Current Issue : Article / Volume 2, Issue 2
- Research Article | DOI:
- https://doi.org/10.58489/2836-3604/010
Prevalence of Hydatidosis in Cattle, Sheep and Goats slaughtered in Haramaya Municipal Abattoir Eastern Part of Ethiopia
1,2 Jimma University, and Addis Ababa University, Aklilu Lemma Institute of Pathobiology
Feyera Gemeda Dima
Dr. Feyera Gemeda Dima (2023), Prevalence of Hydatidosis in Cattle, Sheep and Goats slaughtered in Haramaya Municipal Abattoir Eastern Part of Ethiopia, Covid Research and Treatment 2(1). DOI: 10.58489/2836-3604/010
© Dr. Feyera Gemeda Dima1. this is an open access article distributed under the CreativeCommons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 25-03-2023
- Accepted Date: 14-04-2023
- Published Date: 18-04-2023
Hydatidosis, Ruminant, Prevalence, cyst cattle, sheep
Abstract
A cross sectional study was carried out from November 2019 to March 2020 with the aims of determining the prevalence and associated risk factors for transmission of the cystic echinococcosis between ruminant animals, and organ distribution of cysts in cattle, sheep and goats at Haranya municipal abattoir, eastern Ethiopia. During this study, 298 cattle, 164 sheep and 88 goatsâ carcass were examined thoroughly for the occurrence of the disease and Cattle, 47(15.8%), sheep 31(18.9%) and goats 3(3.4%) were found to be positive for hydatid cyst by postmortem inspection. Out of examined organs in all species (livers, lungs, kidney and hearts), the infection prevalence was higher in lung (60.1%) lung, than the rest of the organs whereas the liver is second affected organ in all the three species of animals with (p< 0.05) respectively. The prevalence and the number of cysts in ruminants were found different when the cattle, sheep and goats examined were selected randomly based on their origin, sex, age, and body condition. The Highest prevalence was observed (P<0.05) in all cattle, sheep, and goatsâ of poor body condition (19.7%), followed by medium (17.7%) and good body condition (10.5) respectively. There was also a statistically significant difference (P<0.05) in the prevalence of ruminant hydrosis in different age groups considered. The highest prevalence was in adult (16.9%) animals and the lowest (10.5%) was found in young animals. The prevalence of hydatidosis recorded among animal origins with Haranya, Babile, Kersa and Kombolcha were (17.5%), (15.8%) (15.3%) (11.7) respectively and no statistical variation was observed (P> 0.05). However statistical significance variation (P = 0.001) was observed in the prevalence of hydatidosis among female and male. The result showed that out of 153 cysts assessed (56.8%), (31.4%) 18(12%) were fertile, sterile and calcified respectively. Among the 87 fertile cysts, 54(62.1%) and 33(37.9%) of them were found to be viable and non-viable respectively. The present study showed that hydatidurias is considerably prevalent disease of ruminants in the study area. Therefore, it needs to avoid backyard slaughter practice, unsafe offal feeding of dogs, and proper west disposal. Moreover, Public awareness on the use of abattoirs.
Introduction
Ethiopia naturally endowed with different agro-ecological zones and environmental conditions suitable for livestock production. The country is considered to have numerous livestock population in Africa. The country has about 54 million cattle, 25.5 million sheep, and 24.06 million goats by which majority of them are indigenous breeds (CSA, 2017). The livestock sector is an important means of livelihoods of many Ethiopian farmers and has a significant contribution to the national economy. It has a contribution of around 16.5% to the national Gross Domestic Product (GDP) and about 35.6% to the agricultural GDP (Metaferia et al., 2011). Despite the large animal population, productivity in Ethiopia is low and even below the average for most countries in eastern and sub-Saharan African countries, due to poor nutrition, reproduction insufficiency, management constraints and prevailing animal diseases (Bekele et al., 2010). Among them, parasitic diseases are considered as a major obstacle in the health and product performance of livestock. Amongst these parasitic diseases, hydatidosis is one of the most important parasitic diseases, which affects the efficiency of both animals and human being (Francias, 2004; Cringoli et al., 2007).
Cystic Echinococcosis (hydatidosis) is a zoonotic parasitic infection of many mammalian species caused by the larval stage of Echinococcus granulosus (Regassa et al., 2010). Dogs are the usual definitive hosts whilst a large number of mammalian species are intermediate hosts, including domestic ungulates and man (El-Ibrahim, 2009). They are acquiring infection by ingestion of eggs from contaminated grass and water. Upon ingestion, the oncospheres penetrate the intestinal wall and reach visceral organs such as the liver, lung, heart, and kidney of animals and humans (Fakhar and Sadjjadi 2007). The cycle completed when a suitable carnivore eats an intermediate host or its infected organ (Carmena et al., 2008). The adult tapeworm in the definitive host is harmless, unlike the hydatid cyst in the intermediate host animals – that is of immense economic and medical importance in the infected host (Azlaf & Dakkak 2006; Ibrahim 2010).
The disease occurs throughout the world and causes considerable economic losses by decreased livestock production and condemnation of organs containing hydatid cysts in slaughterhouses (Eckert and Deplazes, 2004). Despite substantial research and control efforts, hydatidosis remains endemic and public health problems in many livestock rearing area of the world such as Middle East, Mediterranean, Asia, Africa, Central and South America (Kebede et al., 2009; Endrias et al., 2010). In Africa, E., granulosus has been recognized from most countries including Ethiopia. Previous and recent report has described the endemic occurrence of E. granulosus in dogs and livestock (Eckert et al., 2002). Based on abattoir reports, the prevalence of echinococcosis in animals in hyper-endemic areas ranges from 20%-95% (Bulto, 2015). The disease caused by different species of Echinococcus spp. and its prevalence various among regions due to climate difference and agro ecology, level of education, and development condition (Buishi et al., 2005; Jenkins et al., 2008).
In Ethiopia, abattoir reports from different regions indicated that hydatidosis is highly prevalent disease incurring economic loss and affecting public health (Fufa et al., 2012; Tadesse et al., 2013; Tadesse et al., 2014; Asmerom and Birhanu, 2014; Atsede and Belay,2015; Berhanu and Toffik, 2015). According to abattoir-based studies, the prevalence range of echinococcosis is from 6% in Central Oromia, Adama (Getaw et al., 2010) to 53% in Hawasa (Regassa et al., 2010) in cattle, sheep and goats and also 21% in North Gondar Zone (Abebe et al., 2014). This abattoir survey in different part of the country indicated that the present damage and warning alarm of the future economic and public health consequences.
Certain deep-rooted traditional activities have been described as factors associated with the spread and high prevalence of the disease in the country. These can include the widespread backyard slaughter of animals, the corresponding absence of rigorous meat inspection procedures, feeding domesticated dogs with condemned offal and the subsequent contamination of pasture and grazing fields. This can facilitate the maintenances of the life cycle of the parasite (Jobre et al., 1996). The study of ruminant hydatidosis not so far conducted in Haramaya Municipal Abattoir. Hence, it is essential to obtain baseline data concerning prevalence of the disease and to know public perception about the diseases to specific agro-ecological zones with respect to socio-economic status before contemplating any rational control and prevention programs. Therefore, the objective of the present study was to determine the prevalence and associated risk factors for transmission of the cystic echinococcosis between ruminant animals, to determine organ distribution of cysts and to identify fertility of cysts in study area.
Literature Review
Etiological agents and Taxonomy
Echinococcus granulosus is a small tapeworm of carnivorous with larval (metacestode) stage in the intermediate hosts. It is an etiological agent of disease known as hydatidosis/ echinococcosis. It is a zoonotic parasite (Chandler, et al., 1961; Soulsby, 1986; Siemens, 2003, Millard, et al., 2007). Its belongs to Kingdom Animalia; Phylum Platyhelminthes; Class Cestoda; OrderCyclophyllidea; Genus Echinococcus; Species E. granulosus (Hossein et al., 2014).At present, four species of the genus Echinococcus are recognized and regarded as taxonomically valid: E. granulosus (cystic hydatidosis), E. multilocularis (multivesicular hydatidosis), E. vogeli (polycystic hydatidosis) and E. oligarthrus (Soulsby, 1982). The first of the four and the most common form found in humans and animals is CE (also known as unilocular echinococcosis), which is caused by E. granulosus. The second is alveolar echinococcosis, which is caused by E. multilocularis and, the third is polycystic echinococcosis (in human also known as polycystic hydatid disease, neo-tropical echinococcosis), which is caused by E.vogeli and very rarely, E. oligarthrus. Alveolar and polycystic echinococcoses are rarely diagnosed in humans and domestic animals and are not as widespread as CE. However, polycystic echinococcosis is relatively new on the medical scene. Alveolar echinococcosis is a serious disease that not only has a significantly high fatality rate but also has the potential to become an emerging disease in many countries, (CDC, 2010a). Based on the extent of the genetic, morphological and biological similarity and heterogeneity, they have G1-G3, strains of E. granulosus and are called E. granulosus sensu stricto, while G4, G5, G6-G10 are grouped under the species names Echinococcus equines, Echinococcus ortleppi and Echinococcus canadensis, respectively (CFSPH, 2011).
