine and BUN levels decreased and approached the baseline levels indicating a reversal in nephrotoxicity.
Following glycerol administration via intra-muscular route, serum creatinine and BUN levels (mg/dL) significantly (p0.05) increased from baseline to 6 h post intra-muscular glycerol administration: mean serum creatinine levels increased from 0.51 at baseline to 1.00, and mean BUN levels increased from 12.54 at baseline to 26.26 post 6 h intra-muscular glycerol administration. These levels (mg/dL) continued to increase up to 24 h post-administration (at 24 h: serum creatinine, 1.62; BUN, 42.58; p 0.05 vs baseline). The elevated levels were maintained at least for 48 h post glycerol administration.
Table 1. Serum creatinine and BUN levels in intra-peritoneal and intra-muscular glycerol treated groups
| Route of administration of glycerol | ||||
| Time (h) | Intra-peritoneal | Intra-muscular | ||
| Serum creatinine (mg/dL) | BUN (mg/dL) | Serum creatinine (mg/dL) | BUN (mg/dL) | |
| Baseline | 0.68±0.05 | 19.39±4.03 | 0.51±0.10 | 12.54±3.39 |
| 6 | 0.98* ±0.16 | 37.56* ±5.60 | 1.00*±0.24 | 26.26*±5.50 |
| 12 | 0.76±0.09 | 35.50* ±4.46 | 1.11*±0.31 | 29.86*±5.39 |
| 24 | 0.63±0.10 | 17.84±1.17 | 1.62*±0.30 | 42.58*±4.81 |
| 48 | 0.60±0.06 | 15.03±1.51 | 1.34*±0.26 | 46.90*±6.08 |
| 72 | 0.70±0.06 | 16.08±4.10 | 0.97*±0.26 | 27.48*±4.54 |
All values are expressed as mean±SD (n=6); *significant (p <0> BUN, blood urea nitrogen; SD, standard deviation | ||||
3.2. Phase II: validation of the intramuscular glycerol model
As intra-muscular administration of glycerol showed significant and sustained increase in serum creatinine and BUN levels compared to control animals, which is suitable for evaluation of pharmacokinetics of drugs, we selected the intra-muscular glycerol model in phase 2 of our study for the validation of the model.
There was a significant increase (p0.05) in mean plasma exposure (AUC0-t) to metformin and digoxin in animals with ARF (10361.6 and 3365.3 ng*h/mL, respectively) versus control (2592.2 and 1369.5 ng*h/mL, respectively). The plasma drug concentration versus time profiles of metformin and digoxin in control and ARF animals are presented in Figures 1 and 2.
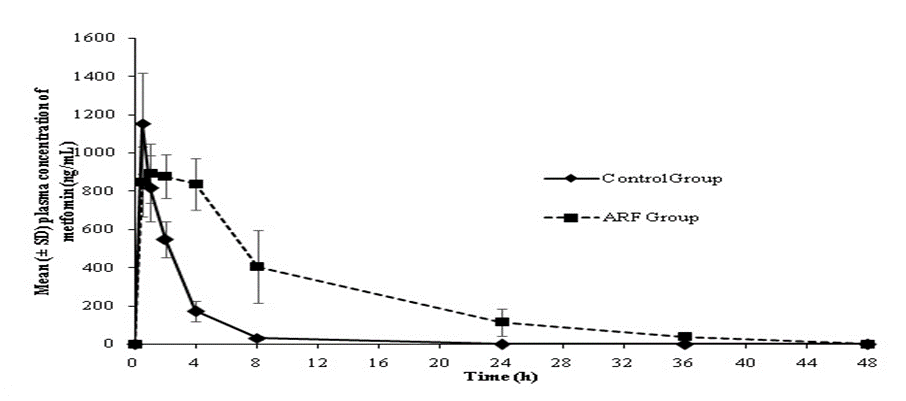
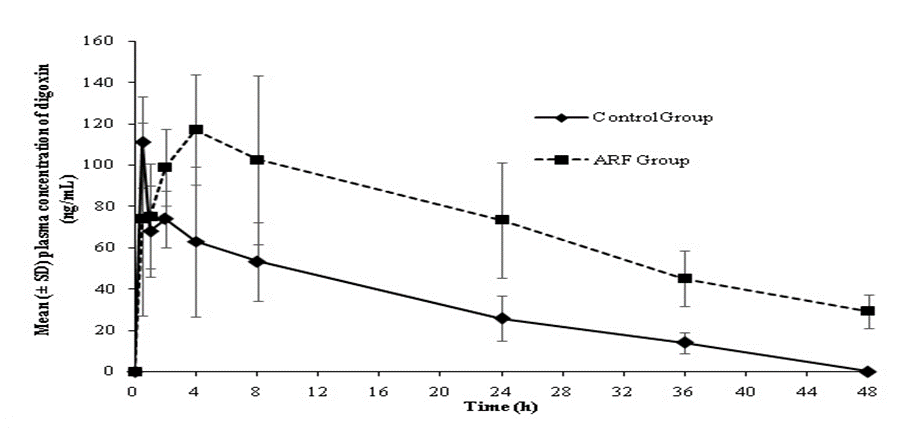
Further, a significant increase in t1/2 and decrease in plasma Cl of both drugs in ARF animals was observed compared to the control animals. The average t1/2 of metformin increased from 1.46 h in control animals to 7.1 h in ARF animals. Similarly, the t1/2 of digoxin increased from 11.71 h in control animals to 21.35 h in ARF animals. The mean plasma Cl (mL/min/kg) of metformin (15.69 vs. 63.29) and digoxin (40.7 vs. 112.5) decreased in ARF animals versus control animals (Table 2).
