Current Issue : Article / Volume 2, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-3604/007
Prevalence of Equine Lung Worm and Its Associated Risk Factors in Kersa district Jimma Zone, South West Ethiopia.
1 Jimma University, and Addis Ababa University, Ethiopia.
2 Aklilu Lemma Institute of Pathobiology, Ethiopia.
Fayera Gemeda Dima
Feyera Gemeda Kibinesh Alemu (2023), Prevalence of Equine Lung Worm and Its Associated Risk Factors in Kersa district Jimma Zone, South West Ethiopia. Journal of Covid Research and Treatment, 2(1): DOI: 10.58489/2836-3604/007
© 2023 Feyera Gemeda. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Received Date: 25-03-2023
- Accepted Date: 05-04-2023
- Published Date: 12-04-2023
Dictyocaulus arnifieldi; equines; kersa district; prevalence; risk factors
Abstract
Dictyocaulus arnfieldi parasites directly affect the health and production of working equines, which contributes to the reduction in their work output and ultimately in the income of the owner and the community. A cross-sectional study was conducted from November 2019 to march2020 in and around kersa distinct south west Ethiopia. With the objectives of determining the prevalence and assessing the possible risk factors of lungworm infection in equine. A total of 384 faecal samples from equine species (124 donkeys, 200 horses, 60 mules) were collected and examined for the presence of eggs of parasites using modified Bearmann technique. Out of these, 384(53.1%) equines were found positive for lung worm. The prevalence of lung worm in donkeys, horses, and mules was64.5 %, 49.0%, and43.3% respectively with statistical significant variation (x2 =10.14, P = 0.006). Age of equines was found to have a significant association with the prevalence of Dictyocaulus arnfieldi infection (P<0.05), the prevalence to being in age of greater in young animal. Assessment of the two body condition scores with their prevalence revealed a significant variation, the prevalence was very high in poor body condition groups (x2 =299.99, P = 0.000). However, there was no statistically significant (p>0.05) between the occurrence of equine lungworm and the factors sex. It is concluded that prevalence of equine lungworm in the study area associated with young and emaciated equines were more affected by the lung worm infection. Therefore, Due attention needs to be given to equine health services by district veterinary services office so that equines are handled well in order to earn their maximum potential benefits and grazing management and regular strategic deworming of the whole herd with anthelminthic rather than treating infested individuals is recommended.
Introduction
The world’s equine population reaches 98.3 million (40 million donkeys, 15 million mules, 43.3 million horses). The population of equines in Africa is known to be 17.6 million (11.6 million donkeys, 2.3 million mules and 3.7 million horses). The number of equines in Ethiopia is estimated to be 8.4 million (2.75 million horses, 5.02 million donkeys, and 0.63 million mules). (Taye, 2017))In the livestock sector equines play an important role in the economy of the nation (Mearg et al., 2015).
In addition, equines are important animals to the resource-poor communities in rural and urban areas of Ethiopia, providing traction power and transport services at low cost. Therefore, the health and welfare of equines should be of crucial importance to Ethiopia. Despite technological advancement in transportation industry, equines, donkeys in particular remain the backbone of rural transportation in the region. Donkeys are mainly used for transportation of farm products from farmstead to home, to and from market, grain to and from grinding mill houses, fire wood and charcoal for household use or sale, stone and blocks for construction, water for manual-irrigation like growing cash crops including chat, fertilizers, seedlings, aid-supplies, for ploughing and as cash income for the family. Mules in the urban settings are used for carting with horses (Solomon et al., 2016).
In comparison with other equines, the horse plays a dominant role due to its physical and physiological characteristics and easily demonstrates drought ability and often shows great willingness to undertake such works. Hence, cart horses are a business of way of life and generate a large amount of revenue in the area as a source of sustainable daily income for many people in the town ( Robera et al., 2016). Equines are one of the most important and mostly intimately associated with man ( Yitna et al.,2015).
Despite their great importance donkeys have been suffering from overwork and malnutrition as most of their owners are poor and resource limited and depend on these animals for their
livelihood. In household without donkeys the women take the responsibilities of doing heavy work. Donkeys are often described as sturdy animals, hence are exposed to a variety of diseases and many other adverse conditions. A poorly designed or ill-fitted harness can cause inefficient transfer of power from the donkeys to the implement and cause fatigue, discomfort or injury the donkey (Kassaye and Bedaso, 2015).
The donkey able to survive on poor quality food and many families leave their donkeys to scavenge. Donkeys are hardy and will live longer than other species in the same conditions. Donkeys and horses are herd animals and will happily live in groups with donkeys or animals of a different species such as, sheep and goats. Donkeys and horse are very friendly animals and enjoy the company of humans. They are easily trained and are suitable for handling by children especially donkey (Zeleke, 2017).