Morphology and Structural Characteristics
The egg
Echinococcus eggs contain an embryo that is called an oncosphere or hexacanth. The name of this embryo stems from the fact that it has six hooklets (John et al., 2006). They are ovoid (30μm-40μm diameter) and have highly resistant keratinized embryophore which gives the eggs dark striated appearances, the outer capsule quickly disappear once the egg is liberated (Thompson, 1986). Under ideal conditions, it remains viable for several weeks or months in pastures or gardens, and on fomites. They survive in water and damp sand for three weeks at 30°C, 225 days at 6°C and 32 days at 10-21°C (CFSPH, 2011). However, at a relative humidity of 25%, eggs of E. granulosus were killed within 4 days and at 0% within 1 day. Heating that ranges from 60-800C kills the eggs of E. granulosus in less than 5 minutes. On the other hand, Echinococcus eggs can survive at freezing temperature (Reinecke, 1989).
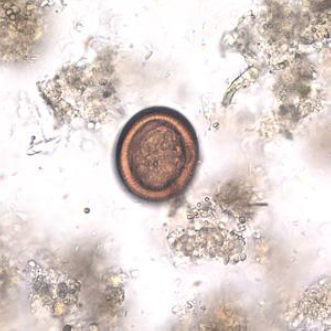
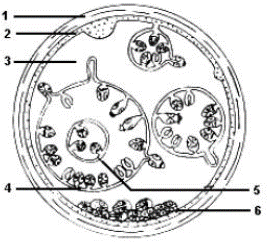
Hydatid cyst (metacestodes)
The cysts are composed of two derived layers of membrane: an inner, nucleated, germinal membrane, and an outer, acellular, laminated layer (Gottstein, 2000). The inner layer is the germinative layer, which gives rise to the hydatid fluid and small secondary cysts (brood capsules), which bud internally from this layer. Fragment of the germinative layer and brood capsules gives rise to daughter cysts. Daughter vesicles (brood capsules) are small spheres that contain the protoscolices and are formed from rests of the germinal layer. Ten to twelve months after infection, protoscolices are produced in the broad capsules. The outer layer is a dense and furious layer and composed of modified host cells. It is the protective layer (Pedrosa et al., 2000).
The structure and development of the metacestode differs among the four species of Echinococcus. In the liver and lungs, the cyst may have diameter of up to 20 cm but in rare sites such as abdominal cavity, where unrestricted growth is possible, it may be very large and contain several liters of fluid (Charles and Hendrix, 1998). For example, the hydatid cyst of E.granulosusis termed a "unilocular cyst" because it has one main expanding, non-invasive cyst body and keeps its brood capsules internalized while Echinococcus multilocularis, spread aggressively through the intermediate host animal's internal tissues and generates external buds of its wal (daughter cysts) on the outside of the main cyst structure, such that they (CFSPH, 2011).
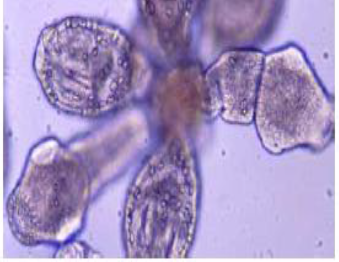
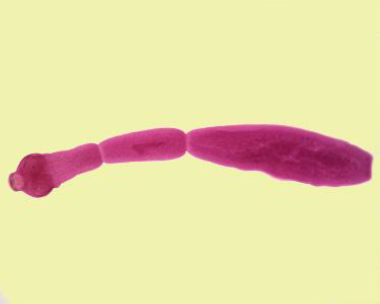
Adult
The adult Echinococcosis granulosus inhabits small intestine of dogs and wild canids. It is small tape worm, measures 3mm-6mm(rarely more than 7 mm) in length and usually has no more than six segments whereas species of Taenia can grow to several meters in length and consist of several thousand segments. It consists of head (scolex), neck and body (strobila). The neck is short and thick, the body usually consists of three proglotids (segments). The first is immature segment, the second is mature and the third is terminal gravid segment (Thompson & McManus, 2002; John et al., 2006). Interiorly an adult Echinococcus possesses a specialized attachment organ, the scolex that has four muscular suckers and two rows of hooks, one large and one small on the rostellum. The body or strobila segmented and consists of a number of reproductive units (proglottids), which may vary in number, from two to six (Mandell et al., 2010). The adult worm is hermaphrodite with reproductive ducts opening at a common, lateral, genital pore, the position of which may vary depending on species and strain. The uterus dilates after fertilization eventually occurring most of the terminal segment when the eggs are fully developed (Eckert and Deplazes, 2004).
Life cycle of Echinococcus granulosus
Echinococcus spp. requires two mammalian hosts for completion of its life cycle. The adult tapeworm found in parts of small intestine of the definitive host, and the segments containing eggs passed with their feaces (Schantz, 2005). The egg ingested by an intermediate host, then hatches in the small intestine of the intermediate host, and releases an oncosphere that penetrates the intestinal wall and moves through the circulatory system into different organs, in particularly the liver and lungs (Gemmell and Lawson, 1986). ). In these organs, the onchosphere encysted within 9 weeks reaching 1cm in diameter. The cysts (2-30 cm) in diameter are constituted by an external acellular cuticle and an inner cellular “germinal layer” (10-25 µm) thick that produce brood capsule containing 6-12 protoscolices or single protoscolex (for fertile cysts). The protoscolex develops into adult tapeworm up on ingestion of the infective metacestode(s) by definitive host usually with viscera from affected intermediate hosts and the protoscolices attach to the intestine. They then develop into adult worms and the cycle starts all over again (CDC, 2010).The hydatid cyst develops slowly over several months, forming an outer laminated membrane called the germinal layer (Schantz, 2005).The definitive host then becomes infected after ingesting the cyst-containing organs of the infected intermediate host (Gemmell and Lawson, 1986).
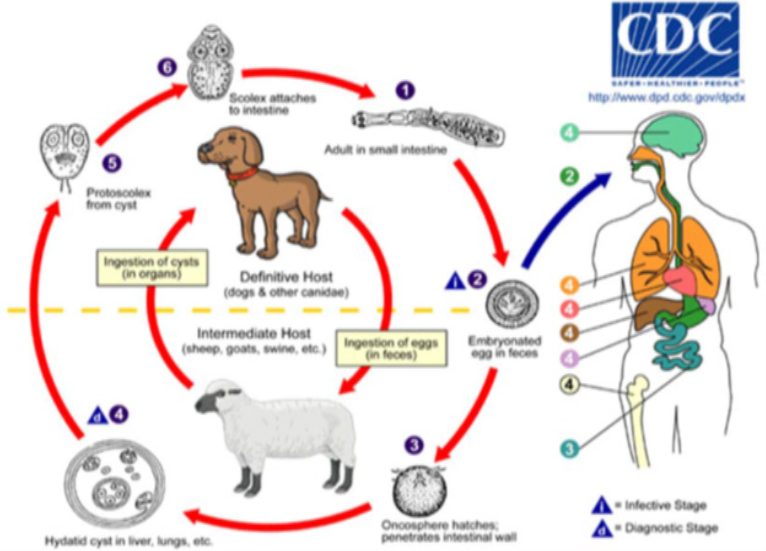
Epidemiology
Global distribution
Hydatidosis has cosmopolitan distribution which was found to have global distribution with differences by its magnitude of occurrence. It is the highest prevalence in parts of Eurasia (especially Mediterranean countries, the Russian federation and adjacent independent states, and China), North and East Africa and South America (Dalimi et al., 2002). The strain specificities of E. granulosus in domestic cycles include, dog/sheep in the Mediterranean region, South America (Argentina, Brazil, Chile, Peru and Uruguay), Africa (Ethiopia, Kenya and Sudan), the middle East and Levant regions, Russia, Central Asia (Kazakhstan, Kyrgyzstan and Uzbekistan), Mongolia, the people’s Republic of China, Oceania and the United Kingdom; dog/horse in Belgium, Ireland and the United Kingdom; dog/cattle in Belgium, Germany, South Africa and Switzerland; dog/swine in Poland; and dog or wolf/reindeer in sub-Arctic regions of Norway, Finland and Alaka (Fasihi et al., 2002; Bart et al., 2006; Carmena and Cardona, 2014).
