Current Issue : Article / Volume 3, Issue 2
- Research Article | DOI:
- https://doi.org/10.58489/2836-497X/024
Phase III Study to Evaluate the Efficacy and Safety of Two Novel Fixed-Dose Vaginal Ovule Formulations in the Treatment of Various Vaginal Infections: One-Shot Study Running Title: Phase III study of two novel vaginal ovules
1Department of IVF Research and Training Center, Ege University Faculty of Medicine, Izmir, Türkiye
2Department of Obstetrics and Gynecology, Kanuni Sultan Suleyman Research and Training Hospital, Istanbul, Türkiye
3Department of Obstetrics and Gynecology, Antalya Research and Training Hospital, Antalya, Türkiye
4Department of Obstetrics and Gynecology, Bakırköy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Türkiye
5Department of Obstetrics and Gynecology, Kartal Dr. Lutfi Kırdar Research and Training Hospital, University of Health Sciences, Istanbul, Türkiye
6Department of Obstetrics and Gynecology, Ataturk Training and Research Hospital, Katip Celebi University Faculty of Medicine, Izmir, Türkiye
7Department of Obstetrics and Gynecology, Etlik Zübeyde Hanim Women's Health Training and Research Hospital, University of Health Sciences, Ankara, Türkiye
8Department of Obstetrics and Gynecology, Medicana International Hospital, Izmir, Türkiye
9Department of Obstetrics and Gynecology, Medipol University School of Medicine, Istanbul, Türkiye
10Department of Medical Microbiology, Ege University Faculty of Medicine, Izmir, Türkiye
Erol Tavmergen
Erol Tavmergen, et.al., (2024). Phase III Study to Evaluate the Efficacy and Safety of Two Novel Fixed-Dose Vaginal Ovule Formulations in the Treatment of Various Vaginal Infections: One-Shot Study. Archives of Gynecology and Women Health. 3(2); DOI: 10.58489/2836-497X/024
© 2024 Erol Tavmergen, MD, Professor, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 09-05-2024
- Accepted Date: 01-06-2024
- Published Date: 05-06-2024
Bacterial vaginosis, lidocaine, tinidazole, trichomonas vaginitis, vulvovaginal candidiasis
Abstract
Objective: This study aimed to evaluate the efficacy and safety of two novel formulations: one containing fenticonazole nitrate 600 mg + tinidazole 1000 mg + lidocaine 100 mg (Formulation A) and the other with fenticonazole nitrate 600 mg + tinidazole 2000 mg + lidocaine 100 mg (Formulation B) in the treatment of bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), trichomonas vaginitis (TV), and mixed vaginal infections (MVIs) in comparison to comparator medication (Gynomax® XL).
Materials and methods: This phase III study enrolled 562 adult, pre-menopausal patients with BV, VVC, TV, or MVIs. Of these, 544 received the study medication (included in the safety analysis), and 440 completed the study (included in the efficacy analysis). Patients were randomized into Formulation A, Formulation B, or comparator medication arms. Follow-up visits were conducted two weeks post-treatment, with clinical and microbiological evaluations.
Results: Initial clinical diagnoses indicated BV (62.0%), VVC (19.8%), MVIs (15.5%), and TV (2.7%). High clinical recovery rates were observed with Formulation A (96.7% of BV, 81.8% of VVC, and 96.3% of MVIs), Formulation B (97.7% of BV, 88.2% of VVC, 90.9% of MVIs), and comparator medication (98.9% of BV, 90.3% of VVC, 100.0% of MVIs). None of the patients discontinued medications due to an adverse event or intolerability.
Conclusion:
All formulations are considered as safe, well-tolerated, and highly effective for treating BV, VVC, and MVIs. Formulation A or B may be particularly advantageous due to anticipated greater patient compliance with their single-dose administration.
Introduction
Vaginal and vulvar infections, including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and trichomonas vaginitis (TV), are among the most prevalent medical conditions in the field of general gynecology practice [1]. BV is highly prevalent, impacting up to 30% of the population. In the United States, data from the 2001-2004 National Health and Nutrition Examination Survey showed that 29% of women were positive for BV [2]. Seventy-five percent of sexually active adult women experience VVC at least once, and 45% encounter at least two episodes of infection annually [3]. Furthermore, mixed vaginal infections (MVIs), resulting from at least two pathogens, are common, with a prevalence of up to 35%, as per a recent review [4].
Effective vaginitis treatment relies on accurate diagnosis, efficient treatment administration, and patient compliance. In routine practice, diagnosis often depends on clinical findings, leading to treatment initiation without microbiological confirmation. In cases lacking confirmation or with mixed etiology, fixed-dose combination medications offer a practical and cost-effective approach for treating BV, VVC, and TV by addressing a broader range of causative pathogens compared to monotherapies.
Fenticonazole, an imidazole derivative, exhibits broad antimycotic activity against dermatophytes and yeasts [5]. Tinidazole is an antibiotic widely used for various vaginal infections [6], and lidocaine is a well-absorbed local anesthetic with a proven safety record [7]. Exeltis İlaç (Istanbul, Turkey) has developed two novel single-shot fixed-dose combinations in the form of vaginal ovules: fenticonazole nitrate 600 mg + tinidazole 1000 mg + lidocaine 100 mg (Formulation A) and fenticonazole nitrate 600 mg + tinidazole 2000 mg + lidocaine 100 mg (Formulation B). The study aimed to assess the efficacy and safety of Formulations A and B in the treatment of BV, VVC, TV, and MVIs, comparing results with a registered formulation containing 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine (Gynomax® XL, referred to as comparator medication).