Even though mules and donkeys have often been described as sturdy animals; they succumb to a variety of diseases and a number of other unhealthy circumstances. Among these, parasitic infection is a major cause of illness. Different parasite has been reported to cause respiratory problem in equines. Of these Dictyocaulus species has been reported to be the major cause due to the fact that the equine is natural reservoir of the parasite and the parasite being ubiquitous in nature. In horses its prevalence is difficult to establish since infection is rarely become patent, although it is frequently incriminated as the cause of chronic coughing
and increase respiratory rate. Young animal suffer more as compared to the older animals (Tole et al., 2017).
Lungworms are widely distributed throughout the world providing nearly perfect conditions or their survival and development but are particularly common in countries with temperate climates and in the high lands of tropical and subtropical countries. Dictyocaulidae are known to exist in East Africa (Ethiopia, Kenya and Tanzania) and South Africa. Pneumonia can be caused by parasites in the horse. Dictyocaulus arnfieldi is the true lungworm found in the horses, belonging to the super family of Trichostrongyloidea (Mukerem et al.,2017).
The prevalence was found to be 35.3%, 21.1% and 5.8% in donkeys, mules and
horses, respectively with statistical significance difference among study animals (P<0>, 2016). Apart from few studies in other parts of Ethiopia, there has not been any previous information on equine lungworm in kersa woreda where equines are back bone of the economy. The present
study therefore conducted to determine the prevalence of equine lungworm in naturally infected horses, donkeys and mules in kersa woreda and assess the associated risk factors of lungworm infection in the stud area
Despite the above investigations, there is scanty of information related to this parasite and its economic losses in different part of Ethiopia. In the study area there was no so far study was conducted on the prevalence equine lung worm.
Therefore, the aims of this study were;
To determining the distribution and prevalence of Equine Lung Worm (Dictyocaulus Arnfieldi) and Its Associated Risk Factors in selective area of kersa werda.
Literature Review
Definition and Etiology Lungworms are parasitic nematode worms of the order Strongylida that infest the lungs of vertebrates. The taxonomy of this parasite is belonging to kingdom Animalia, phylum Nematode, class Secementea, family Dictyocaulidae, genus Dictyocaulus and species of Dictyocaulus airfieldi (Sudan et al., 2012 ) Dictyocaulus airfieldi is the true lungworm affecting donkeys, horses, mules and zebras and is found throughout the world (Reed, and Bayly.,2017). It is a relatively well adopted parasite of donkeys but tend to be quite pathogenic in horses, where this parasite is endemic (Bowman, 2020).
General Description of Lungworm Parasites
Lungworms are parasitic nematode worms of the order Strongylida that infest the lungs of vertebrates. The name is used for a variety of different groups of nematodes,
some of which also have other common names; what they have in common is that they migrate to their hosts' lungs or respiratory tracts, and cause bronchitis or
pneumonia( Costa, et al 2019.). The lungworm will gradually damage the airways or lung tissue by inciting an inflammatory reaction inside the tissue. Ultimately, the Parasites survive and reproduce in the respiratory tissues. The most common lungworms belong to one of two groups, the super family Trichostrongyloidea or the super family Metastrongyloidea, but not all the species in these super families are lungworms Kahn, and Line,.(2005.)
Donkeys have been found to be the major host and most important reservoir for equine lungworms. They are considered to act as the source of infection; horses play only an ancillary role and become infected after pastured with donkeys (Tihitna et al., 2012). The pathogenic effects of lungworm depend on their location within the respiratory tract, the number of infective larvae ingested, the animal immune status, the nutritional status and age of the host and Larvae migrating through the alveoli and bronchioles produce an inflammatory response, which may block small bronchi and bronchioles with inflammatory exudates. The bronchi contain fluid and immature, latter adult worms and the exudates they produce also block the bronchi. Secondary bacterial pneumonia and concurrent viral infections are of the complication of Dictyocaulosis (Kamil et al., 2017).
General morphology
Adult Dictyocaulus worms are slender, medium sized roundworms which have a whitish to grayish color. Females are about one third longer than males (Ibrahim, 2017). This parasite has both digestive system and nervous system but have no excretory system. Animals become infected with lung worm infection mainly while grazing, but infection can also happen indoors through contaminated hay or bedding (Adere and Tilahun, 2016)
Pathogenesis
The pathogenic effect of lugworms depend on their location within the respiratory tract, the number of infective larvae ingested, the animal immune state, and on the nutritional status and age of the host (Engdaw, 2015). Larvae migrating through the alveoli and bronchioles produce an inflammatory response, which may block small bronchi and bronchioles with inflammatory exudates. The bronchi contain fluid and immature, latter adult worms and the exudates they produce also block the bronchi. Secondary bacterial pneumonia and concurrent viral infections are of the complication of Dictyocaulosis (Abdulkadir et al,.2017).
