Archive : Article / Volume 1, Issue 2
- Review Article | DOI:
- https://doi.org/10.58489/2836-2322/010
Review on Design of liposomeâs as drug delivery system
Durgamata Institute of Pharmacy, Dharmapuri Parbhani-431401(MS).
Mohammad Zishan Ibrahim
Mohammad Zishan Ibrahim, Shaikh Juned Shaikh Hamid, Sameer S. Sheaikh (2022). Review on Design of liposomeâs as drug delivery system. Pharmacy and Drug Development. 1(2). DOI: 10.58489/2836-2322/010
© 2022 Mohammad Zishan Ibrahim, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 26-07-2022
- Accepted Date: 10-08-2022
- Published Date: 08-12-2022
Liposomes, drug molecules, hydrophilic molecules
Abstract
Liposomes wasinitially described by the British haematologist Dr. Alec D. Bangham and collaborators at the University of Cambridge in the 1960s and the first report was publicized in 1964.Liposomes are a form of vesicles that consist of many, few or just one phospholipid bilayer. The polar character of the liposomal core allow polar drug molecules to be capsulize. Amphiphilic (both hydrophilic and hydrophobic) and lipophilic molecules are solubilised within the phospholipid bilayer according to their affinity towards phospholipids.
Introduction
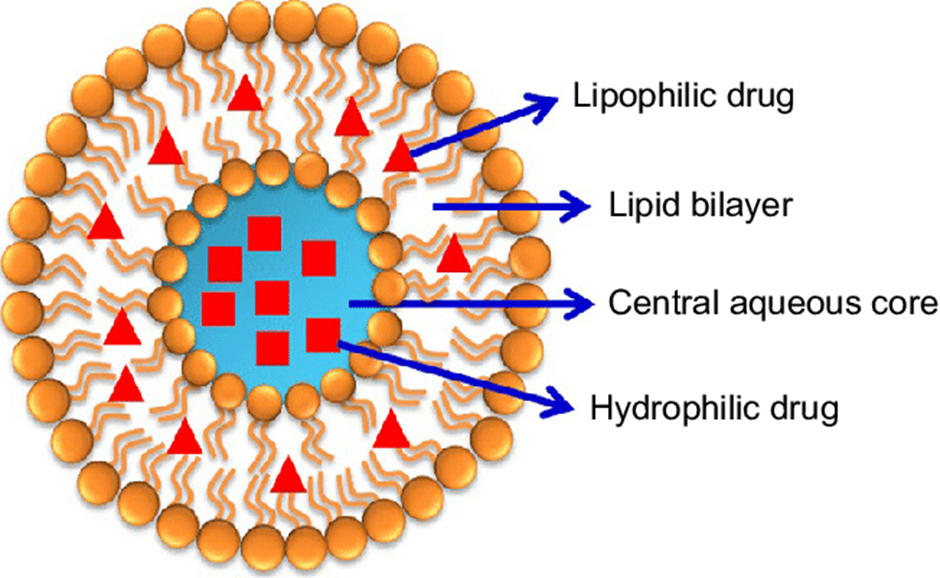
Liposomes wasinitially described by the British haematologist Dr. Alec D. Bangham and collaborators at the University of Cambridge in the 1960s and the first report was publicized in 1964.[1] Liposomes are a form of vesicles that consist of many, few or just one phospholipid bilayer. The polar character of the liposomal core allow polar drug molecules to be capsulize.[2] Amphiphilic (both hydrophilic and hydrophobic) and lipophilic molecules are solubilised within the phospholipid bilayer according to their affinity towards phospholipids. Liposomal membrane can be formed by one or more lipid bilayer arranged around an internal aqueous layer, polar head groups aligned to the inner and outer aqueous phase. Presence of non-ionic surfactants instead of phospholipids in the formation of bilayers results in the formation of niosomes. This structure offers liposomes to ability to load and deliver the loaded moleculeshaving different solubility profiles. Hydrophilic molecules in the internal aqueous layer, aqua phobic molecules into the lipid bilayer and amphiphilic molecules at the water/lipidinterface. (Fig.1) [3]
Liposomes are consider as a powerful drug delivery systems because of their structural versatility as well as their biodegradability, biocompatibility, non-toxicity and non-immunogenicity nature of components used in manufacturing of liposomes.[4]
Merits and Demerits
| Merits | Demerits |
1 | Liposome increased stability through encapsulation | Poor solubility |
2 | Liposomes helps to reduce the exposure of toxic drugs to sensitive tissues | Short half-life |
3 | Liposomes reduce the toxicity of the encapsulated drugs | phospholipid undergoes reaction like oxidation and hydrolysis |
4 | Liposomes enhances the efficacy and therapeutic index of drug | Only few are highly stable |
5 | Flexibility to couple with site-specific ligands to achieve active targeting | High production cost |
6 | Liposomes are non-toxic, flexible, biocompatible and completely biodegradable | Leakage and fusion of encapsulated drug occurs |
7 | Non-immunogenic for systemic and non-systemic administrations |
|
Basic Components of Liposomes
Liposomes or lipid vesicles are colloidal particles having phospholipid moleculeis an importantconstituent in the enclosed lipid bi layer or lipid drug sheet-disk complexes. Most of the liposomes which are intended to be use for human beings contains phosphotidyl choline, with a fatty acyl chain of different lengths and varying in the degree of saturation as a major membrane building blocks.