Materials and methods
This multi-center, open-label, randomized, three-arm, phase III study was conducted across sixteen gynecology and obstetrics clinics in six major cities in Türkiye. Approval (date and number: 25 February 2020, No:20-2.3/2) was obtained from the Clinical Research Ethics Committee of Ege University and the study was conducted in accordance with the principles of the Helsinki Declaration. All patients were informed, and written consent was obtained before performing any study-related procedures. The study was retrospectively registered on ClinicalTrials.gov under the number NCT06056947.
Pre-menopausal, symptomatic females aged between 18-55 years, clinically diagnosed with BV, VVC, TV, or MVIs and requiring treatment per investigator’s decision, were enrolled. Exclusion criteria encompassed pregnancy, lactation, vaginismus, endometriosis, deep dyspareunia, urinary tract infection, undiagnosed vaginal bleeding, bleeding disorders, or confirmed or suspected genital malignancies. The primary efficacy criterion was recovery, assessed as “complete”, “partial”, or “no recovery” based on treating investigators’ evaluations. An independent microbiology specialist confirmed the microbiologic diagnoses and assessed microbiologic recovery as a secondary efficacy criterion. Efficacy outcomes, safety parameters including adverse events (AEs), and tolerability, were assessed by treating physicians in a single follow-up visit approximately two weeks after treatment initiation. Additionally, patient satisfaction was evaluated through a written questionnaire during the follow-up visit.
Following a thorough clinical and gynecological examination, eligible patients were randomized into three treatment arms (1:1:1) using the randomization module, which consisted of a block size of six implemented by the contract research organization within the electronic data capture system. Symptoms and findings were recorded per guidelines outlined by The International Union against Sexually Transmitted Infections/World Health Organization (IUSTI/WHO) [8]. Vaginal pH levels and vaginal swab samples were collected at baseline and follow-up visits. The microbiologic samples were analyzed at a central laboratory (Düzen Laboratuvarlar Grubu, Ankara, Türkiye) using polymerase chain reaction (PCR) tests, gram staining, direct microscopic examination, culture testing for candidiasis, and Nugent Score [9] determination with gram staining. Two distinct PCR kits were employed, and infectious diseases (BV, VVC, TV) were assessed at both baseline and follow-up visits using BD MAXTM Vaginal Panel assays (BD, Sparks, MD 21152-0999 USA). Sexually transmitted diseases were evaluated solely at the baseline visit using the FTD STD9 kit (Fast-track diagnostics, Junglinster, Luxembourg).
Treatment was promptly initiated following the completion of clinical examinations, with microbiological results available approximately 3 days after the physical examination. Patients in the first arm received Formulation A as a single dose (1x1), while those in the second arm received Formulation B as a single dose (1x1), and those in the third arm received a combination of 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine (Gynomax® XL) for three consecutive days (1x1 / 3 days) as comparator medication. Treatment compliance and adverse events were monitored through telephone communication and patient diaries.
It was expected to achieve a complete treatment success rate of 85% in patients, with a non-inferiority margin of -0.10. The significance level was set as 0.05 (two-sided), and the power as 0.95. A dropout rate of 10% was assumed for all treatment groups. Based on these assumptions, a sample size of 146 patients in each arm (resulting in a total of 438 patients) was calculated to achieve 95% power to detect differences between Formulation A and Formulation B when compared to comparator medication. Efficacy analysis was conducted on patients who completed the study in accordance with the protocol, while safety analysis included randomized patients who received at least one dose of the study medications. Kruskal-Wallis and Mann-Whitney U tests were used to compare the parameters that exhibited non-normal distribution. For within-group comparisons, Wilcoxon test was used. Qualitative data were compared using the Chi-Square test.
Results
Between July 2020 and May 2021, 577 patients were screened, and 440 completed the study as per protocol for the efficacy analysis. The safety analysis included 544 patients who received at least one dose of the study medications. Study flow diagram is presented in Figure 1. Demographic data revealed a mean age of 34.1 years (± 8.1 years SD) and a body mass index ranging from 14.9 to 43.7 kg/m2, with a mean of 24.6 kg/m2 (± 4.6 kg/m2 SD).
In the efficacy analysis, 145, 148, and 147 patients were included in Formulation A, Formulation B, and the comparator medication arms, respectively. Initial clinical diagnoses showed a majority with BV (62.0%), followed by VVC (19.8%), MVIs (15.5%), and TV (2.7%). However microbiological diagnoses revealed percentages as follows: BV (45.4%), MVIs (31.4%), VVC (10.9%), and TV (0.2%). Notably, 12.0% (n = 53) of patients in the efficacy population had no detected infectious agents. Diagnoses distribution across randomization groups is detailed in Table 1.
The primary objective was to assess the efficacy of Formulation A, Formulation B, and comparator medication in treating BV, VVC, TV, and MVIs, focusing on complete recovery of clinical findings. Clinical evaluations revealed complete recovery rates of 69.2%, 67.0%, and 69.1% of the patients with BV; 50.0%, 55.9%, and 54.8% for patients with VVC; and 55.6%, 50.0%, and 57.9% for patients with MVIs in the respective treatment arms, showing no statistically significant differences. Complete and partial clinical recovery rates for BV, VVC, TV, and MVIs are presented in Table 2. Microbiological tests indicated complete recovery rates of 48.0%, 49.1%, and 55.7% for BV; 29.4%, 56.3%, and 53.3% for VVC; and 23.3%, 17.0%, and 33.3% for MVIs, with no significant differences observed between treatment arms (Table 3).