Areas of high endemicity are also known to occur in east Africa, including at least part of Sudan, Ethiopia, Kenya, and Uganda (Romig et al., 2011). Studies conducted in Africa indicated that dogs, sheep, goats, cattle, camels, and pigs play a good role in maintaining the life cycle of the parasite (Banks et al., 2006).
Cystic echinococcosis in humans the largest number of CE cases in eastern Africa originates from a coherent Generally, the occurrence of E. granulosus is higher in developing countries, especially in rural communities where there is close contact between the dogs, the definitive host, and various domestic animals, which may act as intermediate host (Radfar and Iranyar, 2004).
region covering southeastern South Sudan, southwestern Ethiopia and northwestern Kenya (Romig et al., 2011).
Transmission
The eggs enter into the intermediate hosts by the ingestion of contaminated grass, water, vegetables, and others (Schantz, 1990).The definitive hosts are infected by the means of eating infected cyst-containing organs condemned at the slaughter houses/abattoirs. Humans are accidental intermediate hosts that become infected by handling soil, dirt or animals’ hair that contains eggs. No biological or mechanical vectors for the adults or larval forms of any Echinococcus species found. However, carrion birds, coprophagic flies and other arthropods can act as mechanical vectors for the eggs (CDC, 2013).
Unhygienic practice plays a major role in the maintenance and transmission of the disease in domestic ruminants and humans. This is particularly true in sub-Saharan Africa countries including Ethiopia. In developing countries, due to lack of effective meat inspection, and a backyard slaughter practices, the hydatid cyst infected viscera’s are deliberately left for home and stray dog’s consumption. In these areas, the infection rate with E. granulosus in dogs was reported to be between endemic and hyperactive endemic (Dakak, 2010).
Host range
Risk factor: Certain deep‑rooted traditional activities have been described as a factor associated with the spread and high prevalence of the disease in some areas of the country. These can include the wide spread backyard slaughter of animals, the corresponding absence of rigorous meat inspection procedures, the long-standing habit of feeding domesticated dogs with condemned offal and the subsequent contaminance of the life cycle of E. granulosus which is the causative of cystic hydatidosis and consequently the high rate of infection of susceptible hosts (Jobre et al., 1996). Three kinds of epidemiological cycles are known: rural type, sylvatic type and urban type. Rural type: this is the most frequent and is concerned with domestic dogs and cats and intermediate hosts, which are usually domestic animals. Man infected, directly or indirectly from dogs and cats in villages or on farms. Sylvatic type: This cycle completed through wild canidae and felidae and their wild prey (Wild rodents). This is the most frequent type for Echinococcus multi locularis and the alveolar form of this disease, from wild canidea and felidae, indirectly infects man. Urban type: This cycle completed through the domestic cat and mice, it has been recognized for some time for alveolar echinococcosis (Soulsby, 1986).
Diagnosis in Animals and Treatments
In the definitive host, a post-mortem examination is the most reliable method of diagnosis. There is usually no early parasitological evidence for the presence of cysts in organs or tissues and in most cases the early stage of infections are asymptomatic. Over the last decade diagnosis of hydatid disease was improved due to the use of imaging techniques including ultrasonography, computed tomography (CT scanning) and magnetic resonance imaging (MRI) supported by immunological assays for confirmation of clinical diagnosis (Zhang and Manus, 2003). Recently, a PCR for specific detection of DNA from E. granulosus egg has been developed (Abbasi et al., 2003). Immune diagnosis involves the detection of parasite antigens in feces (coproantigens) and serum antibody detection. ELISA has been described for several groups for the detection of coproantigens released by cestodes, including Taenia species of dogs, and humans (WHO/OIE, 2002). Treatment comprises mainly surgical intervention or percutaneous treatment and/ or high dose, long-term therapy with Albendazole alone or in combination with praziquantel (Brunetti, 2010).
Prevention and Control
Echinococcosis can controlled through preventive measures that break the life cycle of between the definitive and intermediate hosts. These measures include a complete deprivation of dogs from the access of infected raw offal’s by proper disposal of hydatid cysts possessing condemned offal’s at abattoirs, local slaughterhouses, back yards and on farms. Further control methods include introduction of appropriate meat inspection, establishment of local slaughterhouses, education of the people, effective implementation of legislative measures, burning or burial of condemned offal’s and sterilization of offal’s, if it is going to be used as dog food (Craiget et al., 2007). Specific control measures including stray dogs’ control, registration of all owned dogs, spaying of bitches and treatment of all (or most) dogs with praziquantel at predetermined intervals for example every 6-8 weeks (Pedro and Peter, 2009). Prevention can be achieved by strict hygiene measures like hand washing after animals handling, in particular dogs (Parija, 2004). Control movements of food animals and dogs from the infected areas to the “clean” ones; marking and control of movements of animals from infected flocks or herds (Vuitton et al., 2011).
The status of Hydatidosis in Ethiopia
Studies conducted recently in abattoirs of various locations have indicated that hydatidosis is widespread in Ethiopia with great economic and public health significance (Sissay et al., 2008; Fikire et al., 2012; Terefe et al., 2012; Dawit et al., 2013). CE was also reported in humans from the different regions of Ethiopia; northern, central and southern parts of the country (Kebede, 2008). Most of the studies of hydatidosis in Ethiopia were done on cattle, sheep, and goats. Despite the large efforts that have been put into the research and control, it remains a disease of worldwide significance (Torgerson and Budke, 2003a, 2003b). It remains persistent and re-emerging problem in countries of low economic status where a resource for an intensive control program is limited (Schantz et al., 2003).
The highest prevalence (35.15%) was recorded in cattle followed by dromedary (16.79%), ovine (11.87%), caprine (4.9%) and swine (0%). For this, a certain deep-rooted traditional activity have been described as factors associated with the spread and high prevalence of the disease. These can include the wide spread backyard slaughter of animals, the corresponding absence of rigorous meat inspection procedures, the longstanding habit of feeding domesticated dogs with condemned offal and the subsequent contamination of pasture and grazing fields. This can facilitate the maintenance of the life cycle of E.granulosus, which is the causative agent of cystic hydatidosis and consequently the high rate of infection of susceptible hosts (Heath et al., 1974).
Materials and Method of Study
Study Area
The study was conducted from November 2019 to March 2020 on cattle, sheep and goat slaughtered in Haramaya town municipal abattoir Eastern Hararghe. Eastern Hararghe was located in Oromia regional state, which is 500 km far too East of Addis Ababa, the capital city of Ethiopia. Geographically, is situated at 410 51' 58'' N latitude and 90024'10'ꞌS longitude. The area is located at 2000 m altitude above sea level and receives an average annual rain fall of approximately 900 mm, with a bimodal distribution pattern, peaking in mid-April and mid-August. There are four seasons, such as a short rainy season (from mid-March to mid-May), a short dry season (from end May to June), a long wet season (July to mid-October) and a long dry season (end of October to February). Main pasture production expected after the short rainy season, continuing until the end of the long wet season. Mixed type agriculture is the main occupation of the population of the area. Ecologically, the area has 65% midland and 35% lowland zones (Aman and Sitotaw, 2014). The two predominant soil types are 60% rigo soils and 40 % heavy black clay soil. The mean annual temperature ranges from 10°c to 18°C with a relative humidity of 65%. The husbandry system was sedentary system and the animals reared mainly for marketing (HADB, 2010).
Study Population and Sample size
The study populations were cattle, sheep and goats of all ages brought from different parts of eastern hararghe zone to Haramaya municipal abattoir. The origins of slaughtered animals were from different parts of zone, mainly Haramaya, Kombolcha, Kersa and Babile. However, it was difficult to precisely indicate the specific geographical origin of all animals slaughtered and relate the findings on hydatidosis to particular locality. The sample size was determined using a method recommended by Thrusfield (2007):
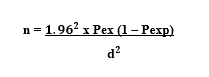
Where: n = required sample sample size,
Pexp = expected prevalence, and
D = desired absolute precision
Since there is no reasonable research done in these areas so far, the sample size calculated using a method recommended by Thrusfield (2007), with 95% confidence interval, at 5
Results
Overall prevalence of ruminants hydatidosis
A cross sectional study was conducted on cattle, sheep and goats from October 2019 to April 2020 at Haramaya municipal abattoir to determine the prevalence identify risk factors and cyst characterization of hydatidosis.