Phospholipids
The general chemical structure of the phospholipids shows a glycerol backbone. At position 3 of the glycerol molecules, the hydroxyl is esterified to phosphoric acid. The hydroxyl group at position 1 and 2 of the glycerol are usually esterified with long chain fatty acids. The lipid nature of the phospholipids can be attributed to these long chain fatty acids. The phosphate moiety along with the attached hydroxyl group which represents the head group of phospholipid. The most common phosphatides in case of animals and plants are phosphatidyl ethanolamine and phosphatidyl choline which also called as lecithin.These two components contributes the major structural part ofmost biological membranes. The main component of liposomes are glycero-phospholipids, which are amphiphilic (both hydrophilic and hydrophobic) lipids made-up of a glycerol molecule which is bound to the phosphate group and two fatty acid chains that may be saturated or unsaturated in nature.[5] The finalproperties of liposomes are affected by structure and the characteristics of phospholipids.
The phosphate group can be also bonded to another organic molecule. [6,7]. According to these organic groups, the natural phospholipids are categorised as phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphati-dylglycerol (PG) and phosphatidylserine (PS).[9] Glyc-erophospholipids which are responsible forformation of liposomes can be categorised in two different formsi.e. natural and synthetic. Mostlythe natural phospholipidswhich are use to designed liposomes, are phosphatidylcholine (PC) and phosphatidylethanolamine (PE), that are abundant phosphatides in plants and animals.[9]
Steroids
Cholesterol has a steroid back bone and its derivative involved in preparation of liposomes. This derivative are used in formulation of liposomes to improve there bilayer characteristics, to improve the stability of bilayer membrane in presence of biological fluids like blood and plasma. And also it reduces the permeability of water soluble molecules through the membrane. The cholesterol molecule oriented itself in between the phospholipid molecule with its hydroxyl group phasing towards the water phase and the tricyclic ring sandwiched between the first few carbon of the fatty acyl chains into the hydrocarbon core of bilayer.
Classification of liposomes
As compared to others colloidal delivery systems liposomes offers the advantage to alter their physicochemical characteristics and their structural features. Therefore, it is possible to modify liposomes behaviour in-vivo and passive delivery to body organs depends on size and the surface charge of liposomes or the incorporation of antibodies or other ligands to targeting liposomes to a specific site in the organism. Liposomes can be classified based on their composition, functionalization and structure. In addition to conventional, stealth and targeted liposomes, the recent improvement on the design of liposomes leads to a different types of liposomes such as immuno-liposomes and stimuli responsive liposomes.[30] The differences between these categories of liposomes will be highlighted below.