In the efficacy population, baseline mean vaginal pH was 5.95, which subsequently reduced to 5.33 during follow-up. Patients clinically diagnosed with BV showed a significant pH reduction in each treatment arm at follow-up compared to baseline. However, patients with VVC and TV did not exhibit statistically significant reductions (p values in Table 4). Additionally, mean pH changes from baseline were assessed between treatment arms, revealing no statistically significant differences (Table 5).
The baseline mean Nugent score in the efficacy population was 3.74, decreasing to 2.89 at follow-up. Patients clinically diagnosed with BV and VVC demonstrated significant mean Nugent score reductions at follow-up (p < 0 xss=removed xss=removed xss=removed>
In the safety population of 544 patients (177 in Formulation A, 182 in Formulation B, and 185 in the comparator medication arms), two patients experienced two serious adverse events that determined to be unrelated to the study medications: one pregnancy and one pelvic inflammatory disease. Overall, 56 patients (10.3%) experienced 104 AEs, which were distributed evenly among the three treatment arms (17 patients in Formulation A, 18 patients in Formulation B, and 21 patients in the comparator medication group). The most frequently reported AEs were categorized under the group of reproductive system disorders (4.8%), followed by musculoskeletal and connective tissue disorders (3.3%), gastrointestinal disorders (2.8%), and nervous system disorders (2.0%). The majority of the AEs (67.3%) were of mild severity and 66.4% of them were unrelated to the study medications. None of the patients temporarily or permanently discontinued the study medications due to an AE or non-tolerability.
All three formulations were reported as easy to use by almost all patients. Only 1% rated ovule insertion difficulty as poor, while 54.3% found it easy. 79.8% of the patients experienced satisfaction or high satisfaction with the medications. Prior experience with similar vaginal products was reported by 75.7% of patients, and 65.9% indicated a preference for the treatment used in this study compared to the previous products. Furthermore, 90% of the patients expressed willingness to consider using this treatment again in the future.
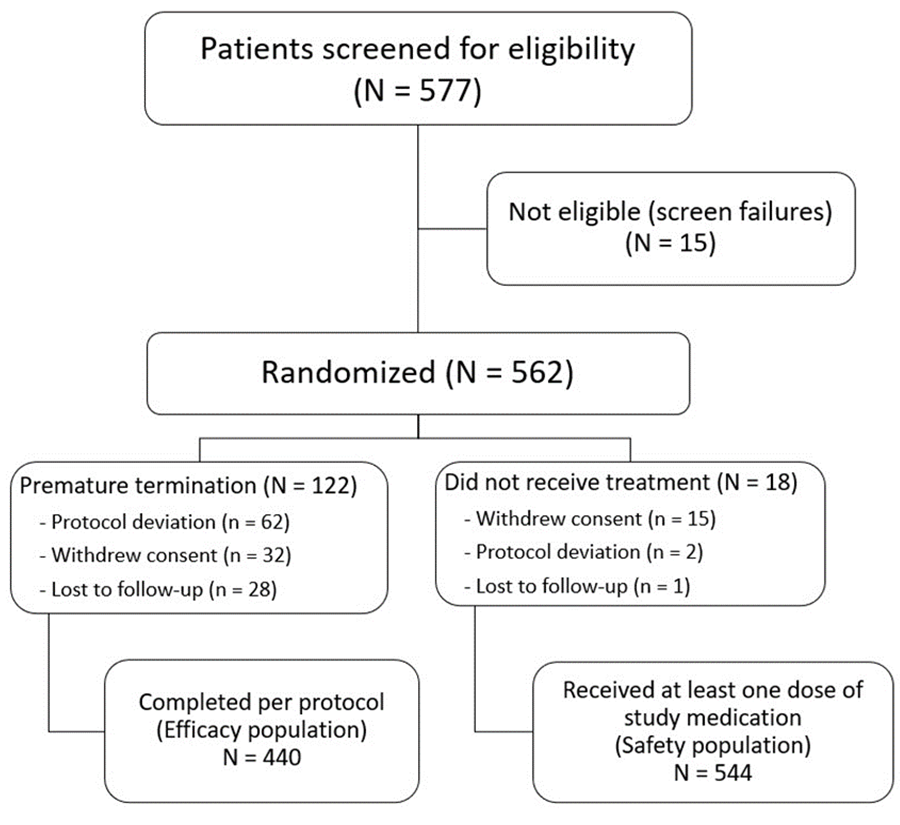
Fig 1: Study flow diagram
Table 1: Distribution of patients according to the clinical and microbiological diagnoses, and the treatments administered
| Formulation Aa | Formulation Bb | Comparator Medicationc | Total | |
| n (%) | n (%) | n (%) | n (%) | |
| Clinical Diagnosis | ||||
| BV | 91 (62.8%) | 88 (59.5%) | 94 (63.9%) | 273 (62.0%) |
| VVC | 22 (15.2%) | 34 (23.0%) | 31 (21.1%) | 87 (19.8%) |
| TV | 5 (3.4%) | 4 (2.7%) | 3 (2.0%) | 12 (2.7%) |
| MVIs | 27 (18.6%) | 22 (14.9%) | 19 (12.3%) | 68 (15.5%) |
| Total | 145 (100.0%) | 148 (100.0%) | 147 (100.0%) | 440 (100.0%) |
| Microbiological Diagnosis | ||||
| BV | 75 (51.7%) | 55 (37.2%) | 70 (47.6%) | 200 (45.4%) |
| VVC | 17 (11.7%) | 16 (10.8%) | 15 (10.2%) | 48 (10.9%) |
| TV | - | - | 1 (7.0%) | 1 (0.2%) |
| MVIs | 43 (29.7%) | 53 (35.8%) | 42 (28.6%) | 138 (31.4%) |
| None detected among investigated infections | 10 (6.9%) | 24 (16.2%) | 19 (12.9%) | 53 (12.0%) |
| Total | 145 (100.0%) | 148 (100.0%) | 147 (100.0%) | 440 (100.0%) |
a Formulation A: 600 mg fenticonazole nitrate + 1000 mg tinidazole + 100 mg lidocaine b Formulation B: 600 mg fenticonazole nitrate + 2000 mg tinidazole + 100 mg lidocaine c Comparator medication: 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine BV, bacterial vaginosis; MVIs, mixed vaginal infections; TV, trichomonas vaginitis; VVC, vulvovaginal candidiasis. | ||||
Table 2: Recovery rates according to the clinical evaluations in patients with vaginal infections following treatments
Formulation Aa n (%) | Formulation Bb n (%) | Comparator Medicationc n (%) | P value* | Total n (%) | |