The major pathologic changes which results from primary infection may be divided in to three stages. These are the prepatent stages, where blockage of small bronchi and bronchioles by eosinophilic exudates produced in response to the developing and migrating larvae. The patent stage, where adult worms causes bronchitis and is primary pneumonia development. The post patent phase is when adult worms are expelled and majority of animals gradually recover. The pathological changes seen in the lungs during necropsy are atelectasis, emphysema, petechial hemorrhage and lung consolidation (Susan, and Aiello,1998)
Epidemiology
Epidemiology depends more on pasture contamination by carrier animals. Pasture infectivity is related to rainfall which stimulates the activity of both the larvae and the mollusk. Moisture is essential for the survival and development of the larvae (Engdaw, 2015). The epidemiology of lungworm disease is largely concerned with factors determining the number of intensive larvae on the pasture and the rate at which they accumulate. The third stage larvae are long living in damp and cool surroundings. Horses are not the favorite host of this parasite and do not usually transmit the disease to other horses. In most instances, horses acquire this disease when pastured with donkey’s .Under optimal condition the larvae may survive in the pasture for a year. They are quite resistant to cold although it generally delays their maturations. Larvae can over winter in cold climates. Most outbreak of verminous pneumonia occurs during cool season specially autumn and early winter because the larvae stages of the causative worms tolerate and prefer low temperatures (Ibrahim, 2017).
Life Cycle
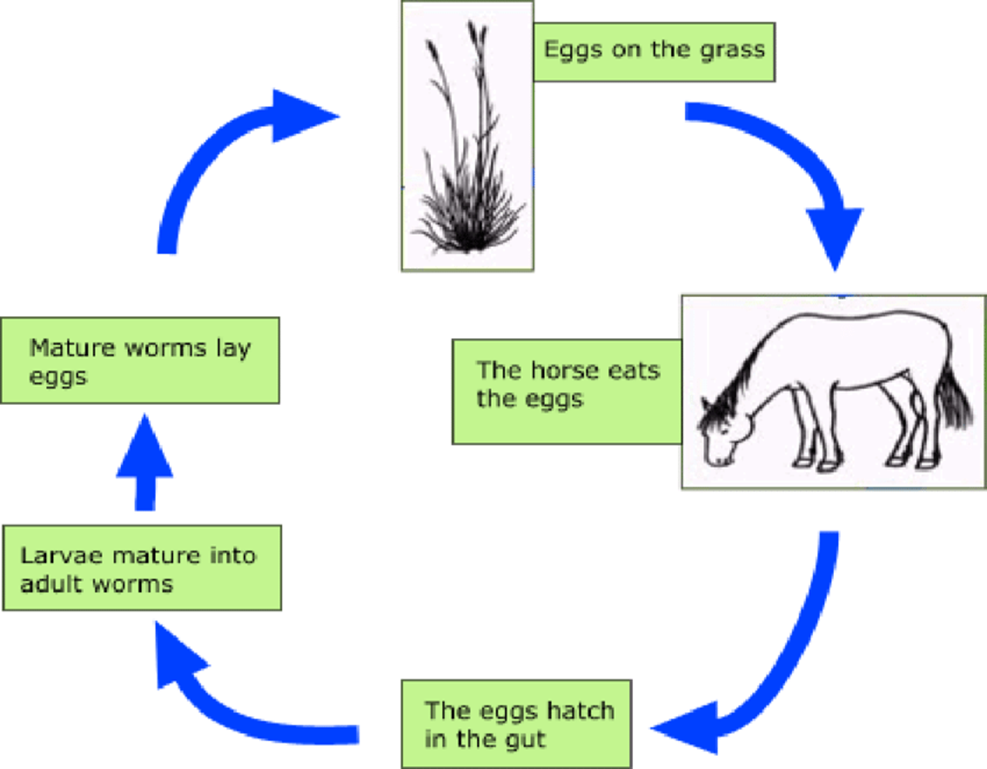
The detailed life cycle is not fully known, but is considered to be similar to that of bovine lungworm,Dictyocaulus viviparus except in the following respect. The adult worms are most often found in the small bronchi and their eggs, containing the first stage larvae, hatch soon after being passed in the faeces. Dictyocaulus worms have a direct lifecycle, i.e. there are no intermediate hosts involved. Adult females lay eggs in the airways of infected hosts. These eggs are transported to the pharynx within respiratory secretions. From the pharynx these eggs are coughed out, into the mouth to be swallowed or directly to the outside (Abdisa, 2018).
Those that are swallowed release the larvae one (L1) in the gut, which are shed in the faeces. Once in the environment, L1-larvae develop to infective L3 larvae in about 1 week. These larvae show a low motility and remain close to the droppings. Animals become infected mainly while grazing, but infection can also happen indoors through contaminated hay or bedding. Once ingested and in the host's gut infective larvae penetrate into the gut's wall and reach the lymphatic nodules where the molt to L4 larvae (Gm et al., 2003).
Fecal Examination
The faecal samples were prepared with the necessary materials. Place a double layer of cheesecloth or gauze on a disposable paper towel or equivalent on the bench. Using a spoon or spatula weigh or measure approximately 5-10 grams of faecal material. Place the faecal material in the centre of the cheesecloth. Form a pouch containing the faecal material by holding the four corners of the cheesecloth together and moulding the cloth around the faecal material. Using a rubber band or length of string close the cheesecloth pouch. Push the stick or short metal rod under the rubber band or string so that the pouch can be suspended. Place the pouch containing the faecal material in the plastic cone. Trim off the excess cheesecloth using scissor. Fill the plastic cone with lukewarm water. Make sure the faecal material is covered. Leave the apparatus to stand for 24 hours.