Base on the structure
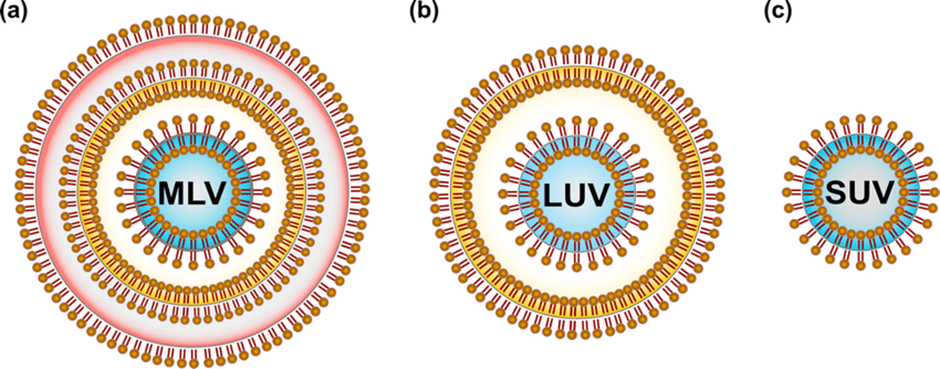
Liposomes are clinically used as a delivery system for biological macromolecules, chemotherapeutic and diagnostics agents. Because of their flexibility in size and composition, different types of liposomes have been developed which are different in surface and structural characteristics. According to their structure, liposomes are categorised on the basis of number of lipid bilayers which are known as lamellae and on the vesicle size (Fig. 2). On the basis of their lamellarity, liposomes can be classified as unilamellar (ULV, all size range), multi-lamellar (MLV, >500 nm) and multi-vesicular (MVV, >1000 nm) vesicles. [10,11] ULV can also be further classified according to their size into three categories, small unilamellar vesicles (SUVs, 20 – 100 nm), large unilamellar vesicles (LUVs, >100 nm) and giant unilamellar vesicles (GUVs, >1000 nm). ULVs are distinguished by the presence of a single bilayer, having higher ability to encapsulate the hydrophilic molecules. MLVs present two or more concentric lipid bilayers sorted by an onion like structure, favourable for the encapsulation of lipophilic molecules. MVVs include several small non-concentric vesicles trapped in a single lipid bilayer and are ideally suitable for the encapsulation of large volume of hydrophilic material. [11,12] Additionally to the size of vesicles, the number of lamellae also decide the amount of drug which is to be encapsulated in liposomes.[10,13] Alternatively, an innovative vesicle-type formulation is the multi-compartment liposome (MCL). The Multi-compartment liposomes are structurally composed of two distinct types of vesicles connected by a tight bilayer interface and are developed as single-vehicle delivery systems for combinatory compounds. [14,15] Multi-compartment liposomes constitute an attractive drug carrier system offering advantages in terms of inner vesicle protection, sustained drug release and possibility for combinatory (cocktail) therapies through a single delivery system.
Based on composition and functionalization
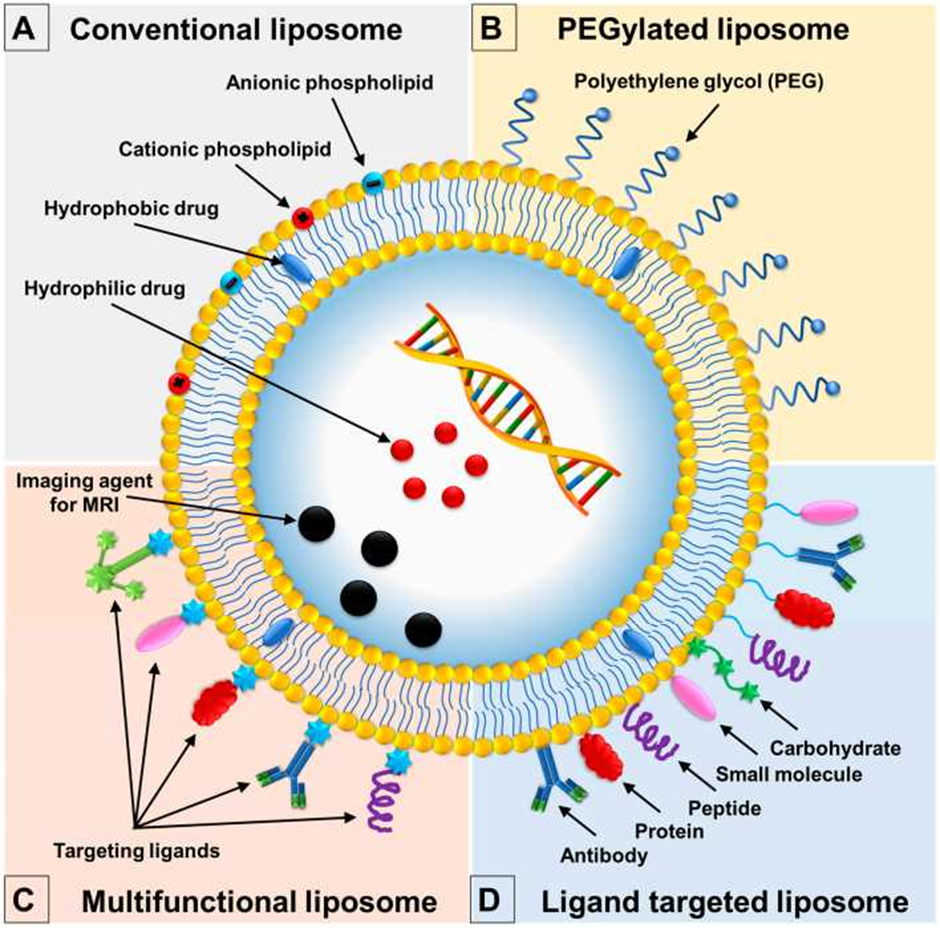
liposomes are formed with different characteristics like their composition and functionalization (Fig. 3). Conventional liposomes are the first generation of liposomes. They are composed of lipid bilayer molecules surrounding the aqueous chamber and are the basic layerfor all other liposomes. [32,33]There are neutral, cationic or anionic charged phospholipids are use in liposomes preparation generally in combination with CH to give the stability to the liposomal bilayer.[34,35]Although, this type of liposomes are to be subjected to many type of problems, such as the instability in blood plasma which results in short half-life blood. Liposomes are rapidly identified by RES and removed out from the blood circulatory system.[36] After binding with opsonins, serum proteins, the liposomes are eliminated from body fluid. Opsonins, recognize the conventional liposomes as foreign particles, and consequently they stars to destroyed liposomes by phagocytes of the MPS.[37] To reduce the disadvantages of conventional liposomes, a second generation liposomes was developed which guide to the creation of so called stealth strategy, long-circulating or PEGylated liposomes.[38] The stealth strategy involves the possibility to coat the liposomal surface with biocompatible water-hating polymer conjugates, like, polyethyleneglycol, chitosan, and others, increasing repulsive forces in between liposomes and serum-components.[39] For that reason, results in the reduction of immunogenicity and macrophage uptake, increasing its blood circulation half-life and reducing the toxicity of encapsulated compound.