In this study, about 550 animals included, among that 298 cattle, 164 sheep and 88 goats’ carcass were examined thoroughly inspected at postmortem examination for the occurrence of hydatidosis. Out of those sampled animals 47 cattle, 31 sheep and 3 goats were found to be infected with larval parasite (hydatid cyst). Thus, the overall prevalence of ruminants hydatidosis in the study area was found to be 14.7% (95% CI: 4.9%- 10.2%). A prevalence of 15.8%, 18.9% and 3.4% were recorded from cattle, sheep and goats respectively. Prevalence was also determined based on species, age, sex, origin and BCS of the study animals
Prevalence of ruminants hydatidosis by species and origin of animal
In Table 1 summarizes the prevalence of hydatidosis in cattle, sheep and goats. The rate of infection in sheep is higher than cattle that followed by goats. It was statistically significant among the species (Fishers exact = 0.005, P = 0.003).
The highest rate of infection was observed in animals with Haramaya (17.5%), Babile (15.8%) followed by Kersa (15.3%) and Kombolcha (11.7). Although a rate of infection in those four districts is different, no statistical variation was observed (P> 0.05) as indicated in Table 1.
Table 1: Prevalence of hydatidosis by species and origin of animals examined
| Variable | No. of examined | No positive | Prevalence (%) | 95%CI | X ² | P-value | ||||
Species Cattle Ovine Caprine |
298 164 88 |
47 31 3 |
15.8 18.9 3.4 |
|
|
11.5 |
0.003 | |||
Origion Kombolcha Babile Haramaya Kersa |
187 164 114 85 |
22 26 20 13 |
11.7 15.8 17.5 15.3 |
|
2.2
|
0.53
| ||||
Prevalence of ruminants hydatidosis by sex, age and body condition of animals examined
Sex was found to be positively associated with hydatidosis (P = 0.001) with female animal being more likely to test positive than males (Table 2).
From total animal sampled 359 were adult and 191 were young animals. From adult 359 animals sample 298(83%) were infection free and 61 (16.99%) were infected. From young 171 (89.5% were free and 20 (10.5%) were infected; and statistical variation was observed (P <0>
The BCS was found to be positively associated with hydatidosis (P = 0.02) with high rate of infection in poor body condition (19.7%), followed by medium (17.7%) and good (10.5) body conditions as indicated in Table 2.
Table 2: Prevalence of hydatidosis by species, age and sex of animals examined
| Variable | No. of examined | No positive | Prevalence (%) | 95%CI | X ² | P-value |
Sex Male Female |
302 248 |
31 50 |
10.3 20.2 |
|
10.6 |
0.001 |
Age Young Adult |
191 359 |
20 61 |
10.5 16.9 |
|
4.2 |
0.025 |
BCS Poor medium Good Total |
117 175 258 550 |
23 31 27 81 |
19.7 17.7 10.5 14.7% |
|
7.2 |
0.027 |
Distribution of Hydatid Cysts in different organs of Infected Animals
Out of these total examined animals 47 (15.8%), 31 (18.9%) and three (3.4%) cattle, sheep and goats, respectively were found infected with hydatid cyst. Out of 298 examined cattle 34 (11.4%), 18 (6.0), 7 (2.4%) and four (1.3) lung, liver, kidney and heart, respectively were found infected with hydatid cyst. On the other hand, out of 164 examined sheep 23 (14.0%) lung, 15 (9.2%) liver, 3 (1.8%) kidney and 1 (0.6%) heart were found infected with hydatid cyst, out of 88 examined goats 7 (7.9%), 3 (3.4%), 1(1.1%) and 0(0%) lung, liver, kidney and heart were found infected respectively (Table 3).
Table 3: Distribution of hydatid cyst in different organs in cattle, sheep and goats slaughtered in Haramaya municipal abattoir
| Species of animals | No. of animal infected | Infected organs | |||||||||||
| Lung | Liver | kidney | Heart | Total | |||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | ||
| Cattle | 47 | 15.8 | 34 | 11.4 | 18 | 6.0 | 7 | 2.4 | 4 | 1.3 | 64 | 21.1 | |
| Sheep | 31 | 18.9 | 23 | 14.0 | 15 | 9.2 | 3 | 1.8 | 1 | 0.6 | 36 | 25.6 | |
| Goat | 3 | 3.4 | 7 | 7.9 | 3 | 3.4 | 1 | 1.1 | 0 | 0 | 11 | 12.4 | |
| Total | 81 | 38.1 | 64 | 33.3 | 36 | 18.6 | 11 | 5.3 | 5 | 1.9 | 5 | 59.1 | |
Cyst Characterization
Out of the total 93 cysts recorded in the organs of slaughtered cattle 48 (51.6%) small, 28(30.1%) medium and 17(18.3%) large cysts were found. On the other hand, out of 53 total cysts recorded in sheep 33(62.3%) small, 15(28.3%) medium and 5(9.4%) large cysts were found, whereas of 7 cysts recorded in goats 5 (71.4%) small, 1 (14.3%) medium and 1 (14.3%) large were found. The highest numbers of the cyst were recorded in the lungs of cattle 64 and have 41.8% average of occurrence in this organ. In this study, total 153 cysts were found from the slaughtered ruminant organs; 92(60.1%) lung, 39(25.5%) liver, 11(7.2%) kidneys, and 11(7.2%) hearts of slaughtered ruminants in general as presented in table 4.
Table 4: Cyst size and count in relation to involved organs in infected ruminants slaughtered at Haramaya municipal abattoir
Animal species
|
Organs | No. of cyst examined
| Cyst count in terms of size |
Total | ||||||
| small | medium | Large
| ||||||||
| No | % | No | % | No | % | No | % | |||
Cattle
| Lung | 64 | 28 | 30.1 | 22 | 23.6 | 14 | 15.1 | 64 | 68.8 |
| Liver | 22 | 15 | 16.1 | 4 | 4.3 | 3 | 3.2 | 22 | 23.6 | |
| Kidney | 3 | 2 | 2.1 | 1 | 1.1 | 0 | 0 | 3 | 3.3 | |
| Heart | 4 | 3 | 3.2 | 1 | 1.1 | 0 | 0 | 4 | 4.3 | |
Sheep
| Lung | 26 | 14 | 26.4 | 9 | 16.9 | 3 | 5.7 | 26 | 49 |
| Liver | 14 | 8 | 15.1 | 5 | 9.4 | 1 | 1.9 | 14 | 26.4 | |
| Kidney | 6 | 5 | 9.4 | 1 | 1.9 | 0 | 0 | 6 | 11.3 | |
| Heart | 7 | 5 | 9.4 | 2 | 3.8 | 0 | 0 | 7 | 13.2 | |
| Goat | Lung | 2 | 1 | 14.3 | 0 | 0 | 1 | 14.3 | 2 | 28.6 |
| Liver | 3 | 2 | 28.6 | 1 | 14.3 | 0 | 0 | 3 | 42.9 | |
| Kidney | 2 | 2 | 28.6 | 0 | 0 | 0 | 0 | 2 | 28.6 | |
| Heart | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | Cattle | 93 | 48 | 51.6 | 28 | 30.1 | 17 | 18.3 | 93 | 100 |
| Sheep | 53 | 32 | 60.3 | 17 | 32.1 | 4 | 7.5 | 53 | 100 | |
| Goat | 7 | 5 | 71.4 | 1 | 14.3 | 1 | 14.3 | 7 | 100 | |
Cyst viability test result
The cyst were collected from different infected organs and subjected to fertility and viability test. From the total cysts examined 87(56.8%) were fertile, 48(31.4%) were sterile and 18(12%) were calcified. Out of 87 fertile cysts in slaughtered ruminants 54(62.1%) were viable and 33(37.9%) were non-viable (Table 5).