The supernatant was discarded and sediment was taken .Use a Pasteur pipette to transfer a small droplet of the sediment fluid from the Petri dish to a microscope slide. Add drop of iodine to fix the larvae and gently place a cover slip over the drop. Let Examine under compound microscope at 10 times magnification. Using a Pasteur pipette, remove a drop of the sediment at the bottom of the tube and place it on a microscope slide for examination. Kaufmann, J., (1996).
Clinical Signs
Despite the prevalence of patent D. arnfieldi infection in donkeys, overt clinical signs are rarely seen; however, on close examination slight hyperpnoea and harsh lung sounds may be detected. This absence of significant clinical abnormality may be partly a reflection of the fact that donkeys are rarely required to perfume sustained exercise. Infection is much less prevalent in horses. However, patent infections may develop in foals and these are not usually associated with clinical signs. In older horses infections rarely become patent but are often associated with persistent coughing and an increased respiratory rate (Gm, et al., 2003). Donkeys usually show no disease and can be silent carriers and shedders of this parasite, which causes clinical signs in horses (Johnson et al., 2003).
Clinically characterized by respiratory distress and pathologically by bronchitis and broncho pneumonia due to infection colonize the lower respiratory tract, resulting in bronchitis or pneumonia or both (Amana ., 2019).
Post Mortem Findings
The morphological change in the lungs include wide spread areas of collapsed tissue of dark pink color, hemorrhagic bronchitis with much fluid filling all the air passed and enlargement of the regional lymph nodes. Histological, the characteristic lesions are edema,
eosinophilic infiltration, debris and larvae in the bronchioles and alveoli. The bronchial epithelium is hyperplasic and heavily infiltrated by inflammatory cells,
particularly eosinophils (Abdisa, 2018).
Diagnosis
Diagnosis is based on clinical signs, epidemiology, presence of first-stage larvae in feces, and necropsy of animals in the same herd or flock. Bronchoscope and radiography may be helpful. Larvae are not found in the faeces of animals in the prepatent or post patent phases and usually not in the re infection phenomenon. ELISA tests are available in some laboratories. Bronchial larvae can reveal Dictyocaulus arnfieldi infections in horses (Bekele, and Shibbiru, 2017)
Clinical diagnosis
Typical signs and symptoms are heavy coughing (often paroxysmal), accelerated and/or difficult breathing and nasal discharge. Affected animals lose appetite and
weight. Severe infections can also cause pneumonia (lung inflammation), emphysema (over inflation of the alveoli), and pulmonary edema (liquid accumulation in the airways). Adult livestock usually develops resistance and if re-infected may not show clinical signs but continue shedding larvae that contaminate their environment Junquera, (2014)
Treatment
The equine which has been infected by dictyocaulosis can be treated by administration of anthelmintic like, the benzimidazoles (Fenbendazole) and macrocyclic lactones (ivermectin). Fenbendazole can against all stages of D.arnifield. (Kahn, and Line, 2005).
Control and Preventions
Routine deworming of horses and donkeys may help prevent cross infection when kept together. Pastures that housed donkeys may be infected with lungworm larvae. As a result, horses and donkeys should not be grazed together (Johnson et al., 2003).Reducing pasture contamination with infective larvae is a key preventative measure that can be achieved to a large extent with adequate management measures. Obviously, by very moist weather or where pastures are almost permanently moist survival may be longer. Alternate grazing with sheep and/or horses may be considered, since Dictyocaulus species are quite host-specific (for cattle, sheep & goats, horses).
The longer the absence of the specific host, the higher will be the reduction of its specific lungworm However; this may not be advisable in places infected with gastrointestinal roundworms that are simultaneously parasitic of cattle and sheep or horses. For their first grazing season it is highly advisable that young stock does not share the pastures with older stock that has been exposed earlier to infected grounds and can therefore shed larvae. It must also be avoided that young stock uses pastures already used by older stock during the same season. It must also be considered that heavy rains and flooding can disseminate infective larvae inside a property or from one property to neighboring ones.
Keeping the pastures as dry as possible and keeping livestock away from places excessively humid are additional key measures to reduce the exposure of livestock to infective larvae. In endemic regions preventative strategic treatment of young stock is often recommended just prior to their first grazing season, followed by additional treatments depending on the infestation level of the pastures and the residual effect of the administered anthelmintic (Junquera,2014)
Age and Body Condition Estimation
The age of the selected equines was determined using the incisor eruption times and wear and by asking owner (Etana et al., 2011). Equines were grouped into three age categories namely equines under two years old were classed as young (n=59), those in range of two to ten years were classed as adult (n=250) and those beyond ten years were classed as old (n=75). Body Condition score was assessed subjectively using a scale from 1 (emaciated), 2 (thin), 3 (average), 4(fat) to 5 (very fat) (Abebew, et al., 2012).