Table 2: Pharmacokinetic parameters of metformin and digoxin in control and ARF induced rats
| Pharmacokinetic parameter | Metformin | Digoxin | |||
| Control | ARF | Control | ARF | ||
| AUC0-t, ng*h/mL | 2592.22±345.30 | 10361.59±2793.81* | 1369.51±447.56 | 3365.28±1062.75* | |
| Cl, mL/min/kg | 63.29±7.70 | 15.69±4.43* | 112.53±29.60 | 40.72±8.33* | |
| t1/2, h | 1.46±0.18 | 7.10±1.18* | 11.71±2.31 | 21.35±8.01* | |
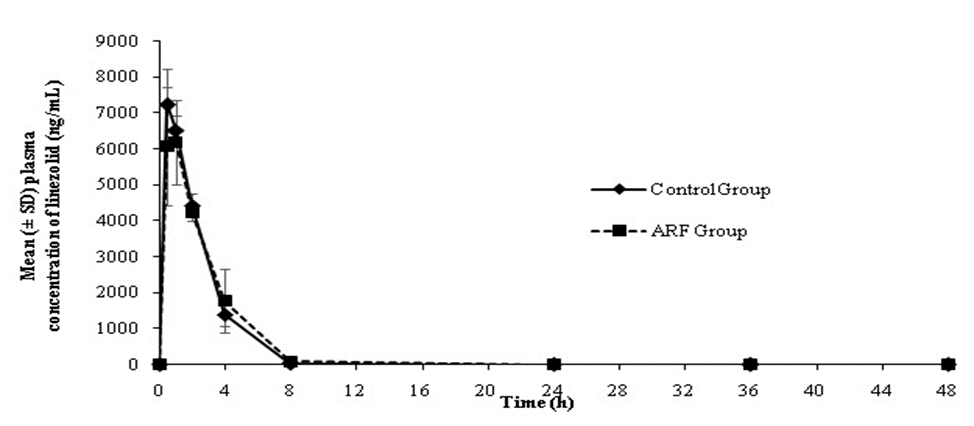
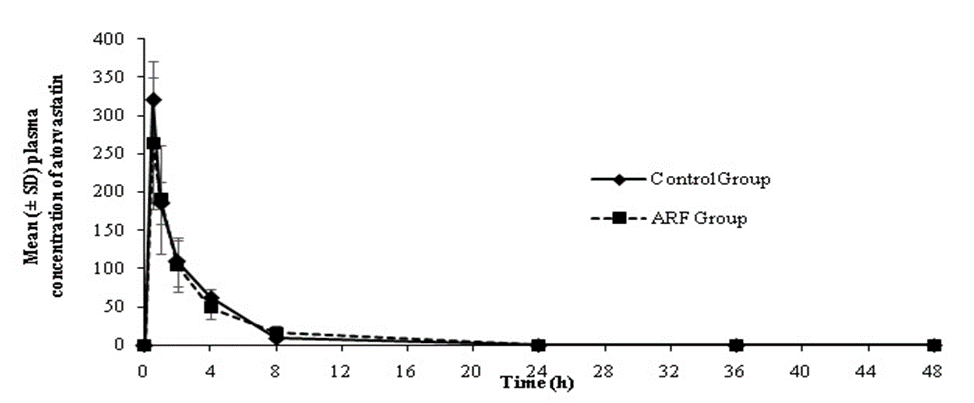
All values are expressed as mean±SD (n=4). *Significant (p<0> ARF, acute renal failure; AUC, area under the curve; Cl, clearance; SD, standard deviation; t1/2, half life On the other hand, AUC0-t, Cl and t1/2 for both linezolid and atorvastatin were found to be comparable between ARF and control animals (Table 3).