[40] The methods to anchor the PEG in the liposome membrane involves the physical adsorbing of the polymer onto the surface of the liposomes, the incorporation the PEG-lipid conjugate during liposome preparation, or by the covalent attachment of reactive groups on the surface of preformed liposomes.[36] Still, an important limitation of stealth liposomes is their largebiological distribution. Consequently, thecompound to be encapsulated is cannot be selectively delivered to particular target cells,[41]because of this limitation, ligand-targeted liposomes was designed for targeted delivery of compounds at the desired tissues organisation, promoting higher and more selective therapeutic activity.[36] Additionally to the surface modification of liposomes with Poly-Ethylene Glycol,targeted liposomes are also functionalized by using poly-saccharides, glycoproteins, or a ligand for particular receptors, like wise antibodies, small molecules or peptides [37,41] The ligands can be target the specific receptors which are expressed on the surfaces of the disease cells, binding, results in reduce side effect due to minimum off-target effects to healthy cells.[42,43]
Now, the multifunctional liposomes have been identified for their efficacy to perform multiple functions by liposomal surface modification techniques, which results in formulation of liposomes with a broad range of functions.[37] Example is the thera-nostic liposomes, these are used as imaging agent and therapeutic agent at the same time. (Diagnosis and treatment functions) (Li et al., 2012; [35,44] Another example is the dual-targeting liposomes having ability to bind two different types of ligands.[37]
Design o liposomes
There are a several techniques which are applied for liposome production, Like liposomal productionmethods itself and the size reduction methods. The different techniques will have affect the ultimate properties of liposomes, like size, encapsulation potency (EE) and lamellarity.[16] The methods which are used to formulate the liposomal can be categorized as conventional or novel. In the following section will be explored some of these methods.
Conventional methods
The most commonly used methods for the preparation of liposome are the reverse phase evaporation, thin film hydration,solvent injection, and detergent removal method.[17,18] These methods involve the subsequent basic stages: [10]
- lipids dissolved in organic solvents
- removal of organic solvent
- purifying and isolation of liposomes and
- analysis of final liposomes
Reverse phase evaporation method
This method provides a high progress in liposome technology, since it allowed initially for the preparation of liposomes with a capability to entrap a large amount of the aqueous material and a high aqueous/ lipid quantitative relation. Reverse-phase evaporationrelies on the formation of inverted micelles. These inverted micelles are formed q upon sonication of a mixture of a buffered aqueous phase, which contains the water-soluble molecules which is to be encapsulated into the liposomes and an organic part in which the amphiphilic molecules are solubilized. The slow elimination of the organic solvent enhance the conversion of these inverted micelles into viscous state and gel. At a critical point in such process, the gel state fall down, and the few inverted micelles was disturbed. The large amount of phospholipids in the environment donates to the formation of a complete bilayer around the residual micelles, which ultimately result in the formation of liposomes. Liposomes formed by reverse phase evaporation method can be made from lipid formulations and having an aqueous volume-to-lipid ratio which are four times greater than hand-shaken liposomes or multi-lamellar liposomes [19,20].