Materials And Methods
The study was conducted at Kersa district of the Jimma Zone South western Ethiopia. The site is located at about 318 km from Addis Ababa and 28 km East from Jimma town (7° 40′ 0″ N latitude and 36° 50′ 0″ E longitude) at an altitude of 1740 masl while the climatic condition of the area is “weynadega”. The average annual maximum and minimum air temperatures are 28.80 C and 11.8 0C, respectively .The area has a bimodal rainfall occurring from March to April (a short rainy season) and from July to October (long rainy season).the area receives adequate amount of rain fall, ranging from 1,200 to 2,800 mm per annum. (CSA, 201- )
The study population includes all age groups and both sexes of equine population (horses, mule and donkeys) in kersa Distinct. They were all local breeds, kept under extensive management system used for packing and transportation.
A cross-sectional study was conducted from November 2019 to march 2020 to identify and estimate the prevalence of equines lung warm parasites and the associated risk factors
The desired sample size was calculated using single population proportion formula as given by Thrusfield (2005) at 50% of expected prevalence and 5 Prevalence of Dictyocaulus Arnfieldi among Studied Equines The examination of fecal samples of 384 equines revealed that 204 (53.1%) samples were positive for Dictyocaulus arnfieldi, implying overall prevalence of the disease in the study area. The prevalence of Dictyocaulus arnfieldi by animal species was 64.5%, 49.0% and 43.3%in donkeys, horse and mules, respectively. The highest prevalence was recorded in donkeys followed by horses then mule. Higher prevalence (59.4%) was observed in young animals while the among the adult age groups was 41.5%. Similarly, the prevalence of Dictyocaulus Arnfieldi the male equines was higher (56.1%) compared to that of (46.0%) equines in the study area. The prevalence of the lung worm infection markedly varies by body condition scores that the prevalence score was 98.4% and10.2% among equines with poor and good body conditions, respectively (Table 1). Characteristics Categories Test results, N (%) Positive Negative Total Species Horse 98 (49.0) 102 (51.0) 200 (100) Donkey 80 (64.5) 44 (35.5) 124 (100) Mule 26 (43.3) 34 (56.7) 60 (100) Total 204 (53.1) 18(46.9) 384 (100) Age Young 148 (59.4) 101 (40.6) 249 (100) Adult 56 (41.5) 79 (58.5) 135 (100) Total 204 (53.1) 180 (46.9) 384 (100) Sex Female 52 (46.0) 61 (54.0) 113 (100) Male 152 (56.1) 119 (43.9) 271 (100) Total 204 (53.1) 180 (46.9) 384 (100) Body condition Poor 184 (98.4) 3 (1.6) 187 (100) Good 20 (10.2) 177 (89.8) 197 (100) Total 204 (53.1) 180 (46.9) 384 (100) Total 204 (53.1) 180 (46.9) 384 (100) Table 1: prevalence of dictyocaulus arnfieldi by animal characteristics There was statistically significant difference in distribution of Dictyocaulus arnfieldi among species of the equines (X 2 =10.14; P = 0.006), age of the animals (X 2 =11.33; P= 0.001)and body condition score of the equines (X 2 =299.99; P <0.001). However, there was no statistical significant difference in prevalence of lung wormparasites between sex groups of the equines (X 2= 3.25; P = 0.072) (Table 2). Prevalence of Lungworm Infection in Equines by Age Age wise prevalence of the parasites was observed and its rate was 59.4% in young and 41.5 Prevalence of Dictyocaulus arnfieldi Infection in Equines by body condition score Based on body condition, animals were categorized into two groups, namely good and poor body conditioned animals. The lungworm infection rate according to the physical body condition was Prevalence of Lungworm Infection in Equines by sex There is no statistically significant difference p=0.072, x2 3.25 the prevalence of lungworm with sexes of equine species in this study. Prevalencewas higherin males (56.1%) than in females (46%) observed. Characteristics Categories Test results, N (%) Positive Negative X 2 p-value Species Horse 98 (49.0) 102 (51.0) Donkey 80 (64.5) 44 (35.5) 10.14 0.006 Mule 26 (43.3) 34 (56.7) Total 204 (53.1) 1846.9) Age Young 148 (59.4) 101 (40.6) 11.33 0.001 Adult 56 (41.5) 79 (58.5) Total 204 (53.1) 180 (46.9) Sex Female 52 (46.0) 61 (54.0) 3.25 0.072 Male 152 (56.1) 119 (43.9) Total 204 (53.1) 180 (46.9) Body condition Poor 184 (98.4) 3 (1.6) 299.99 0.000 Good 20 (10.2) 177 (89.8) Total 204 (53.1) 180 (46.9) Total 204 (53.1) 180 (46.9) Table 2: prevalence of Dictyocaulus Arnfieldi in Equine Species with Associated Risk Factors Species of the equines as predictor of the lung worm was statistically insignificant. Although horses (AOR = 0.63; 95% CI: 0.139, 2.890 and donkeys (AOR = 0.38; 95% CI: 0.08, 1.89) seem to have been less affected compared to mules, the differences were statistical insignificant. Again, even though the prevalence of Dictyocaulus arnfieldi was higher among male (56.1%) animals, the role of the animals sex was insignificant (AOR= 0.84; 95% CI: 0.32, 2.20) as predictor of the disease in this study. On the other hand, age and body condition of the equines were found to be predictors of the lung worm infection among the study animals. Young animals were more than six times (AOR = 6.60; 95% CI: 1.87, 23.32) higher chance of being infected compared to adult equines.Again, equines with good body condition were by far less affected (AOR = 0.001; 95% CI: 0.000, 0.003) compared to those with poor body conditions (Table 3). Characteristics Categories P value AOR (95% C.I.) Species Horse 0.556 0.63 (0.14, 2.89) Donkey 0.239 0.38 (0.08, 1.89) Mule 1 Age Young 0.003 6.60 (1.87, 23.32) Adult 1 Sex Female 0.721 0.84 (0.32, 2.20) Male 1 Body condition Good 0.000 0.001 (0.000, 0.003) Poor 1 Table 3: the association between independant logistic variable and lung worm of equine Lung worm infection (verminous pneumonia) is a chronic and prolonged infection caused by nematodes that affects the lungs of equine. This disease results in substantial economic losses due to the reduction of growth rate, morbidity and mortality as the disease exposes animals to secondary bacterial infection. In this study, attempts were conducted to know the current over all prevalence of lung worm infection using Carpological examination of faecal samples of 384 equine in and around kersa District. Examination of faecal samples revealed 53.1% of overall prevalence of lung worm infection in the study area, which is higher than the previous findings (Tihitna etal.,2010) who reported a prevalence of 13.8% in Jimma Town, Tilahu and Adere,(2016)who reported a prevalence of11.2% in Jimma Town,(Mukerem et al, 2017),who reported a prevalence of (25.0%) Omo nada District. The difference could be due to the difference in environmental conditions, sample size, study duration and management practice favoring the survival of the larvae of the parasite (Tihitna et al., 2012). In this study relatively higher prevalence of Dictyocaulus arnfieldi was recorded in donkey (64.5%) than in mule (43.3%) and horses (49.0%).The present prevalence of lungworm infection in donkeys (64.5 %) is similar (65.0%) with finding of a study conducted Brazil (Costa, A.,1996). Yitna et al 2015 also reported similar findings of a study conducted in Lode Hetosa district, southeastern Ethiopia that there was higher occurrence rate of Dictyocaulus arnfieldi donkeys (57.8%) than in mules (45.31%) and in horses (9.37%). The current study is higher than the previous findings of 42.7%, 18.21% and 27.8% which were reported by (Nuraddis et al 2016) from Sudie district, southeastern Ethiopia, (18.21%) Tolesa et,al(2017) from Ambo Town and Mukerem et al, 2017) from Omo nada district, Southwest Ethiopia, respectively. The observed higher prevalence in donkeys might be due to the fact that they are a reservoir host for lung worm (Solomon,et al., 2012.) and attributed to the fact that less attention is given to these animals by far lower than their workload (Tesfaye and Curran, 2005).Additionally, donkeys are considered more common hosts for this parasite, with patent infections sometimes persisting in donkeys throughout their lives. These animals therefore provide the most important sources of pasture contamination (Aram et al., 2018). The present prevalence of lungworm infection in mules (43.3%) is closely in concord with previous finding (45.31%) reported by Yitna,et al 2015from a study in Lode Hetosa District, southeastern Ethiopia. and much more higher than findings (21.1%, 25% and 29.26%) in Jimma Town (Tilahu and Adere, 2016) in Omo-nada District (Mukerem, et al, 2017) and Jimma Town (Tihitna et al 2012), respectively. The prevalence report in horses in the current study is higher than the 31 % prevalence of D. arnfieldi reported by Tolesa, et al. (2017) from Ambo Town. The relatively higher prevalence in horses might be attributed to the difference in the purpose of these animals that in the present study area most of the horses are used for transporting and carts. The difference in prevalence could be due to management differences in the area because most of the horses in the previous area were better managed than donkeys. In comparison with other equines, the horse plays a dominant role due to its physical and physiological characteristics and easily demonstrates drought ability and often shows great willingness to undertake such works Robera et al (2016). In the current study of sex on the overall prevalence of infection showed insignificant difference in susceptibility to infection of lung worms, even though the prevalence in male (56.1%) was found to be relatively higher than in female (46.0%).In addition, farmers are kept male animal for different purposes like transporting and cart as a result male weregiven more dewormed than females. The level of prevalence was compared between animals of different age groups. Higher