| Patients diagnosed with a single infection | |||||
| BV | |||||
| Complete recovery | 63 (69.2%) | 59 (67.0%) | 65 (69.1%) | 0.868 | 187 (68.5%) |
| Partial recovery | 25 (27.5%) | 27 (30.7%) | 28 (29.8%) | 80 (29.3%) | |
| No recovery | 3 (3.3%) | 2 (2.3%) | 1 (1.1%) | 6 (2.2%) | |
| Total | 91 (100.0%) | 88 (100.0%) | 94 (100.0%) | 273 (100.0%) | |
| VVC | |||||
| Complete recovery | 11 (50.0%) | 19 (55.9%) | 17 (54.8%) | 0.920 | 47 (54.0%) |
| Partial recovery | 7 (31.8%) | 11 (32.4%) | 11 (35.5%) | 29 (33.3%) | |
| No recovery | 4 (18.2%) | 4 (11.8%) | 3 (9.7%) | 11 (12.7%) | |
| Total | 22 (100.0%) | 34 (100.0%) | 31 (100.0%) | 87 (100.0%) | |
| TV | |||||
| Complete recovery | 3 (60.0%) | 3 (75.0%) | 2 (66.7%) | 0.894 | 8 (66.7%) |
| Partial recovery | 2 (40.0%) | 1 (25.0%) | 1 (33.3%) | 4 (33.3%) | |
| No recovery | - | - | - | - | |
| Total | 5 (100.0%) | 4 (100.0%) | 3 (100.0%) | 12 (100.0%) | |
| Patients diagnosed with mixed infections | |||||
| MVIs | |||||
| Complete recovery | 15 (55.6%) | 11 (50.0%) | 11 (57.9%) | 0.718 | 37 (54.4%) |
| Partial recovery | 11 (40.7%) | 9 (40.9%) | 8 (42.1%) | 28 (41.2%) | |
| No recovery | 1 (3.7%) | 2 (9.1%) | - | 3 (4.4%) | |
| Total | 27 (100.0%) | 22 (100.0%) | 19 (100.0%) | 68 (100.0%) | |
a Formulation A: 600 mg fenticonazole nitrate + 1000 mg tinidazole + 100 mg lidocaine b Formulation B: 600 mg fenticonazole nitrate + 2000 mg tinidazole + 100 mg lidocaine c Comparator medication: 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine BV, bacterial vaginosis; MVIs, mixed vaginal infections; TV, trichomonas vaginitis; VVC, vulvovaginal candidiasis. * Chi-square Test | |||||
Table 3: Recovery rates according to the microbiological evaluations in patients with vaginal infections following treatments
| Formulation Aa | Formulation Bb | Comparator Medicationc | P value* | Total | |
| n (%) | n (%) | n (%) | n (%) | ||
| Patients diagnosed with a single infection d | |||||
| BV | |||||
| Complete recovery | 36 (48.0%) | 27 (49.1%) | 39 (55.7%) | 0.087 | 102 (51.0%) |
| Partial recovery | 24 (32.0%) | 13 (23.6%) | 9 (12.9%) | 46 (23.0%) | |
| No recovery | 15 (20.0%) | 15 (27.3%) | 22 (31.4%) | 52 (26.0%) | |
| Total | 75 (100.0%) | 55 (100.0%) | 74 (100.0%) | 200 (100.0%) | |
| VVC | |||||
| Complete recovery | 5 (29.4%) | 9 (56.3%) | 8 (53.3%) | 0.128 | 22 (45.8%) |
| Partial recovery | 4 (23.5%) | 5 (31.3%) | 1 (6.7%) | 10 (20.8%) | |
| No recovery | 8 (47.1%) | 2 (12.5%) | 6 (40.0%) | 16 (33.3%) | |
| Total | 17 (100.0%) | 16 (100.0%) | 15 (100.0%) | 48 (100.0%) | |
| TV | |||||
| Complete recovery | - | - | - | - | - |
| Partial recovery | - | - | - | - | |
| No recovery | - | - | 1 (100.0%) | 1 (100.0%) | |
| Total | - | - | 1 (100.0%) | 1 (100.0%) | |
| Patients diagnosed with mixed infections* | |||||
| MVIs | |||||
| Complete recovery | 10 (23.3%) | 9 (17.0%) | 14 (33.3%) | 0.462 | 33 (23.9%) |
| Partial recovery | 26 (60.5%) | 36 (67.9%) | 23 (54.8%) | 85 (61.6%) | |
| No recovery | 7 (16.3%) | 8 (15.1%) | 5 (11.9%) | 20 (14.5%) | |
| Total | 43 (100.0%) | 53 (100.0%) | 42 (100.0%) | 138 (100.0%) | |
a Formulation A: 600 mg fenticonazole nitrate + 1000 mg tinidazole + 100 mg lidocaine b Formulation B: 600 mg fenticonazole nitrate + 2000 mg tinidazole + 100 mg lidocaine c Comparator medication: 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine BV, bacterial vaginosis; MVIs, mixed vaginal infections; TV, trichomonas vaginitis; VVC, vulvovaginal candidiasis. d None of the investigated infections were detected in 53 patients * Chi-square Test | |||||
Table 4: Vaginal pH values and comparison analysis between baseline and follow-up visits within each treatment arm
Clinical Diagnosis Treatment a | Mean pH at Baseline | Mean pH at Follow-up | Mean Change from Baseline | P values* |
| BV | 6.12 | 5.35 | -0.77 | <0> |