| |||||
Table 3: Pharmacokinetic parameters of linezolid and atorvastatin in control and ARF induced rats
| Pharmacokinetic parameter | Linezolid | Atorvastatin | ||
| Control | ARF | Control | ARF | |
| AUC0-t, ng*h/mL | 19413.53±1490.09 | 19565.65±1394.80 | 665.83±107.97 | 608.62± 183.57 |
| Cl, mL/min/kg | 8.60 ± 0.67 | 8.48 ± 0.60 | 246.37±38.31 | 277.68± 118.41 |
| t1/2, h | 0.84 ± 0.12 | 1.07 ± 0.16 | 1.66 ± 0.35 | 2.18 ± 0.70 |
All values are expressed as mean±SD (n=4). ARF, acute renal failure; AUC, area under the curve; Cl, clearance; SD, standard deviation; t1/2, half life | ||||
The plasma drug concentration versus time profiles of linezolid and atorvastatin in control and ARF animals are presented in Figures 3 and 4.
4. Discussion
It is very important to understand the potential absorption, distribution, metabolism, and excretion challenges of new molecules at the early stages of drug discovery to minimize the attrition rate at the clinical development stage. Acute renal failure is one of the most common pathological conditions and it affects the pharmacokinetics of drugs. So, in cases where the drug is likely to be administered in renal impaired patients, pharmacokinetics should be assessed in these special population patients to provide dose adjustments.
There was a significant increase in serum creatinine and BUN levels within 6 h following intra-peritoneal and intra-muscular administration of glycerol; however, both these parameters decreased within 24 h following intra-peritoneal administration, whereas with intra-muscular administration, a sustained increase was observed up to 72 h post glycerol administration. Therefore, intra-muscular administration of glycerol provided a sustained ARF in rats, which is suitable for evaluation of pharmacokinetics of drugs.
Subsequently we validated the i.m glycerol induced ARF model by evaluating the pharmacokinetic parameters of both the classes of drugs that are prescribed with (metformin and digoxin) and without (atorvastatin and linezolid) dose correction in renal impaired patients in clinic.
Metformin does not undergo hepatic metabolism, and approximately 90% of the absorbed drug is eliminated unchanged via the renal route. Metformin can accumulate to toxic levels in patients with renal impairment because of its greater systemic exposure in renal failure condition versus normal renal function. Therefore, dose adjustment is recommended in these patients [13]. Digoxin, is primarily excreted as unchanged drug via renal route [14-16] and the t1/2 of digoxin gets prolonged in patients with renal failure [17] resulting in increased systemic exposure. Therefore, a dose correction is recommended in patients with renal impairment.
In the current study, there was a significant increase (p<0>
Of the total amount of linezolid in the body, only 30% was eliminated unchanged via the kidneys in humans [18], whereas a major part of the linezolid gets metabolized by oxidation of its morpholino ring, resulting in two metabolites: an aminoethoxy acetic acid metabolite (metabolite A), and a hydroxyethyl glycine metabolite (metabolite B) that was formed by nonenzymatic oxidation in an in vitro setting [19]. Linezolid had a total Cl of 7 L/h and a terminal elimination t1/2 of approximately 5 h in humans [20]. In patients with impaired renal function, no significant changes in total Cl were observed; thus, a dose adjustment was reported not to be necessary in this patient population [21].
Atorvastatin and its metabolites are eliminated primarily in bile following hepatic and/or extra-hepatic metabolism. Mean plasma elimination t1/2 of atorvastatin in humans is approximately 14 hours [22]. Less than 2% of a dose of atorvastatin is recovered in urine following oral administration. In patients with impaired renal function, no significant change in pharmacokinetics of atorvastatin is observed; thus, a dose adjustment was reported not to be necessary in this patient population [23].
In this study, no significant change (p <0>
In order to understand the role of metabolizing enzymes in the Cl of drugs, several in vitro tools are available for predicting possible drug interactions due to competing pathways. Preclinical models with dosing of a pan-CYP inhibitor 1-aminobenzotriazole [25] have also gained wide acceptance in understanding the role of hepatic CYP450 enzymes in the Cl of drugs. In a similar manner, the intra-muscular glycerol induced rat ARF model may have some utility in early drug discovery work to understand the potential for pharmacokinetic changes in renal impairment and especially for drugs which are cleared both by renal and hepatic routes.
5. Conclusion
In the present study, we optimized a rat model of ARF which can be used in early drug discovery stages to predict the possibility of pharmacokinetic changes of drugs in renal impaired patients. The results showed that intra-muscular administration of glycerol to rats provided a more sustained ARF model that is suitable to evaluate the pharmacokinetics compared to intra-peritoneal route. The results obtained from the pharmacokinetic studies of the selected probe drugs in the control and ARF rats are in correlation with the clinical data generated in renal impaired patients. These results demonstrate the utility of ARF model in rats in predicting pharmacokinetic changes that may occur in patients with renal impairment. This model may prove useful in early discovery work to screen and prioritize compounds based on potential for dose adjustment requirement in renally impaired subjects.