In detail, the water-in-oil emulsion is formed by sonication of a two-phase system, containing phospholipids in organic solvent such as diethyl ether or isopropyl ether or a mixture of isopropyl ether and chloroform with aqueous buffer. The organic solvents are detached under reduced pressure which resulting in formation of a viscous gel. The liposomes are shaped when residual solvent is detached during continues rotary evaporation under reduced pressure. Through this method, high encapsulation efficiency up to 65
Thin film hydration
It is also known Bangham method, film hydration is the easiest and oldest method used in liposome preparation. In this technique, lipids molecules are initially get dissolved in a appropriate organic solvent, and leave it till it dried to form a thin film in flask bottom. The formed lipid film is hydrated by using an suitable aqueous medium to produce a liposomal dispersion. The hydration condition can affect the structural organization of the formed vesicles. A hash hydration forms aMulti-lamellar vesicles (MLV) with poor size homogeneity, which requires an additional pulverization step. The most widely used size reducing methods include probe and bath sonication that produce the small unilamellar vesicles (SUV).[10] Despite it is highly effective, probe sonication is often blamed for potential contamination by titanium because the titanium-based nozzles are used for agitation, and production of local heat that can affect lipids and create drugs stability problems.[45] Although the two sonication methods produce liposomes with identical characteristics, the use of bath sonication remains a better option because it is easy to operate. Another technique used for liposome sizing are consecutive extrusion of the liposomal formulation through polycarbonate filters of definite pore sizes. In this method, the number of extrusion cycles is the important parameter to control for effective homogenization. [31]
Size reduction techniques
Liposomes formed by various techniques needs additional methods to reduce their size, such as sonication, homogenization or extrusion.[23] There are two different sonication techniques that can be used to reduce the size of oversized liposomes and bath and probe sonication.[10] The sonication process may have disadvantages as the difficult to provide identical ultrasonic energy in a large volume of liposomal suspension (scale-up) and potential metal contamination from the probe tip. Furthermore, there is possible risk of degradation on phospholipids and even on compound to be encapsulated, as well as low EE. [24,25] In homogenization techniques, liposomes can be forced to go through the small orifice under high pressure to reduce their size, resulting in a concept of high-velocity collision. Number of techniques can be included in this class of size reduction, such as micro-fluidization, high-pressure homogenization, and shear force-induced homogenization processes.[26] Another method of pulverization of liposomes is the extrusion process. After formulation, the liposomes pass several times (extrusion cycles) by the membrane of definite pore size, normally a polycarbonate filter, to uniform size distribution. [27,28] This process needs much lower pressure and less volume of liposomal suspension compared with homogenizers.[29]
Detergent removal technique
The detergent removal method is another known technique for formation of liposomes. In this methodremoval of detergent molecules from the aqueous dispersion of phospholipid occurs, detergent mixed micelles represents a different approach. When the detergent molecules are removed, the micelles become progressively rich with phospholipid and ultimately coalesce to form a packed, single-bilayer vesicles (liposome). The detergent is then removed using a diffusion-based process such as gel chromatography or dialysis. Ionic detergents like deoxy-cholate and cholate or Non-ionic detergents, mainlyTriton-X 100 and octylglucoside are used.
This approach has considerable advantages over other methods by allowing close control of the final size of the liposomes, which have a homogenous size range.But thedisadvantage are the encapsulation efficiency is lower then with other methods of preparation of large unilamellar vesicles (LUVs) and detergent removal by ordinary dialysis techniques is a time consuming process which can take several days to reduce the residual detergent to a negligible level.
Solvent Injection Method
Ethanol Injection, Ether Infusion and Fluorocarbon injection are the three known techniques of solvent injection method used for preparation of liposomes
Ethanol Injection Method
The lipids are dissolve in ethanol are rapidly injectable into concentrated buffer solution. The procedure is rapid, simple and time consuming to both lipids and the which is to be entrapped. The final concentration of ethanol is in between 10-20% by volume as the small unilamellar vesicles (SUVs) me either will not form or will grow in size soon after formation. Another disadvantage is that certain biologically active macromolecules tend to inactivated in small amount of alcohol.