| Formulation A | 6.21 | 5.20 | -1.02 | <0> |
| Formulation B | 6.15 | 5.43 | -0.72 | <0> |
| Comparator medication | 5.99 | 5.43 | -0.57 | 0.004 |
| VVC | 5.43 | 5.05 | -0.37 | 0.093 |
| Formulation A | 5.43 | 4.84 | -0.59 | 0.141 |
| Formulation B | 5.44 | 5.21 | -0.24 | 0.357 |
| Comparator medication | 5.40 | 5.03 | -0.37 | 0.419 |
| TV | 5.83 | 5.42 | -0.42 | 0.514 |
| Formulation A | 5.30 | 5.30 | 0.00 | 1.000 |
| Formulation B | 5.75 | 5.00 | -0.75 | 0.593 |
| Comparator medication | 6.83 | 6.17 | -0.67 | 0.593 |
| MVIs | 5.99 | 5.58 | -0.41 | 0.019 |
| Formulation A | 6.06 | 5.61 | -0.44 | 0.197 |
| Formulation B | 6.02 | 5.55 | -0.48 | 0.121 |
| Comparator medication | 5.87 | 5.58 | -0.29 | 0.419 |
BV, bacterial vaginosis; MVIs, mixed vaginal infections; TV, trichomonas vaginitis; VVC, vulvovaginal candidiasis. a Formulation A: 600 mg fenticonazole nitrate + 1000 mg tinidazole + 100 mg lidocaine, Formulation B: 600 mg fenticonazole nitrate + 2000 mg tinidazole + 100 mg lidocaine, Comparator medication: 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine * Baseline and follow-up values compared using Wilcoxon Signed Ranks Test | ||||
Table 5: Comparison of mean change of pH values from baseline to follow-up visit between treatment arms
| Diagnosis | Compared Treatments a | Significance* | 95% CI | |
| Lower Bound | Upper Bound | |||
| According to clinical diagnosis | ||||
| BV | ||||
| Formulation A – Formulation B | 0.598 | -0.85 | 0.26 | |
| Formulation A – Comparator medication | 0.145 | -0.99 | 0.10 | |
| Formulation B – Comparator medication | 1.000 | -0.70 | 0.40 | |
| VVC | ||||
| Formulation A – Formulation B | 1.000 | -1.65 | 0.93 | |
| Formulation A – Comparator medication | 1.000 | -1.53 | 1.09 | |
| Formulation B – Comparator medication | 1.000 | -1.04 | 1.31 | |
| TV | ||||
| Formulation A – Formulation B | 1.000 | -4.04 | 5.54 | |
| Formulation A – Comparator medication | 1.000 | -4.55 | 5.89 | |
| Formulation B – Comparator medication | 1.000 | -5.54 | 5.37 | |
| MVIs | ||||
| Formulation A – Formulation B | 1.000 | -1.09 | 1.16 | |
| Formulation A – Comparator medication | 1.000 | -1.33 | 1.02 | |
| Formulation B – Comparator medication | 1.000 | -1.41 | 1.04 | |
| According to microbiological diagnosis | ||||
| BV | ||||
| Formulation A – Formulation B | 1.000 | -0.68 | 0.73 | |
| Formulation A – Comparator medication | 1.000 | -0.74 | 0.58 | |
| Formulation B – Comparator medication | 1.000 | -0.82 | 0.61 | |
| VVC | ||||
| Formulation A – Formulation B | 0.925 | -2.35 | 0.97 | |
| Formulation A – Comparator medication | 1.000 | -1.72 | 1.72 | |
| Formulation B – Comparator medication | 0.996 | -1.06 | 2.43 | |
| MVIs | ||||
| Formulation A – Formulation B | 0.855 | -1.21 | 0.47 | |
| Formulation A – Comparator medication | 0.150 | -1.61 | 0.16 | |
| Formulation B – Comparator medication | 0.940 | -1.20 | 0.49 | |
| No Infection | ||||
| Formulation A – Formulation B | 1.000 | -1.41 | 1.02 | |
| Formulation A – Comparator medication | 1.000 | -1.68 | 0.84 | |
| Formulation B – Comparator medication | 1.000 | -1.21 | 0.77 | |
BV, bacterial vaginosis; CI, Confidence interval; MVIs, mixed vaginal infections; TV, trichomonal vaginitis; VVC, vulvovaginal candidiasis. a Formulation A: 600 mg fenticonazole nitrate + 1000 mg tinidazole + 100 mg lidocaine, Formulation B: 600 mg fenticonazole nitrate + 2000 mg tinidazole + 100 mg lidocaine, Comparator medication: 300 mg tinidazole + 200 mg tioconazole nitrate + 100 mg lidocaine * Bonferroni test | ||||
Discussion
The study aimed to evaluate the efficacy of two new formulations in achieving complete recovery of clinical findings in the treatment of BV, VVC, TV, and MVIs. Each single-dose formulation was compared with one another and with the three-day treatment regimen of a comparator medication through clinical and microbiological assessments.