Table 5: Fertility rates and viability of protoscolices of fertile cysts recovered from different organs
Organs
| No of cyst examined
| Fertile | Sterile | Calcified | |||||||
| Total No | Viable | Non-viable | |||||||||
| No | % | No | % | No | % | No | % | No | % | ||
| Lung | 92 | 57 | 37 | 37 | 65 | 20 | 35 | 29 | 19 | 6 | 3.9 |
| Liver | 39 | 25 | 16 | 16 | 64 | 9 | 36 | 7 | 4.6 | 7 | 4.6 |
| Kidney | 11 | 2 | 1.3 | _ | _ | 2 | 100 | 7 | 5 | 2 | 1.3 |
| Heart | 11 | 3 | 2 | 1 | 33 | 2 | 67 | 5 | 3.3 | 3 | 2 |
| Total | 153 | 87 | 56.8 | 54 | 62.1 | 33 | 37.9 | 48 | 31.4 | 18 | 12 |
Discussion
Based on data collected from Haramaya Municipal Abattoir, the prevalence of hydatidosis during the study period was found to be 15.8%, 18.9% and 3.4% were recorded from cattle, sheep and goats respectively with over all prevalence of 14.7%. Statistical analysis shows that there is significant difference among the species with higher prevalence in sheep (18.9%) than cattle (15.8%), and with the lowest prevalence recorded in goats (3.4%) (P= 0.003). The prevalence from the present study were comparable with the findings of Yilma Jobere, (1985) (12.2 % in cattle, sheep and goats) at Bishoftu and Yilma Jobre et al. (1996), (18.8%) in eastern part of Ethiopia. However, the prevalence of hydatidosis in ruminants from this study was higher than reports of that of Alemayehu Lemma, (1990) (2.2% in cattle, sheep and goats) at Arsi. In addition, our study result was lower than the previous reports of (28.5%) (Bisrat Hailu, 2001) Ethiopia.
The main reason for the occurrence of relatively higher prevalence of hydatidosis could be most of the slaughtered goats, sheep, and cattle were adults. It was known that adult animals might be more exposed to the parasite and thus increased possibility of acquiring the infections. Similarly, previous studi strongly suggested prevalence is heavily influenced by age (WHO/OIE, 2001). In addition other factors such as difference in socio-cultural structures and the degree of close association among the society, livestock and dogs are important factor that have been responsible for high prevalence of hydatidosis elsewhere (Macpherson, 1985).
The Prevalence of cattle hydatidosis (15.8%) in the present study agrees with previous findings in different corners of the country and other parts of the World. Such as the works of Kebede et al., (2011), (15.2%) in Birre Sheleko and Dangila areas of Northwestern Ethiopia, Jemere Bekele and Berhanu Butako (2011) (16.85%) in Wolayita Sodo, Kassem et al. (2013) (15%) in Libya, Eisa et al., (1982) (19.14%) in Sudan and Dhote et al., (1992) (12.4%) in India.
However, the prevalence of hydatidosis recorded in cattle in this study can be seen as a lower level of infection when compared to the highest recent prevalence reports elsewhere in Ethiopia (Getaw et al., 2010; Nigatu Kebede et al., 2009; Tadele Tolosa et al., 2009; Shimeles et al., 2018). with this report 46.8%, 34.05%,31.44% and 42.86%, respectively and Cattle hydatidosis studies in other countries also show higher prevalence than the present study including that of 38.9% in Pakistan(Khan et al., 1990), 56.6% in Greece (Hi-Monas et al., 1994) and 48.7% in Tanzania (Ernest et al., 2008).
The variation in the prevalence may be due to the difference in the origin of animals brought for slaughter. Generally, the density, infectivity and availability of the eggs in the environment, the feeding behavior of the intermediate host, together with the external environment have a vital role in determining the dynamics of transmission of parasite (FAO, 1994).
The prevalence of sheep hydatid cyst in current study was found to be 18.9%, this value is in agreement with prevalence reports of Kebede et al. (2010), Mezgebu, (2003), Lahmar et al., (2013) who reported 19.9%, 15.0 % and 16.42% respectively. In Ethiopia and other countries however, Haridy et al. (2000) and Njoroge et al. (2002) recorded lower hydatid cyst infection in sheep; (0.33%) and (3.6%) respectively.
The current overall prevalence of goats hydatidosis found in this study was 3.4%,close agreement with the study of Haridy et al. (2000) and Njoroge et al.(2002), who reported (4.5%) and (3.4%)respectively. It is also relatively agree with prevalence reports of Lahmar et al., (2013), Getachew et al. (2010), Fromsa and Jobre (2011), Sabri et al. (2005) and Kassem et al. (2013) who reported 2.88%, 6.7 %, 4.9%, 5.27% and 2.4% respectively.
The discrepancy and similarity in the prevalence between the various areas might be attributed principally to strains difference and relationship in E. granulosus that exist in different geographical situations (Arene, 1995). Moreover, additional reasons could be the difference in the level of awareness of the community with regard to methods of its transmission as people used to slaughter small ruminants at home and throw the offal’s to the dogs around their villages. Furthermore, difference in culture, social activity and attitude to dog in different regions might have contributed to such inconsistency (Arbabi and Hooshyr, 2006).
The current overall goats hydatidosis reported in this study is lower than the prevalence of 17.4%, 16%, 24.8.1%, and33.3% % recorded in the goats reported in Adama (Habtamu, 2010) Addis Ababa (Kebebe et al 2010), Jimma Town (Kumsa, and Mohammed-Zein, 2012), Egypt (Mosaab et al., 2013), and in Soroti part of Uganda (Nyero et al., 2015) respectively.
This variation might have be resulted from different geographical area of the animals where they were brought to the abattoirs; which implies the distribution of cyst generally varies according to different agro-ecology. Furthermore, the difference in the prevalence indicated above might be attributed to the age, breed of study animals, stocking rates, movements of animals, animal husbandry systems, culture and religion of the society, and number of dogs in different regions of the country (Kebede et al., 2010).
The origin factors showed no significance association with the prevalence’s of the disease (P > 0.05). This could be due to the similarity in the socio-economic status and animal husbandry practices of community in all areas from where animals were bought for slaughter.
Sex was found to be positively associated with hydatidosis (P = 0.001) with female animal being more likely to test positive than males. Blancas et al. (2007) have reported a similar finding. The reason might be associated with keeping of female longer than males for reproductive purposes and therefore have a higher probability of having more encounters with the ova of E. granulosus during their longer life span than their male counterparts have.
The result of the current study showed that age has significant effect on the prevalence of cystic echinococcosis; being higher in adult animals than the young. It was reported that cystic echinococcosis infection was higher for older animals than the younger animals (Azlaf and Dakkak, 2006; Alembante, 2009). Animals with more than one years of age were found to be highly infected that statically significant (P-value = 0.02). This could be mainly because of aged animals have longer exposure time to Echinococcus granulosus eggs.
The other risk factors were body condition score and the result indicated that there was a significant difference (P < 0>et al. (2012), Zelalem et al (2012) and Miheret et al. (2013).
In this study, the lungs and liver were the organs most commonly affected by hydatid cysts in cattle, sheep and goats. This is in agreement with literature, which states that hydatid cysts are most commonly found in the liver and lungs of ungulates Getaw et al. (2010), Kebede (2010), Ibrahim (2010) and Shimeles and Awole (2018). This could be justified by the fact that lungs and liver possess the first greater capillary fields which acts as partial barriers for the ingested oncosphere, Which adopt the portal vein route and primarily negotiates the hepatic and the Pulmonary filtering action sequentially before any other peripheral organ is invaded Matosain (1977). The kidneys and hearts are the least affected organs. Various workers also obtained similar findings and it was indicated that liver and lungs are the most commonly affected organs with hydatid cyst due to the reason that they are the first capillary fields encountered by the blood borne oncospheres of the parasite (Angus, 1987).
From the total 153 hydatid cysts examined in this study, 85 (55.6%) were small, 46 (30.1%) medium and 22 (14.4%) large. The higher proportion of small cyst may indicate late infection of the animals because of heavy rainfall and continuous grazing in the past raining season or due to immunological response of the hosts, which might preclude expansion of cyst size. Rainfall and moisture favour the survival of eggs of Echinococcus species and at the same time eggs may get chance to be disseminated by flood (Urquhart et al., 2003).
In Examination for the condition of cyst fertility and viability (56.8%) fertile, (31.4%) sterile and (12%) calcified cysts were obtained. Out of 87 fertile cyst 62.1% were viable and 37.9% were non-viable. This indicates ruminant animals are an important intermediate host for the perpetuation of the life cycle of the parasite. In comparison with the fertility rate among the organs, the fertility rate of cysts was higher in lungs than liver. This is an agreement with the result of other workers and it has been stated that, the relatively softer consistency of the lung allows easier development of the lung pressure cysts and fertility of hydatid cysts may show a tendency to increase with advancing age of host Hubbert et al. (1995). Additionally,this may be related with reduced immunological compatibility of the hosts at their old age of infections (Getaw, et al 2010; Ibrahim, 2010). However, the percentage of cyst calcification was found high in the liver than in the lungs. This could attribute to relatively high reticuloendothelial cells and abundant connective tissue reaction of the liver (Gemmel et al., 2002).