Ether Infusion
Liposomes are prepared by slowly introducing a solution of dissolved lipids in diethyl ether in to slightly warm water. The lipid mixture is injected into an aqueous of a material which is to be encapsulated with the help of a syringe type infusion pump at near about 55-65°C or under lower pressure. The single layer vesicles having small diameter i.e.,50-200nm is formed by vaporisation of ether.
Fluorocarbon Injection
There are some disadvantages of using diethyl ether as a solvent for preparation of Liposomes. So, to overcome the hazards of using diethyl ether, A fluorocarbon solvent has been use for injection. Freon 21 was found to be an good solvent for phospholipid; it is hyrophobicand boils at 9°Cat equilibrium pressure. Large unilamellar liposomes are formed. The fluorocarbon boils off at almost the same rate as it is introduced, leaving the lipid behind to hydrate and form liposomes.
Application
Antimicrobial agents:
Liposomes are broadly investigated as a delivery systems for the treatment of bacterial, fungal, viral and parasitic diseases. Liposomes have been use for passive delivery of immunomodulatory compounds to microphages, which brings about non specific resistance to infections in general.
AmbisomeTMis a liposomal form that consists of SUVs composed of hydrogenated soya PC, disearoyl phosphotidyl glycerol and cholesterol, drug are believedto align in parallel lines to form cylindrical structures, two of which fit end to end to form a bilayer spanning pore across which ions and other solute can pass. These pores can be inserted into the fungal cell membrane, allowing rapid loss of cell contents. In vitro studies have shown binding of Ambiaome liposomes to fungal cells, followed by liposomes disintegration and subsequent cell disruption.
Anticancer agent:
Liposomeshavebeen used for the treatment of cancer because of their tumor targeting potential. A variety of cytotoxic drugs encapsulated in liposomes have been administered through various routes to a range of animal tumour models. DaunosomesTMconsists of daunorubicin incorporated into an SUV with a diameter of 40-50 nm and composed of DSPC and cholesterol and is supplied as a ready to use aqueous liposomes suspension. The most widely used and studied isAdriamycin (commercial name for doxorubicin HCl; byBen Venue Laboratories, Bedford, Ohio).[46]
It has been well-known that a efficacious anticancer drug, especially one that targets the cytoplasm or cell nucleus, doesn't workbecause of low permeability of drug across a plasma membrane, degradation bylysosomal enzymes by an endocytosis-dependentpathway, and other reasons. Thus, too much attention onthe application of drug delivery systems is focused on reducing these problems, finally leading to the inductionof higher ability of anti-cancer drug. In this respect, anew model for cancer therapy through a novel drug delivery system, fusogenic liposome [45], was developed. Fusogenic liposomes are poised of the ultraviolet inactivated Sendai virus and conventional liposomes.
Fusogenic liposomes effectively and directly deliver theirencapsulated drug into the cytoplasm through a fusionmechanism of the Sendai virus, whereas conventionalliposomes are phagocytosis by endocytosis through phagocyticcells of the reticulo-endothelial system, for examplemacrophages and neutrophils. Thus, fusogenic liposomeis a good vehicle to deliver drugs into thecytoplasm of Sendai virus in an endocytosis-independent manner [45].
Site Avoidance mechanism:
Liposomes do not disperse in certain organs such as kidney, heart, lungs, Brain and nervous system which reduces the nephro, cardio, neuro toxicities. Typical example are it reduces the toxicity of Amphotericin B and reduces cardiovascular toxicity of Doxorubicin liposome.
Site specific targeting:
In certain cases liposome with surface attached ligands can bind to target cells i.e. lock and key mechanism or can be delivered into the target tissue by local anatomical conditions such as leaky and badly formed blood vessels, their basal lamina, and capillaries. Examples are anticancer agent, anti-infection and anti-inflammatory agents ; improved transfer of hydrophilic, charged molecules like anti biotic, plasmids, chelating agents and gens into cells; and improved penetration into tissues, especially in the case of dermally applied liposomes dosage forms e.g. including anaesthetics, corticosteroids and insulin. [47,48]
Diagrams
References
- Bangham, A.D., Horne, R.W., (1964). Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 8, 660–668.
- Nisini, R., Poerio, N., Mariotti, S., De Santis, F., Fraziano, M., (2018). The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. https:// doi.org/10.3389/fimmu.2018.00155.