Bacterial, candidal, and trichomonas vaginitis collectively constitute over 90% of all cases. BV was reported as the most prevalent (30-35%), followed by VVC at 20-25%, MVIs at 15-20%, and TV at around 10% [10]. Recent reviews indicated even higher prevalence rates, with BV reaching up to 50%, VVC up to 39%, and TV up to 35% [11]. In another study, frequencies were 52.2% for BV, 40.6% for VVC, and 7.2% for MVIs [12]. Notably, in the Gyno-Türk study conducted in Türkiye [13], MVIs were diagnosed in 54.1% of the patients, followed by BV (35.7%) and VVC (31.6%) at baseline, with TV was diagnosed in only one patient (1.0%). Cepický et al. [14] reported varying rates, like 30% of the symptomatic patients had MVIs, while 15% remained undiagnosed. In our study, the initial clinical diagnosis revealed BV in 62.0% of patients, followed by VVC (19.8%) and MVIs (15.5%), which resembles findings from several studies. Only one case of trichomoniasis was microbiological detected in our study, and oral treatment was initiated for the patient and her partner. Since there were only one TV case, this paper does not discuss treatment outcomes for TV infections.
We strengthened our study by conducting both clinical and microbiological evaluations, comprehensively examining causative organisms covering nearly 95% of common vaginitis infections. It is noteworthy that microbiological investigations excluded anaerobic microorganisms. Therefore, the absence of a detected causative agent in 12% of the patients (n = 53) with presenting symptoms may be attributed to the microorganisms not included in our investigation. As seen in previous studies, detecting microbiological findings in every patient with clinical symptoms may not always be possible [11,12]. The Gyno-Türk study reported a similar result, where clinical diagnosis did not align with microbiological test results in 21.4% of the patients [13]. While microbiologic tests typically exhibit high sensitivity, disparities between clinician diagnosis and laboratory test results may occur when not all potential causative agents are assessed, as observed in other studies [15].
A previous study with our comparator medication using the same dosing regimen evaluated clinical recovery at both 10 and 30 days [12]. Recovery rates were 80.6% on day 10 and 86.6% on day 30. While our study results generally align with these findings, our tenth-day cure rate was slightly higher (95.5% vs 80.6%) than what was reported. Clinical recovery rates in Gyno-Türk study [13], sharing a similar study design but being a single-arm study, were comparable to our study (95.5% vs 96.9%).
In the literature, there are also studies on the treatment of BV with oral pharmaceutical agents. Comparisons were made between our study results and those of Schwebke and Desmond, who investigated metronidazole and tinidazole for seven days in 593 women with BV [16]. They reported cure rates of 76.8% at 14 days and 64.5% at one month, with no significant differences between treatment arms. Milani et al. compared oral single-dose 2 g tinidazole with 2% vaginal clindamycin cream for seven days in patients with BV, reporting a clinical cure rate of 84% at week one with no significant difference between groups [17]. In our study, Formulation A achieved a cure rate (complete + partial recovery) of 96.7%, and Formulation B had a rate of 97.7% for BV. These rates were comparable to oral treatments reported in the literature. Despite the anticipation of greater efficacy with seven days of oral treatment, our study suggests that single-dose vaginal treatment with Formulation A or B demonstrated good efficacy profiles, potentially offering a more advantageous treatment option with higher patient compliance.
Studies evaluating fixed-dose combinations for the treatment of mixed infections highlighted the effectiveness of nifuratel and nystatin. A panel from the Polish Gynecologic Society endorsed this combination as an effective treatment for MVIs [18]. Zlatkov and Karag'ozov reported clinical and microbiological cure rates of 89.5% and 84.2% on Day 7, and 83.3% and 72.2% on Day 30, respectively, following treatment with nifuratel and nystatin for vulvovaginal complaints [19]. Similarly, a multicenter study conducted by Karag'ozov et al. reported cure rates of 88.1% and 86.8% on Days 7-10, and 81.1% and 82.4% on Days 30-40 with nifuratel and nystatin combination [20]. Polatti et al. investigated different doses of nifuratel and nystatin combination for trichomoniasis and/or candidiasis, reporting microbiological cure rate of 45%, 84%, and 95% for low, moderate, and high doses, respectively, over five days [21]. Our study observed cure rates of 96.3% for Formulation A and 90.9% for Formulation B in patients with MVIs, suggesting comparable efficacy of a single-dose treatment to multi-day dosages of nifuratel and nystatin combination.
Increased vaginal pH serves as a diagnostic criterion for BV [22]. The Gyno-Türk study reported a baseline mean vaginal pH of 6.1, which significantly decreased to 5.7 on the 10th day visit [13]. Similarly, our study showed improvements in mean vaginal pH values for BV patients across all three treatment arms at the follow-up visit compared to baseline. Nevertheless, we did not observe any significant differences between the treatments.
The therapeutic efficacy and tolerability of fenticonazole were evaluated in a study where symptomatic and mycologically confirmed patients received intravaginal administration of 2percentage fenticonazole cream once a day for 7 days [23]. The results demonstrated a cure rate of 95%, with the treatment exhibiting a favorable safety profile and no reports of local or systemic signs or symptoms of toxicity. In another clinical study, patients with symptomatic candidiasis were treated with a single-dose vaginal ovule containing 600 mg fenticonazole, achieving a cure rate of 70% one week after treatment [24]. This treatment approach was well-tolerated, with no observed local or systemic adverse reactions. These findings align with our study results in terms of efficacy and safety.