Conclusion and Recommendations
In conclusion, the findings reported here in show that cystic echinococcosis is widespread in cattle, sheep and goats in Haramaya woreda eastern Oromia. It is the highly prevalent parasitic disease found in ruminant intermediate host including cattle (15.8%), sheep (18.9%) and goats (3.4%) slaughtered at Haramaya municipal abattoir. The lung and liver were the most affected organs than other visceral organs. The documentation of high fertility of cysts in both organs indicated that ruminant animals play an important role in the life cycle of this serious zoonosis and the presence of potential risks of transmission to other intermediate hosts in the study area. The problem warrants well-organized control intervention.
Based on these facts, the following recommendations forwarded:
- Public education on means of transmission, prevention and control strategies of E. granulosus
- Disposal of affected offal freely for dogs and wild canids (the usual practice in the community) should be stopped and all the condemned organs should be either buried or incinerated.
- Backyard and roadside slaughtering practices should be prevented by putting the law and regulation of meat inspection into action.
- Well-equipped and standardized abattoirs should be Established
- Use of vaccine and improving public awareness by means of education
- Regular testing and treatment of definitive host should be practiced throughout the country and avoid stray dogs are important.
References
- Abbasi I, Branzburg A, Campos PM, Abdel Hafez SK, Roul F, et al. (2003) Copro-diagnosisis of Echinococcus granulosus infection in dogs by amplification of newly identified repeated DNA sequence. American Journal of Tropical Medical Hygiene 69: 3254-3360.
- Abdel-Rahman N, Daragmeh N, Adwan M, Al-Qaoud D, Abdel Hafez SK (2001) Human Cystic Echinococcosis in the west bank of Palestine: Surgical incidence and Sero- epidemiological study. p.19
- Abebe A, Desat B, Kumsa B (2014) Cystic echinococcosis in cattle slaughtered at Gondar Elfora export Abattoir, northwest Ethiopia. J. Parasit Dis 38: 404-409.
- Abebe F, and Yilma J (2013) Estimated annual economic loss from organs condemnation, decreased carcass weight and milk yield due to bovine hydatidosis. Ethiopian Veterinary Journal 16(2): 1-14.
- AcostaJamett, G., S. Cleaveland, A.A.Cunningham, B.M.C.Bronsvoort and P.S. Craig,(2010). Echinococcus granulosus infection in humans and livestock in the Coquimbo region,north-central Chile. Veterinary Parasitology, 169: 102-110.
- Alembante, M. (2009). Study on Prevalence and Economic Significant of Hydatidosis in Cattle Slaughtered at Hawassa Abattoir, DVM Thesis, JUCAVM, Jimma, Ethiopia. Pp. 24
- Arbabi, M. and Hooshyr, H. (2006): Survey of Echinococcosis and hydatidosis in kashan region, central Iran, Iranian public health J., 35:75-81.
- Arene, F.A.I. (1995): Prevalence of hydatidosis in domestic livestock in the Niger Delta,Trop. Anim. Health Prod, 17:3-5.
- Ashenafi Markos (2013), Prevalence of bovine cysticercosis and hydatidosis inWolayita Sodo Municipality Abattoir, southern Ethiopia, MSC Thesis. Department of Biology, college of Natural and computational science, Haramaya University; 34-55
- Asmerom Asfaw and Birhanu Afera (2014), Prevalence of Hydatid cyst in cattle Municipal Abattoir of Shire, J. Veternar Sci Technolo, 5: 186.
- Atsede Brehane and Belay Abeba, (2015) Epidemiological Investigation of HepatoPulmonaryBovine Hydatidosis and its economic and zoonotic importance at Jimma Municipal abattoir, Ethiopia. Agriculture and Health Care, Journal of Biology 5: 11.
- Epidemiological 10. Azlaf, R. and Dakkak, A. (2006study of the cystic echinococcosis in Morocco, Vet.Parasitol, 137: Pp 83 -93.
- Blancas, M.M., Herrera, E.R., Rodrguez, P.C., Tavizn, J.P.,Mercado, R.M., Badillo, A.V., Echavarra, F., Lpez, S.A. and Mondragn, C. (2007). Gender as a factor of susceptibilityto infection in experimental hydatidosis Rev Latinoam Microbiol., 49(1–2): 31–37.
- Bekele, J., Asmare, K., Abebe, G., Ayelet, and G, Esayas, G (2010). Evaluation Deltamethrin applications in the control of tsetse and trypanosomosis in Southern rift valley areas of Ethiopia. Veterinary Parasitology, 168:177-184.
- Bekele, J., and Butako, B., 2011. Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, Southern Ethiopia. Trop. Anim. Health. Prod. 43 (1):222 -228.
- Berhanu Haftu and Toffik Kebede (2015) Study on prevalence and economic significance of bovine hydatidosis in Bako Municipal Abattoir,West shoa zone , Oromia Regional State, Ethiopia . Global Journal of Scientific Research 3: 109-118
- Bouree, P (2001) Hydatidosis: dynamics of transmission. World J. Surg. 25: 4-9.
- Brunetti E, Kern P, Vuitton DA (2010) Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans Acta Trop 114:1–16. doi: 10.1016/j.actatropica. 2009.11.001
- Bulto, GB (2015) Hydatidosis in ruminants and Echinococosis in dogs: Epidemiology, histopathology and public health significance in Central Oromia, Ethiopia: Doctoral thesis of echinococcosis epidemiology research CVMA, University of Addis Ababa, 1-40
- BBuishi I, Njoroge E.M, Bouamra O. and Craig, P.S (2005): Canine echinococcosis in northwest Libya: assessment of coproantigen ELISA and a survey of infection with analysis of risk factors. Vet. Parasitol.130: 223-232.
- B. Lemma F. Gabre-Ab, and S. Tedla, (1985) Studies on fascioliasis in four selected sites in Ethiopia, Veterinary Parasitology, vol. 18, no. 1, pp. 29–37.
- https://www.researchgate.net/profile/Eyasu-Ejeta/publication/279036559_Prevalence_Public_Significance_and_Financial_Loss_of_Hydatid_Cyst_on_Cattle_Slaughtered_at_Nekemte_Municipal_Abattoir_Western_Ethiopia/links/559389df08ae1e9cb429a6ab/Prevalence-Public-Significance-and-Financial-Loss-of-Hydatid-Cyst-on-Cattle-Slaughtered-at-Nekemte-Municipal-Abattoir-Western-Ethiopia.pdf
- CDC (2013), Hydatid Disease, retrieved 20 March 2014
- CFSPH (Center for Food Security and Public Health), (2011): Echinococcosis: Iowa State of University, College of Veterinary Medicine., Iowa, USA. pp. 1-14
- Chandler, A.S., Read, A.C., Clark, P. (1961): Introduction to parasitology with special reference to the parasite of man 10th edition. Jon Wiley and Sons, Inc, New York, pp. 361-367
- CraigP S, Mc Manus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, et al. (2007) Prevention and control of Cystic echinococcosis. Lancet Infectious Disease 7: 385-394.CSA (2017), Agricultural sample survey report on livestock and livestock characteristics, private and peasant holdings, Addis Ababa, Ethiopia, Vol. II, and Statistical Bulletin 585.
- Dakak, A. (2010) Echinococcosis/hydatidosis; a severe threat in Mediterranean countries: Vet. Parasitol, 174: 2-11
- Dalimi A, Motamedi G, Hosseini M, Mohammadian B, Malaki H, Ghamari Z, Ghaffari FF (2002), Echinococcosis / Hydatidosis in western Iraq; Vet. Parasitol. 105:161-171.
- Dawit G.,Adem A., Simenew K. and Tilahun Z. (2013) Prevalence, Cyst characterization and Economic importance of Bovine hydatidosis in Mekelle municipality abattoir, northern Ethiopia. Int. J. Med. Bio-Med. Sci., 5:87-93.
- Delaunta, A. and Habel, R. (1986): Applide veterinary anatomy, USA, philadephina: W.B saunders comp Pp 4-9.