prevalence (59.4%) was recorded in young (less than 3 years) than (41.5%) in adult (3-10 years) age groups. This finding of equine lung worm infection is in agreement with a finding of another study (Nuraddis., et al 2016), in which young animals were found to be more susceptible than adults.This might be related to the condition that younger animals have lower immunity and management practices than the adult ones. Different levels of prevalence were observed in different body condition score. A prevalence of 98.4%, and 10.2% were recorded in poor and good body condition scores, respectively. The difference in prevalence by equine body condition score was statistically significant (x2=299.99, P=0.000) in the study setting. Almost all of the equines which were classified as poor body condition score were tested positive for lung worm infection. This could be due to the fact that animals with poor body condition might be immune compromised probably due to malnourishment and higher workload, which consequently predispose the animals to harbor more parasitic (Seid Guyo,et,al 2015). Only four variables were considered in the analysis that can affect the statistical stability of the effect estimator. Other important variables like living conditions and animal health care (veterinary services) in the settings where the equines used to live were not assessed for practical reasons. The fact that equines are usually sold to/bought from distant places makes it difficult the assessment of real time/place of exposure to risk factors. Conclusions The prevalence of Dictyocaulus arnfieldi is higher among the equines in the study area. The prevalence significantly varies with animal species, age and their body condition score. Age and body conditions of the equines were found to be predictors of the disease where those young and emaciated equines were more affected by the lung worm infection. RecommendationsResults
adult equines. There was statistically significant difference (p<0>
recorded to be10.2% in animals with good body condition while it was 98.4%in animals with poor body condition. As the table below shows this result indicates a significance association (p<0> Discussions
Limitations of the study
Conclusion And Recommendation
treating individuals is recommended.
References
- Robera Chemeda, Negesse Mekonnen, Yimer Muktar and Waktole Terfa. (2016). Study on Prevalence of Internal Parasites of. American-Eurasian J. Agric. & Environ. Sci., , 16 (6), 1051-1057.
- Yitna Tsegaye, Hailu Degefu, Ketema Bogale and Anwar Shifaw. (2015). Academic Journal of Animal Diseases , 4 (2), 104-111.
- Abdisa, T. (2018). REVIEW ON DICTYOCAULOSIS AND ITS IMPACT IN EQUINE. World Journal of Advance Healthcare Research , 2 (1), 22-28.
- Adere Feye, Tilahun Bekel. (2016). Prevalence of Equine Lung Worm (Dictyocaulus Arnfieldi) and. Advances in Life Science and Technology , 40.
- Amana Mama and Tadele Gashaw. (2019). A study on prevalence of small ruminants lung worm infection. International Journal of Advanced Research in Biological Sciences , 6 (7), 2348-8069.
- Aram Saadi1, Mousa Tavassoli2, Bahram Dalir-Naghadeh1, Awat Samiei2. (2018). A survey of Dictyocaulus arnfieldi (Nematoda) infections in. Annals of Parasitology , 64 (3).
- Engdaw, T. A. (2015). A REVIEW ON: LUNGWORM INFECTION IN SMALL RUMINANTS. World Journal of Pharmaceutical and Life Sciences , 1 (3), 149-159.
- Ibrahim, N. (2017). Equine Lung Worm: A Systematic Review. Global Journal of Medical Research: G , 17 (2), 5.
- Kamil Abdulkadir, Nuraddis Ibrahim* and Yosef Deneke. (2017). Prevalence of equine lungworm and associated risk. African Journal of Agricultural , 12 (18), 1526-1531.
- Kamran Sharifi 1, Hassan Borji 2 and Peyman Milani 3. (2010). First Report of Dictyocaulus arnfieldi Infestation. Iranian Journal of Veterinary Science and Technology , 2 (1), 45-50.
- Kassaye, B. K. (2015). Investigation on Factors Associated with Back Sore in Working Donkeys in. Kassaye., J Veterinar Sci Technol , 6 (5), 2.
- Mearg Fitsum1* Kirmani Monzur Ahmed2. (2015). Population Dynamic Production Statistics of Horse and Ass in. Journal of Biology, Agriculture and Healthcare , 5, 7.
- Mebrahten Gebrekidan, Kamil Kemal, Mukarim Abdurahaman*. (2018). Prevalence of Ovine Lung Worm in and around Jimma, South. nternational Journal of Research Studies in Biosciences (IJRSB) , 6 (5), 24-32.
- Mukerem Kaso, Nesradin Yune and Getinet Kebede. (2017). Prevalence of Equine Lung Worm (Dictyocaulus arnfieldi). Europ. J. Biol. Sci , 9 (4), 187-192.
- Solomon Tiruneh*, Mussie H/Melekot, and Fanaye Shiferaw. (2016). Assessment of carting equine welfare and management practice. International Journal of Advanced Research in Biological Sciences , 3 (12), 100-112.
- Taye, A. (2017)). Epidemiological Study on Equine Gastrointestinal Helminth Parasites in Mekelle, North Ethiopia. Open Journal of Veterinary Medicine , 7, 121-130.