- Laouini, A., Jaafar-Maalej, C., Limayem-Blouza, I., Sfar, S., Charcosset, C., Fessi, H., (2012). Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 1, 147–168.
- Mathiyazhakan, M., Wiraja, C., Xu, C., (2018). A Concise Review of Gold Nanoparticles-Based Photo-Responsive Liposomes for Controlled Drug Delivery. Nano-Micro Lett.10 https://doi.org/10.1007/s40820-017-0166-0.
- Pinot, M., Vanni, S., Pagnotta, S., Lacas-Gervais, S., Payet, L.A., Ferreira, T., Gautier, R., Goud, B., Antonny, B., Barelli, H., (2014). Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 345 (80), 693–697.
- Beltran-Gracia, ´ E., Lopez-Camacho, ´ A., Higuera-Ciapara, I., Vel´azquez-Fernandez, ´ J.B.,Vallejo-Cardona, A.A., (2019). Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol.
- Monteiro, N., Martins, A., Reis, R.L., Neves, N.M., (2014) a. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 11, 20140459.
- Tsuji, T., Morita, S.Ya., Ikeda, Y., Terada, T., (2019). Enzymatic fluorometric assays for quantifying all major phospholipid classes in cells and intracellular organelles. Sci. Rep. 9.
- Antimisiaris, S.G., Kallinteri, P., Fatouros, D.G., (2007). Liposomes and Drug Delivery. Pharmaceutical Manufacturing Handbook: Production and Processes. 443–533.
- Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S.W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M., Nejati-Koshki, K., (2013). Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 8, 102.
- Emami, S., Azadmard-Damirchi, S., Peighambardoust, S.H., Valizadeh, H., Hesari, J., (2016). Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 11, 737–759
- Maherani, B., Arab-Tehrany, E.R., Mozafari, M., Gaiani, C., Linder, M., (2011). Liposomes:A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 7, 436–452.
- Olusanya, T.O.B., Ahmad, R.R.H., Ibegbu, D.M., Smith, J.R., Elkordy, A.A., (2018). Liposomal drug delivery systems and anticancer drugs. Molecules 23, 1–17.
- Al-Jamal, W.T., Kostarelos, K., (2007). Construction of nanoscale multicompartment liposomes for combinatory drug delivery. Int. J. Pharm. 331, 182–185.
- Catalan-Latorre, A., Ravaghi, M., Manca, M.L., Caddeo, C., Marongiu, F., Ennas, G., Escribano-Ferrer, E., Peris, J.E., Diez-Sales, O., Fadda, A.M., Manconi, M., (2016). Freeze-dried eudragit-hyaluronanmulticompartment liposomes to improve the intestinal bioavailability of curcumin. Eur. J. Pharm. Biopharm. 107, 49–55.
- Pattni, B.S., Chupin, V.V., Torchilin, V.P., (2015). New Developments in Liposomal Drug Delivery. Chem. Rev. 115, 10938–10966.
- Karn, P.R., Cho, W., Hwang, S.J., (2013). Liposomal drug products and recent advances in the synthesis of supercritical fluid-mediated liposomes. Nanomedicine.
- Meure, L.A., Foster, N.R., Dehghani, F., (2008). Conventional and dense gas techniques for the production of liposomes: A review. AAPS PharmSciTech.
- Himanshu A, Sitasharan P, Singhai AK: Liposomes as drug carriers. IJPLS2011, 2(7):945–951.
- Kataria S, Sandhu P, Bilandi A, Akanksha M, Kapoor B, Seth GL, Bihani SD:Stealth liposomes: a review. IJRAP (2011), 2(5):1534–1538.
- Szoka F Jr, Papahadjopoulos D:, (1978), Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA, 75(9):4194–4198.
- Handa T, Naito S, Hiramatsu M, Tsuboi M:, (2006), Thermal SiO and H13CO+ lineobservations of the dense molecular cloud G0.11-0.11 in the GalacticCenter Region. Astrophys J, 636:261–266.
- Kraft, J.C., Freeling, J.P., Wang, Z., Ho, R.J.Y., (2014). Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. Journal of Pharmaceutical Science.
- Batzri, S., Korn, E.D., (1973). Single bilayer liposomes prepared without sonication. BBA-Biomembr. 298, 1015–1019.
- Tejera-Garcia, R., Ranjan, S., Zamotin, V., Sood, R., Kinnunen, P.K.J., (2011). Making unilamellar liposomes using focused ultrasound. Langmuir 27, 10088–10097.