- Desiye, T. and Mersha, C. (2012): Study on Rumen and Reticulum Foreign Bodies in Cattle Slaughtered at Jimma Municipal Abattoir, South West Ethiopia. American-Eurasian Journal of Scientific Research, 7(4):160-167
- Eckert J, Deplazes P, Craig PS, Gemmell MA, Gottstein B, Heath D, Jenkins DJ, Kamiya M, Lightowlers M (2001): Echinococcosis in animals: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS (eds): WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, 72–100.
- Eckert, J., Schantz, PM. and Gasser, RB, (2002) WHO/OIE manual in echinococcosis in humans’and animals. Geographic distribution and prevalence: World health Organization and World Organization for Animal Health, Paris, Pp: 101-143.
- Eckert J, Deplazes P, (2004). Biological, Epidemiological and Clinical aspects of Echinococcus a zoonosis of increasing concern: Clinical Microbiology Review 17: 107-135.
- El Ibrahim, J.H. (2009) Prevalence of Sheep Hydatidosis in North West Bank Palestine, MSc Thesis, An-Najah National University, Nablus, Palestine.
- Elmahdi, I., Ali, E., Magzoub, Q., Ibrahim, M., Saad, A. and Roming, M. (2004) Cystic echinococcosis of livestock and human in central Sudan, Ann. Trop. Med. Parasitol.,98: 473-479
- Endrias, A., T. Yechale, M. Assefa, (2010) Major Bovine hydatidosis: Ambo municipal abattoir, West Shoa, Ethiopia. Ethiopian Veterinary Journal, 14: 1-14.
- Esatgil, Mu. and Tuzer, E. 2007.Prevalenceofhydatidosisinslaughteredanimals, Turkey.Turkiye Parazitoloji Dergisi, 31 (1): 41 – 45.
- https://www.jstor.org/stable/2389193
- Thesis, FVM, Fikre L (1994) Echinococcosis in konso: an assessment of its prevalence, economic and public health importance, DVM AAU, DZ, Ethiopia, pp. 10.
- Z., Tolosa T., Nigussie Z., Macias C. and Kebede N. (2012): Prevalence and characterization Fikire of hydatidosis in animals slaughtered at Addis Ababa abattoir, Ethiopia. J. Parasitol. Vector Biol., 4:1-6.
- Fufa Abunna, Sisay Fentaye, Bekele Megerese, Alemayehu Regasa, (2012) Prevalence of bovine hydatidosis in Kombolcha ELFORA abattoir, northeastern Ethiopia. Open J Ani Sci.; 2: 281-286.
- Francias, E. (2004): Manual of diagnostic tests and vaccines for terrestrial animals. Echinococcosis/hydatidosis, Pp: 62-73.
- Fromsa A and Jobre Y (2011) Infection prevalence of hydatidosis (Echinococcus granulosus, Batsch, 1786) in domestic animals in Ethiopia: A synthesis report of previous surveys Ethiop Vet J 15: 11-33.
- Gebretsadik B (2009) Abattoir survey on cattle hydatidosis in Tigray Region of Ethiopia. Trop. Anim. Health Prod. 41: 1347-1352.
- Gemmell MA, and Lawson JR (1986) Epidemiology and control of hydatid disease, the biology of Echinococcus and hydatid disease (R. C. A. Thompson, Ed.), Allen and Unwin, London, Pp: 189-216.
- Getaw A, Beyene D, Ayana D, Megersa B, Abunna F (2010) Hydatidosis: Prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, central Oromia, Ethiopia. Acta Trop 113: 221-225.
- Gottstein B. (2000). Hydatid Disease, Major Tropical syndromes by body system, Systemic infections, Chap 169, section 6.
- Habtamu A (2010) Study on prevalence and economic loss of hydatidosis in slaughtered ruminants at Adama abattoir, DVM Thesis, Collage of Health Science, SVM, AAU, pp. 11.
- Haftu and Kebede, (2014) Study on Prevalence and Economic Significance of Bovine Hydatidosis in Bako Municipal Abattoir, West Shoa Zone, Oromiya Regional State. Journal of Veterinary Science & Technology 5: 1-5.
- Haridy, F.M., B.B. Ibrahim and T.A. Morsy, (2000) Sheep-dog-man.The risk zoonotic cycle in Hydatidosis J. Egyptian Society of Parasitol., 30: 423-429.
- Haridy FM, Ibrahim BB, Elshazly AM, Awad SE, Sultan DM, et al. (2006) Hydatidosis granulosus in Egyptian slaughtered animals in the years 2000-2005. J Egypt Soc Parasitol 36(3): 1087-1100.
- Herd, D.B, and L.R. Sprott (1986) Body Condition, Nutrition and Reproduction of Beef Cows; Texas Agricultural Extension Service, B-1526
- Himonas, C., 1987. The Fertility of Hydatid cyst in Food Animals in Greece, Helmenth, Zoonosis, Martin, Nijohoft, Publisher, Neitherland, pp: 12-18.
- Hossein, M., Ebrahim, S., Dezaki, F., Kheirandish, B., Ezatpour, S., Jahanbakhsh, M., Fasihi, H. (2014) Scolicidal Effects of Black Cumin Seed (Nigella sativa) Essential Oil on Hydatid Cysts; Korean J. Parasitol.,52(6), 653-659. doi: 10.3347/ kjp.2014.52.6.653
- Hubbert WT, Culloch WF, Scnurren Beger AA (1995) Diseases Transmitted from Animals to man. Sixth (Edn.) Choreler C, Thomas Publisher, Spring Field Illinosis, USA, pp. 682-692.
- Jenkins, D., Lallen, J. and Goullet, M. (2008): An encroachment of Echinococcus granulosus into urban areas in eastern Queensland, Australia, Aust Vet J. 86: 294-300.
- Jobre, Y., F. Lobago, R. Tiruneh, G. Abebe and P. Dorchie, (1996) Hydatidosis in three selected region in Ethiopia: an assessment trial on its prevalence, economic and public health importance. Revue de Medicine Veterinary147: 797-804.
- John T., David and William, A. Petri, (2006) Markell and Voge's (eds). St. Louis, MI Saunders Elsevier, Medical Parasitology 9: 224-231.
- Kassem HH, AbdelKader AM and Nass SA (2013) Prevalence of hydatid cysts in slaughtered animals inS irte, Libya; J Egypt SocParasitol43(1): 33-40
- Kebede, W., Hagos, A., Girma, Z. and Lobago, F. (2009b): Echinococcosis/hydatidosis: its prevalence, economic and public health significance in Tigray region, North Ethiopia, Trop. Anim. Hlth. and Prod.,41:865-871.
- Kebede, N., Mitiku, A. & Tilahun, G., (2009b) Hydatidosis of slaughtered animals in Bahir Dar Abattoir, Northwestern Ethiopia, Tropical Animal Health and Production 41, 43–50. PMID: 19052901, http://dx.doi.org/10.1007/s11250-008-9152-3
- Kebede, N., Gebre-Egziabher, Z., Tilahun, G. & Wossene, A., (2011) Prevalence and financial effects of hydatidosis in cattle slaughtered in Birre-Sheleko and Dangila abattoirs, Northwestern Ethiopia’, Zoonoses and Public Health 58, 41–46. PMID: 19638161, http://dx.doi.org/10.1111/j.1863-2378.2009.01250.x
- Kebede N (2010) A retrospective survey of bovine hydatidosis in three 325abattoirs of Amhara National Regional State, northwestern Ethiopia. Trop Anim Health Prod42(3): 323-.
- Kebede, E. (2008): Study on prevalence, economic and public health importance of bovine hydatidosis slaughtered animals at Addis Ababa abattoir, MSc Thesis, AAU, Debre Zeit, Ethiopia. Pp: 17-23.
- Kose, M. and F. Kirakli-Sevilmi (2008): Prevalence of cystic echinococcosis in slaughtered cattle in Afyonkarahisar, Tûrkiye. Parasitoloji. Dergisi. 32: 27-30
- Kumsa, B. and Mohammed-Zein, A.L, 2012.Prevalence, organ distribution, risk factors, and financial losses of hydatid cysts in sheep and goats slaughtered in restaurants in Jimma, South Western Oromia.CompendClin.Pathol., 11: 333-339.
- Lahmar, S., Trifi, M., Ben Naceur, S., Bouchhima, T., Lahouar, N., Lamouchi, I., (2013) Cystic echinococcosis in slaughtered domestic ruminants from Tunisia.J. Helminthol., 80: 318–325.
- Lemma, A. (1990) Prevalence of hydatidosis in cattle, sheep and goats, and E. granulosus in dogs in Arsi Administrative region, Ethiopia, DVM Thesis, FVM, AAU, Debre Ziet, Ethiopia, pp. 18.