- Tihitna Solomon, 1Basaznew Bogale, 1Mersha Chanie and 2Achenef Melaku. (2012). Ocuurrence of Lungworm Infection in Equines and Their Associated Risk Factors. Global Veterinaria , 8 (1), 35-38.
- Tolesa Negasa 1, Tegegn Dilbato 2, Daba Gudeta 3. (2017). CROSS-SECTIONAL STUDY ON EQUINE LUNG WORM AND. International Journal of Research - GRANTHAALAYAH , 5 (5), 312-319.
- Zeleke, B. (2017). Status and growth trend of draught animals. Journal of Dairy, Veterinary & Animal Research , 6 (1), 4.
- Junquera, P. (2014). Dictyocaulus species, parasitic lungworms of Cattle, Sheep, Goats and other Livestock. Biology prevention and control .Dictyocaulus filarial, Dictyocaulus viviparous, Dictyocaulus arnfieldi. In: Merck Veterinary Manual, Merck and Co, Inc, Whitehouse Station, N.J., USA, Pp: 885-910
- Gm, U., Armour, J., Duncan, J.L., Dunn, A.M. and Fw, J., 2003. Veterinary parasitology 2nd ed. Black well science Ltd.
- Abebew, D., Endebu, B. and Gizachew, A., 2011. Status of parasitism in donkeys of project and control areas in central region of Ethiopia: a comparative study. Ethiopian Veterinary Journal, 15(2).
- Guyo, S., Legesse, S. and Tonamo, A., 2015. A review on welfare and management practices of working equines. Glob J Anim Sc Livers Prod Anim Breed, 3, pp.203-9.
- Kahn, C.M. and Line, S., 2005. The merck veterinary manual 9th Ed. Fluke infection in Ruminants, Merck and Co, White House Station NJ, USA, pp.273-276.
- Tesfaye, A. and Curran, M. (2005). A longitudinal survey of market donkeys in Ethiopia. Holetta Research Center, Addis Ababa, Ethiopia. Tropical Animal Health Production 37(1):87-100.
- Thursfield, M., 2005. Veterinary epidemiology 3rd ed. UK. Black well science Ltd, p.183.
- Taylor, M. A., R.L. Coop and R.L.Hall, (2007). Veterinary parasitology.3rd edition. USA. Blackwell publishing, 874.
- Costa, P.T., Costa, R.T., Kröning, A.B., Fernandes, T.A., Vaz, R.Z., da Motta, S.P., de Mendonça, G. and Farias, P.P., 2019. Efficacy of antiparasitic drugs in control of gastrointestinal helminthiasis in naturally colored and white lambs from southern Brazil. Medicina Veterinária (UFRPE), 13(4), pp.544-551.
- Kaufmann, J. and Kennedy, T.J., 1996. Parasitic Infections of Domestic Animals: A Diagnostic Manual. Parasitology Today, 12(12), p.496.
- Solomon, T., Bogale, B., Chanie, M. and Melaku, A., 2012. Ocuurrence of lungworm infection in equines and their associated risk factors. Global Veterinaria, 8, pp.35-38.
- Johnson, M., MacKintosh, G., Labes, E., Taylor, J.and Wharton, A. (2003):
- Etana, M., Endeabu, B., Jenberie, S. and Negussie, H., 2011. Determination of reference physiological values for working donkeys of Ethiopia. Ethiopian Veterinary Journal, 15(1).
- Anne, M. and Zajac, G., AC, 2006. Veterinary clinical Parasitology, 7thed. American Association of the Proctolohist, pp.185-210.
- Bowman, D.D., 2020. Georgis' Parasitology for Veterinarians E-Book. Saunders.
- Abdulkadir, K., Ibrahim, N. and Deneke, Y., 2017. Prevalence of equine lungworm and associated risk factors in Sudie district, Oromia region, south eastern Ethiopia. African Journal of Agricultural Research, 12(18), pp.1526-1531.
- Susan, E.A. and Aiello, B.S., 1998. The Merck Veterinary Manual.(8thedn) MERCK and CO. INC., Whitehouse Station, NJ, USA.
- Bekele, T. and Shibbiru, T., 2017. Prevalence of Ovine Lungworm and Associated Risk Factors in and Around Debre Berhan Town, Ethiopia. Int J Vet Health Sci Res, 5(6), pp.190-195.
- Reed, S.M., Bayly, W.M. and Sellon, D.C., 2017. Equine Internal Medicine-E-Book. Elsevier Health Sciences.
- Maria da Glória Quintão e SILVA1; Amáiia Verônica Mendes SILVA2; Hélio Martins de. (1996). Dictyocaulus arnfieldi (Cobbold, 1884): comparative. Braz J . vet. Res. anim. Sci. , 33 (4), 223-225.
- Costa, A., 1996. Dictyocaulus arnfieldi (Cobbold, 1884): comparative analysis of the occurrence in horses, mules and donkeys. Braz J. vet. Res. anim. Sci, 33(4), pp.223-225.