Tinidazole is an agent used in treating trichomonas infections, particularly in cases resistant to metronidazole. High-dose tinidazole was recommended in the literature, either alone or in combination with vaginal administrations for such cases [8,25]. For our study, we selected high-dose tinidazole due to its well-tolerated nature and high cure rates. Data from trials and prescribing information indicate that AEs were reported by 11.8% of patients receiving a single 2 g dose of tinidazole in studies for trichomoniasis and giardiasis, and by 13.8% of patients with multi-day dosing for amebiasis [26]. AEs included metallic / bitter taste, nausea, anorexia (related to the gastrointestinal system), and weakness / fatigue / malaise (associated with the central nervous system). In our study, the AE incidence was 10.3%, slightly lower than in the aforementioned studies. Most AEs were related to reproductive system, assessed as unlikely to be related to the study medications. The majority of AEs were mild, and none of the patients discontinued treatment due to AEs. AE distribution was similar across all three treatment arms. In comparison with the Gyno-Türk study, where 14.6% of the patients experienced AEs following vaginal treatment with the comparator medication [13], our larger study observed a lower percentage (11.4%) in that arm. Additionally, patients in Formulation A and Formulation B arms experienced fewer AEs (9.6 and 9.9%, respectively) compared to the third arm, indicating a better safety profile. Consequently, we conclude that the new formulations exhibit favorable safety profiles, consistent with previously reported data.
Conclusions
The single-dose vaginal treatments used in this study offer several advantages. In cases of common vaginitis, laboratory diagnosis may not always be feasible due to the time-consuming and potentially costly nature of laboratory tests. Therefore, opting for a treatment modality that is effective against a broad spectrum of pathogens, with the option of a single-dose administration, is highly practical. Furthermore, the single-dose vaginal treatment not only reduces the likelihood of gastrointestinal AEs associated with oral administration but also enhances patient compliance compared to multiple-dose treatment regimens. As a result, after evaluating clinical, microbiological, and safety data, both low and high-dose fixed-dose combinations (Formulation A and Formulation B) are considered safe, well-tolerated, and highly effective for the treatment of BV, VVC, and MVIs. Considering their higher patient compliance, either Formulation A or B may be the preferable choice.
Acknowledgements
The authors would like to thank the One-Shot Study Group, which contributed to this study. One-Shot Study Group comprised the following investigators (presented in alphabetical order of surnames):
Aslı Akdöner1, Özgür Aslan2, Hüseyin Aydoğmuş3, Murat Ekin2, Ege Nazan Tavmergen Göker4, Bülent Gülekli1, Elif Cansu Gündoğdu5, Esra Tuştaş Haberal6, Müjde Can İbanoğlu7, Bekir Kahveci8, İsmail Özdemir9, Kemal Sandal10, Sena Sayan11, Yasemin Süzer12, Mustafa Şengül3, Deniz Şimşek13, Özlem Moraloğlu Tekin12, Niyazi Tuğ10, Batuhan Turgay12, Emin Üstünyurt14, Mehmet Ali Vardar8, Tevfik Yoldemir11, Oğuz Yücel15.
1Department of Obstetrics and Gynecology, Dokuz Eylul University School of Medicine, Izmir, Türkiye
2Department of Obstetrics and Gynecology, Bakirköy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Türkiye
3Department of Obstetrics and Gynecology, Ataturk Training and Research Hospital, Katip Celebi University Faculty of Medicine, Izmir, Türkiye
4Department of Obstetrics and Gynecology, Ege University School of Medicine, Izmir, Türkiye
5Department of Obstetrics and Gynecology, Kartal Dr. Lutfi Kirdar Research and Training Hospital, University of Health Sciences, Istanbul, Türkiye
6Department of Obstetrics and Gynecology, Hisar Hospital Intercontinental, Istanbul, Türkiye
7Department of Obstetrics and Gynecology, Etlik Zubeyde Hanim Women's Health Training and Research Hospital, Ankara, Türkiye
8Department of Obstetrics and Gynecology, Balcalı Hospital, Cukurova University Faculty of Medicine, Adana, Türkiye
9Department of Obstetrics and Gynecology, Kanuni Sultan Suleyman Research and Training Hospital, Istanbul, Türkiye
10Department of Obstetrics and Gynecology, Sancaktepe Sehit Prof Dr Ilhan Varank Training and Research Hospital, University of Health Sciences, Istanbul, Türkiye
11Department of Obstetrics and Gynecology, Marmara University Faculty of Medicine, Istanbul, Türkiye
12Department of Obstetrics and Gynecology, Ministry of Health Ankara City Hospital, Ankara, Türkiye
13Department of Obstetrics and Gynecology, University of Health Sciences Bursa Yuksek Ihtisas Training and Research Hospital, Bursa, Türkiye
14Department of Obstetrics and Gynecology, Doruk Hospital, Bursa, Türkiye
15Department of Obstetrics and Gynecology, Ministry of Health Adana City Training and Research Hospital, Adana, Türkiye
Conflicts of Interest
The authors have no conflicts of interest relevant to this article.
References
- Paladine, H. L., & Desai, U. A. (2018). Vaginitis: diagnosis and treatment. American family physician, 97(5), 321-329.
- Allsworth, J. E., & Peipert, J. F. (2007). Prevalence of bacterial vaginosis: 2001–2004 national health and nutrition examination survey data. Obstetrics & Gynecology, 109(1), 114-120.