- Macpherson C NL, Zeyhle E, Roming T (1985) An Echinococcus plot control program for North West Turkan Kenya Ann Trop Med Parasite 78: 188-192
- Maillard, S., Benchikh-Elfegoun, M.C., Knapp, J., Bart, J.M., Koskei, P., Gottstein, B. and Piarroux, R. (2007): Taxonomic position and geographical distribution of the common sheep GI and camel G6 strains of Echinococcus granulosus in three African countries Parasitol Res. 100: 495-503
- Matosain RM (1977) Hydatidosis, A global problem of increasing importance, Bul WHO, Balkans 55: 499-507.
- Ibrahim MM (2010) Study of cystic echinococcosis in slaughtered animals in Al Baha region, SaudiArabia; interaction between some biotic and abiotic factors, Acta Tropica, 113(1): 26-33.
- Lu DY. HIV/AIDS Treatments, Fight for a Cure. LAMBERT Academic Publishing. Ed Da-Yong Lu, 2017, Germany (ISBN-978-3-330-07665-5)
- Metaferia, T. Cherenet, A. Gelan et al., A Review to ImproveEstimation of Livestock Contribution to the National GDP, Ministry of Finance and Economic Development and Ministry of Agriculture, Addis Ababa, Ethiopia, 2011.
- Mezgebu, M., (2003) Survey on hydatidosis and lung infestation in and around Addis Abeba, DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, DebreZeit, Ethiopia (unpublished).
- Miheret M, Biruk M, Habtamu T, Ashwani K (2013). Bovine Hydatidosis in Eastern part of Ethiopia. MEJS 5(1):107-104
- Mohammed J. M.; Maysam N. A. and Mohammed T. D. (2016) Determination the causative strain for hydatid cyst in Iraqi cattle by using ND1 gene The Iraqi Journal of Veterinary Medicine, 41(1), p;11.
- Mosaab Omar, Sultan K, Haridy M and Omran A (2013) Prevalence of cystic echinococcosis in slaughtered ruminants in different abattoirs in Upper Egypt; American Journal of Animal and Veterinary Sciences8(3): 117-121.
- M. Ansari-Lari, 2005, “A retrospective survey of hydatidosis in livestock in Shiraz, Iran, based on abattoir data during 1999-2004,” Veterinary Parasitology, vol. 133, no. 1, pp. 119–123.
- Njoroge, E.M., P.M. Mbithi, J.M. Gathuma, T.M. Wachira, P.B. Gathura, J.K. Magambo and E. Zeyhle, (2002) A study of Cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya.Vet. Parasitol. 104: 85-91
- NRC, (1996) Growth and Body Reserves; Nutrient Requirements of Beef Cattle 7th ed. Washington, D.D: Natl. Acad. Press.
- Nyero D, Zirintunda G, Omadang L and Ekou J (2015) Prevalence of hydatid cysts in Goats and sheep slaughtered in Soroti Municipal Abattoir, Eastern Uganda. Afri J Parasitol and Res 2(9): 148-151.
- Pedrosa, I., Saiz, A., Arrazola, J., Ferreiros, J. and Pedrosa, C.S. (2000): Hydatid Disease: Radiologic and Pathologic Features and Complications. pp. 123-129.
- Pedro M, and Peter MS (2009) Division of Parasitic Diseases, Coordinating Center for Infectious Diseases, Atlanta, Georgia, and USA. Center of Disease Control and Prevention 13: 125-133.
- Regassa F, Mola A, Bekele J (2010) Study on the prevalence of cystic hydatidosis and its economic significance in cattle slaughtered at Hawassa Municipal abattoir, Ethiopia. Trop Anim Health Prod 42: 977-984
- Sabri JH, Hassan MA, Ramadan MY and Khalifa NO (2005) Hydatidosis in sheep, Goats and human contacts, Benha J Vet Med 16: 2-2
- Sabzi, F., Ghasemi, F., Madani, H. and Faraji, R., (2013) Hydrated Cyst of the Right Atrium Wall. East. Mediterr. Health J., 19, 220-223.
- Scala, A. and R. Mazette, (2009) Cystic echinococcosis in the sheep: causes of its persistence in Sardinia. Vet. Res. Commun., 33: 41-45.
- Schantz PM, (1990) Parasite zoonosis in perspective. International Journal of Parasitology 21: 165-166
- Schantz PM, (2005) Progress in Diagnosis, Treatment and Elimination of Echinococcosis and Cysticercosis. Parasitology Internal 20: 30-37.
- Schantz P. M., Wang H., Qiu J., Liu F., Saito E., Emshoff A., Ito A., Roberts J. and Delker C. (2003): Echinococcosis on the Tibetan Plateau: Prevalence and risk factors for echinococcosis in Tibetan populations in China. Parasitol., 127:109-120.
- Seimenis, A. (2003): Overview of the epidemiological situation on echinococcosis in the Mediterranean Region. Acta Trop. 85:191-195.
- Shimeles A, Awole M (2018). Crossectional Study on the Prevalence And Economic Significance of Hydatidosis in Slaughtered Ruminants at DebrezeitElfora Export Abattoir Oromia Region Eastern Showa Zone, Ethiopia, Biomed J Sci&Tech Res 3(3).
- Sisay, M.M., Ugla, A. and Waller, P.J. (2008) Prevalence and seasonal incidence of larval and adult cestodes infections of sheep and goats in eastern Ethiopia, Trop. Anim.Health Prod 40: 87-94.
- Soulsby, E.J.L. (1986). Helminth, Arthropods and Protozoa of Domesticated Animals 7th edition, Baillere Tinidall London, pp. 119-124.
- Surhio A. S., Bhutto B., Gadahi J. A, Akhter N. and Arijo A. (2011) Research opinions in animal and veterinary sciences; Study on the prevalence of caprine and ovine hydatidosis at slaughterhouses of Larkana, Pakistan. Roavs, 1: 40-43
- Terefe D., Kebede K., Beyene D. and Wondimu A. (2012): Prevalence and financial loss estimation of hydatidosis of cattle slaughtered at Addis Ababa Abattoirs Enterprise. J. Vet. Med. Anim. Health., 4: 42-47.
- Thompson, J. and Meyer H., (1994) body condition scoring of sheep and goats, Oregon State University extension service, Pp: 1-4.
- Toulah FH, ElShafaeisAA, and Alsolami MN (2012) Prevalence of Hydatidosis amongslaughtered animals in Jeddah, Kingdom of Saudi Arabia, J Egypt Soc Parasitology 42(3): 563-572.
- Urquhart G. M., Armour J., Duncan J. L, Dunn A. M. and Jennings F.W. (2003) Genus Echinococcus: In Veterinary Parasitology Second ed. Blackwell sciences
- Urquhart, A.M, (1996): Veterinary parasitology, 2nd edn, Blackwell Science puplishing, Scotland Pp. 27-130
- Vuitton DA, Economides P, and WHO-IWGE EurEchinoReg Network (2011) Echinococcosis in Europe Web site, Echinococcosis in Western Europe, a risk assessment/riskmanagement approach.
- Wahlers K, Menezes CN, Wong ML, Zeyhle E, Ahmed ME, et al. (2012) Cystic echinococcosis in sub-Saharan Africa. Lancet Infect Dis 12(11): 871-880.
- WHO, (2015) Echinococcosis; World Health Organization, Fact Sheet No. 377
- Yihdego H.1997. Hydatidosis: Prevalence and economic impact in bovine at Mekele municipal abattoir, zoonosis and infection in dogs, DVM thesis, FVM, AAU, Debrezeit, Ethiopia
- Jobre Y, Lobago F, Tiruneh R, Abebe G, Dorchies P (1996) Hydatidosis in three selected regions in Ethiopia: An assessment trial on its prevalence, economic and public health importance. Review Medicine Veterinary 147: 797-804.
- Zhang W, Li J, Mcand Manus DP (2003). Concepts in immunology and diagnosis of hydatid disease, Clinical.Microbiology.Review, p. 18-36
- Zelalem F, Tadele T, Zelalem N, Chanda M, Nigatu K (2012). Prevalence and characterization of hydatidosis in animals slaughtered at Addis Ababa abattoir, Ethiopia. J. Parasitol. Vector Biol. 4(1):1-6.
- Zewdu, E., Y. Teshome and A. Makwoya, (2010) Bovine Hydatidosis in Ambo Municipality Abattoir, West Shoa, Ethiopia, Ethiop. Vet. J., 14(1): 1-14.