6. Funding
Authors did not receive any funding to carry out this research work. The authors are thankful to Piramal Enterprises Ltd., Goregaon, Mumbai where all the animal experiments were conducted. The authors are grateful to Dr Reddy’s Laboratories for supplying gift sample of Atorvastatin.
7. Conflicts of interest
Authors have no conflicts of interest to declare.
8. Acknowledgements
RS was involved in study conduct, study design, data analysis and interpretation. MS was involved in study conduct and data analysis.
9. Animal rights
Legal and ethical approvals were obtained prior to initiation of the research work. This study was approved by Institutional Animal Ethics Committee (IAEC) and conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPSCEA), India (1246/09/CPCSEA dated May 11, 2009). The procedures followed in this study were in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals by the National Academy of Sciences, The National Academies Press, Washington, D.C.
10. Availability of data and materials
All relevant data related to the manuscript has been presented. Any additional data supporting the findings published in the manuscript will be made available upon request.
References
- Kaitin KI. (2008), Obstacles and opportunities in new drug development. Clin Pharmacol Ther. 83:210-12.
- Chaturvedi PR, Decker CJ, Odinecs A. Prediction (2001), of pharmacokinetic properties using experimental approaches during early drug discovery. Curr Opin Chem Biol. 5(4):452-63.
- Strougo A, Yassen A, Krauwinkel W, Danhof M, Freijer J. A (2011), semiphysiological population model for prediction of the pharmacokinetics of drugs under liver and renal disease conditions. Drug Metab Dispos. 39(7):1278-87.
- Boxenbaum H. (1982) Interspecies scaling, allometry, physiological time and ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 10: 201-27.
- Lin JM. (1998) Applications and limitations of interspecies scaling and in vitro extrapolation in pharmacokinetics. Drug Metab Dispos. 26:1202-12.
- Paine SW, Ménochet K, Denton R, McGinnity DF, Riley RJ. (2011), Prediction of human renal clearance from preclinical species for a diverse set of drugs that exhibit both active secretion and net reabsorption. Drug Metab Dispos, 39:1008-13.
- Agrawal M, Swartz R. (2000), Acute renal failure. Am Fam Physician. 61(7):2077-88.
- Bellomo R1, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004); Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 8:R204-12.
- Oken DE, Arce ML, Wilson DR. (1966) Glycerol-induced Hemoglobinuric Acute Renal Failure in the Rat. I. Micropuncture Study of the Development of Oliguria. J Clin Invest. 45:724-35.
- Curry SC, Chang D, Connor D. (1989), Drug-and toxin-induced rhabdomyolysis. Ann Emer Med. 18:1068–84.
- Wolfert AI, Oken DE (1989). Glomerular hemodynamics in established glycerol-induced ARF in the rat. J Clin Invest. 84:1967–73.
- Finckh ES. (1959) The indirect action of subcutaneous injections of glycerol on the renal tubules in the rat. J. Path. Bact. 78:197-202.
- Graham GG, Punt J, Arora M, Day RO, Doogue MP (2011), et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 50:81-98.
- Doherty JE, Perkins WH, Mitchell GK. (1961), Titrated digoxin studies in human subjects. Arch Intern Med. 108:531-39.
- Doherty JE, Perkins WH. Studies with tritiated digoxin in human subjects after intravenous administration. Amer Heart J. 1962; 63:528-36.
- Doherty JE, Perkins WH and Wilson MC. Studies with tritiated digoxin in renal failure. Amer J Med. 1964; 37:536-44.
- Doherty JE, Bissett JK, Kane JJ, de Soyza N, Murphy ML (1975) et al. Tritiated digoxin: studies in renal disease in human subjects. Int J Clin Pharmacol Biopharm. 12(1-2):89-95.
- Moellering RC. Linezolid (2003): the first oxazolidinone antimicrobial. Ann Intern Med. 138:135–42.
- Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB (2001) et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [14C]linezolid to healthy human subjects. Drug Metab Dispos. 29:1136–45.
- Perry CM, Jarvis B. Linezolid (2001): a review of its use in the management of serious gram-positive infections. Drugs. 61:525–51.
- Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK (2003), et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 47:2775–80.
- LIPITOR® (atorvastatin calcium) tablets, Pfizer: Highlights of Prescribing Information (2019).
- Stern RH, Yang BB, Horton M, Moore S, Abel RB, Olson SC. (1997), Renal dysfunction does not alter the pharmacokinetics or LDL-cholesterol reduction of atorvastatin. J Clin Pharmacol. 37(9):816-19.
- Dryden MS. (2011), Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother. 66(Suppl 4): iv7–iv15.
- Balani SK, Zhu T, Yang TJ, Liu Z, He B, Lee FW. (2002), Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in rats, dogs, and monkeys. Drug Metab Dispos. 30(10):1059-62.