- Balci, O., & Çapar, M. (2005). Vajinal enfeksiyonlar. Turkish Journal of Obstetrics and Gynecology, 2, 14–20. [in Turkish]
- Qi, W., Li, H., Wang, C., Li, H., Zhang, B., Dong, M., ... & Xue, F. (2021). Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Frontiers in cellular and infection microbiology, 11, 759795.
- Veronese, M., Salvaterra, M., & Barzaghi, D. (1981). Fenticonazole, a new imidazole derivative with antibacterial and antifungal activity. In vitro study. Arzneimittel-forschung, 31(12), 2133-2137.
- Fung, H. B., & Doan, T. L. (2005). Tinidazole: a nitroimidazole antiprotozoal agent. Clinical therapeutics, 27(12), 1859-1884.
- Martell, B., Kushner, H., Richardson, E., Mize, A., & Mayer, P. (2017). Pharmacokinetics of lidocaine and its metabolites following vaginal administration of lidocaine gel to healthy female subjects. Clinical Pharmacology in Drug Development, 6(1), 27-35.
- Sherrard, J., Wilson, J., Donders, G., Mendling, W., & Jensen, J. S. (2018). 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. International journal of STD & AIDS, 29(13), 1258-1272.
- Nugent, R. P., Krohn, M. A., & Hillier, S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of clinical microbiology, 29(2), 297-301.
- Sobel, J. D. (1990). Vaginal infections in adult women. Medical Clinics of North America, 74(6), 1573-1602.
- Anderson, M. R., Klink, K., & Cohrssen, A. (2004). Evaluation of vaginal complaints. Jama, 291(11), 1368-1379.
- Regidor, P. A., & Sailer, M. (2019). Open Prospective Study to Evaluate the Efficacy of a New Vaginal Pessary Containing 300mg Tinidazole, 200mg Tioconazole and 100mg Lidocaine with a 3-day Regime (GynomaxXL®) in the Treatment of Vaginal Infections due to Bacterial Vaginosis, Candidiasis and Mixed Infections. Biomedical Journal of Scientific & Technical Research, 12(5), 1-11.
- Tavmergen, E., Acet, F., Taner, C. E., Durmuşoğlu, F., Üstün, Y., Dilbaz, B., & Çilli, F. F. (2021). The efficacy and safety of Gynomax® XL vaginal ovule in the treatment of common vaginal infections: a single-arm clinical trial, Gyno-Türk. Clin Obstet Gynecol, 31(4), 120-8.
- Cepický, P., Malina, J., Líbalová, Z., & Kuzelová, M. (2005).
- Schwebke, J. R., Gaydos, C. A., Nyirjesy, P., Paradis, S., Kodsi, S., & Cooper, C. K. (2018). Diagnostic performance of a molecular test versus clinician assessment of vaginitis. Journal of clinical microbiology, 56(6), 10-1128.
- Schwebke, J. R., & Desmond, R. A. (2011). Tinidazole vs metronidazole for the treatment of bacterial vaginosis. American journal of obstetrics and gynecology, 204(3), 211-e1.
- Milani, M., Barcellona, E., & Agnello, A. (2003). Efficacy of the combination of 2 g oral tinidazole and acidic buffering vaginal gel in comparison with vaginal clindamycin alone in bacterial vaginosis: a randomized, investigator-blinded, controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology, 109(1), 67-71.
- Polish Gynecological Society Expert Group. (2012). Stanowisko Zespołu Ekspertów Polskiego Towarzystwa Ginekologicznego dotyczace preparatu Macmiror Complex 500 [Statement of the Polish Gynecological Society Expert Group on the use of Macmiror Complex 500]. Ginekologia Polska, 83(12), 956–9.
- Zlatkov, V., & Karag'ozov, I. (1998). The treatment of vaginal infections with Macmiror and Macmiror Complex. Akusherstvo i Ginekologiia, 37(2), 57-59.
- Karag'ozov, I., Shopova, E., Poriazov, K., Abaliev, I., Kozovski, I., Ianeva, R., ... & Stoĭkov, S. (1999). A multicenter study of the antimicrobial effect of Macmiror and Macmiror Complex in the treatment of vaginal infections. Akusherstvo i Ginekologiia, 38(3), 61-62.
- Polatti, F., Nappi, R. E., Brundu, B., Fantuzzi, M., & Frisenda, L. (2003). Clinical study on the dose-effect relationship of a nifuratel-nystatin combination in the treatment of vulvo-vaginal infections. Arzneimittelforschung, 53(10), 730-737.
- Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C., Eschenbach, D., & Holmes, K. K. (1983). Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. The American journal of medicine, 74(1), 14-22.
- Brewster, E., Preti, P. M., Ruffmann, R., & Studd, J. (1986). Effect of fenticonazole in vaginal candidiasis: a double-blind clinical trial versus clotrimazole. Journal of international medical research, 14(6), 306-310.
- Studd, J. W. W., Dooley, M. M., Welch, C. C., Vijayakanthan, K., Mowat, J. M., Wade, A., & Newell, M. (1989). Comparative clinical trial of fenticonazole ovule (600 mg) versus clotrimazole vaginal tablet (500 mg) in the treatment of symptomatic vaginal candidiasis. Current Medical Research and Opinion, 11(8), 477-484.
- Sobel, J. D., Nyirjesy, P., & Brown, W. (2001). Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clinical Infectious Diseases, 33(8), 1341-1346.
- Tindamax® (tinidazole) Prescribing Information. Mission Pharmacal Company, U.S. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021618s003lbl.pdf Accessed August 01, 2021.