- Wagner, A., Vorauer-Uhl, K., (2011). Liposome Technology for Industrial Purposes. Journal of Drug Delivery 2011, 1–9.
- Olson, F., Hunt, C.A., Szoka, F.C., Vail, W.J., Papahadjopoulos, D., (1979). Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. BBA - Biomembr. 557, 9–23.
- Meure, L.A., Foster, N.R., Dehghani, F., (2008). Conventional and dense gas techniques for the production of liposomes: A review. AAPS Pharmaceutical Science and Technology.
- Kraft, J.C., Freeling, J.P., Wang, Z., Ho, R.J.Y., (2014). Emerging research and clinicaldevelopment trends of liposome and lipid nanoparticle drug delivery systems. Journal Pharmaceutical Science.
- Allen, T.M., Cullis, P.R., (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews
- Pattni BS, Chupin VV, Torchilin VP., (2015), New developments in liposomal drug delivery. Chemical Reviews.115:10938-10966.
- Abra, R.M., Bankert, R.B., Chen, F., Egilmez, N.K., Huang, K., Saville, R., Slater, J.L.,Sugano, M., Yokota, S.J., (2002). The next generation of liposome delivery systems: Recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. Journal of Liposome Research. 1–3.
- Cattel, L., Ceruti, M., Dosio, F., (2004). From conventional to stealth liposomes: A new frontier in cancer chemotherapy. J. Chemother. 94–97.
- Monteiro, N., Martins, A., Reis, R.L., Neves, N.M., (2014). Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 11, 20140459.
- Sercombe, L., Veerati, T., Moheimani, F., Wu, S.Y., Sood, A.K., Hua, S., (2015). Advances and challenges of liposome assisted drug delivery. Front. Pharmacology.
- Immordino, M.L., Dosio, F., Cattel, L., (2006). Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nano medicine.
- Riaz, M.K., Riaz, M.A., Zhang, X., Lin, C., Wong, K.H., Chen, X., Zhang, G., Lu, A., Yang, Z., (2018). Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. International Journal of Molecular Science
- Saraf, S., Jain, A., Tiwari, A., Verma, A., Panda, P.K., Jain, S.K., (2020). Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol.
- Hatakeyama, H., Akita, H., Harashima, H., (2013). The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull.
- Madni, M.A., Sarfraz, M., Rehman, M., Ahmad, M., Akhtar, N., Ahmad, S., Tahir, N., Ijaz, S., Al-Kassas, R., Lobenberg, ¨ R., (2014). Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 17, 401–426.
- Torchilin, V.P., (2005). Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery
- Fathi, S., Oyelere, A.K., (2016). Liposomal drug delivery systems for targeted cancer therapy: Is active targeting the best choice? Future Med. Chem.
- Le, N.T.T., Cao, V.Du, Nguyen, T.N.Q., Le, T.T.H., Tran, T.T., Thi, T.T.H., (2019). Soy lecithin-derived liposomal delivery systems: Surface modification and current applications. Int. J. Mol. Sci.
- Li, S., Goins, B., Zhang, L., Bao, A., (2012). Novel multifunctional theranostic liposomedrug delivery system: Construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjug. Chem. 23, 1322–1332.
- Hamilton RL, Guo LSS., (1984), Liposomes preparation methods. J ClinBiochem Nut, 7:175.
- Banerjee R, Tyagi P, Li S, Huang L:, (2004), Anisamide-targeted stealth liposomes: potent carrier for targeting doxorubicin to human prostate cancer cells.Int J Cancer, 112:693–700.
- William, BD, MM sulliva, KE williams, JR Morgan., (1986), Imaging in rheumatoid arthritis using liposomes labelled with technetium., Br.Med. Journal.,293:1144-1145.
- Svenson, CE, MCPopescu and RC Ginsberg; (1988), liposomes treatment of viral, bacterial and protozoal infection; Crit.Rev.Microbiol. 15: S1-31.
Quick links
- Abstract
- Introduction
- Merits and Demerits
- Basic Components of Liposomes
- Phospholipids
- Steroids
- Classification of liposomes
- Base on the structure
- Based on composition and functionalization
- Design o liposomes
- Conventional methods
- Reverse phase evaporation method
- Thin film hydration
- Size reduction techniques
- Detergent removal technique
- Solvent Injection Method
- Ethanol Injection Method
- Ether Infusion
- Fluorocarbon Injection
- Application
- Diagrams
- References


