Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-2314/002
Stopping Addiction Before It Begins: The Future is now
Invited Perspective.
Correspondng Author: Elizabeth D. Gilley
Citation: Elizabeth D. Gilley (2022). Stopping Addiction Before It Begins: The Future is Now. Journal of Adolescent and Addiction Research. 1(1). DOI: 10.58489/2836-2314/002
Copyright: © 2022 Elizabeth D. Gilley, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-07-30, Received Date: 2022-07-30, Published Date: 2022-09-23
Abstract Keywords: Reward Deficiency Syndrome, Genetic Addiction Risk Severity (GARS), Addiction Prevention.
Abstract
Genetic Addiction Risk Severity (GARS) (Blum et al, 2014; Blum, Badgaiyan, Agan, Frantantonio, Simpatico, et al. 2015; Blum, Baron, Lott, Ponce, Siwicki, et al. 2019) screens for Reward Deficiency Syndrome (RDS) predisposition risk, for polymorphic variance within eleven alleles of the ten most common genes, in mental disorder. The RDS paradigm shift is concerned with treating the underlying neurogenetic and epigenetic challenges of dopaminergic dysfunction, as well as dysfunction in other neurotransmitter channels (Blum, Baron, McLaughlin, & Gold, 2020). Cutting edge psychiatric genomics recognizes that RDS is one preexisting causal influence for addiction (Tsermpini, Adla, & Patrinos, 2022).
Consideration of preexisting neurogenetic challenges which affect low dopamine availability or epigenetic insults are not addressed in traditional old school, Minnesota Model twelve steps treatment modalities (Gilley, 2020), nor it is addressed in the current DSM 5th Edition (APA, 2013) (Gondre-Lewis, Bassey, & Blum, 2020). Scientists in the know are hopeful that RDS will be included in the next edition of the Diagnostic and Statistical Manual of Mental Disorders, as exponential increases in research studies from interactive sciences such as psychology, neurology, genetics and epigenetics have greatly enlarged perspective (Mancheno, Navas-Leon, Fernandez-Calderon, Gutierrez, Sanchez-Garcia, et al 2021). Sometimes progress is slow in funneling progressive cutting-edge applications from the research world into the practitioner world (CASA Columbia, 2012). Unfortunately, it is the patients who suffer, as the opioid overdose deaths of more than 100,000 this year alone, attest (Gupta, Bowirrat, Llanos Gomez, Baron, Elman, Giordano, et al 2022; Blum, Fried, Madigan, Giordano, Modestino, Steinbergy, et al 2017; Moran, Blum, Valdez Ponce, Lott, Gondre-Lewis, Badgaiyan, 2021).
Not only have there been advancements in treatment models, from the Minnesota Model of the 1950âs, the Harm Reduction Model of the 1980âs (Paquette, Daughters, & Witkiewitz, 2022) and the Neurodevelopmental Model of addiction of the 2000âs (Leyton, 2012, 2014), there have been advancements in unifying theory. The evolution of the history of addiction recovery treatment would never be complete without mentioning the foundational dopamine depletion hypothesis (Dackis, & Gold, 1985; Diani, 2011; Volkow, Fowler, & Wang, 2002), which led to way to the current leading theory of Reward Deficiency Syndrome, which includes consideration of genetic (Dick, & Agrawal, 2008; Uhl, Liu, Walter, Hess, & Naiman, 2002) and epigenetic causal influences (Edwards, Roy, Boyett, Badgaiyan, Thanos, Baron, et al 2020; Vaillancourt, Ernst, Mash, & Turecki, 2017).
RDS unifies all addictions, both substance and non-substance under a common rubric (Blum, Bowirrat, Braverman, Baron, Cadet, Kasmi, et al (2021). The Reward Deficiency Syndrome paradigm shift takes into consideration, underlying genetic, biological, physiological, and neurological mechanisms of the brain reward cascade (BRC). In this genomic era of addiction medicine, the new standard of excellence in addiction treatment begins genetic screening (Gilley, 2022 a, b, c). RDS treatment plans are built upon the foundational genetic and epigenetic causal influences (Gilley, 2021, b, c). RDS-Solution Focused Brief Intervention (RDS-SFBI) administers bio-neuro-psychological therapy which assists the client in achieving dopamine homeostasis (Gilley, 2019). Since RDS effects the individual over the entire lifespan, it should be treated as a front-line modality (Blum, Raza, Schultz, Jalali, Green, Brewer, et al 2021), by primary physicians, and teams of RDS specialists (Gilley, Bowirrat, Gupta, Giordano, Dennen, Braverman, Badgaiyan, McLaughin, Baron, & Blum, 2022).
Introduction
Genetic screening is utilized in cancer prevention. Why not also utilize genetic screening for the prevention of mental health disorder, especially in children of addicted and/or mental health disorder diagnosed parents (Moberg, & Humphreys, 2016)? Why wait until addiction or mental health disorders manifest to treat them? Prevention is paramount to stopping the generation cycle from continuing in future generations.
The Elle Foundation 100’s research case study series focuses upon our proband, Case Study #101 (Gilley 2022a) and her nuclear Reward Deficiency Syndrome family, Case Series #102 (Gilley, 2022 b) and Case Series #103 (Gilley, 2022c). Family members share polymorphic variances for pre-dispositional dopaminergic and serotonergic challenge. Addictive behavioral patterns, depression, mood disorders, and obesity are commonalities within this family sample.
Decades of research show support for future manifestation of addictive behavioral patterns, and other mental health disorders, in children who experience trauma and other adverse environmental conditions. Those who experience serious trauma, are most likely to experience future manifestation of addictive behavioral patterns, and any of the other compulsive, obsessive, impulsive and personality disorders associated with Reward Deficiency Syndrome (Gilley, 2021, b, c).
Genes alone do not create addiction, but rather it is the interactive influence of nature and nurture, both heredity and environmental factors which cause addiction (Gilley, 2018a). Genetic risk for RDS creates opportunity for early neurodevelopmental issues, such as Attention Deficit Disorder, Attention Deficit Hyperactivity Disorder (Gilley, 2018, c, d), and the Autism/Asperger Spectrum. Dopaminergic dysfunctions affect development over the entire lifespan. RDS has been proven to have correlation with all addictive behavioral expressions, both substance use and behavior process addictions. Later in life, RDS has been found to be correlational in dementias, such as Parkinson’s and Alzheimer’s Disease. See Figure 1- Lifespan Timeline for RDS phenomenon.
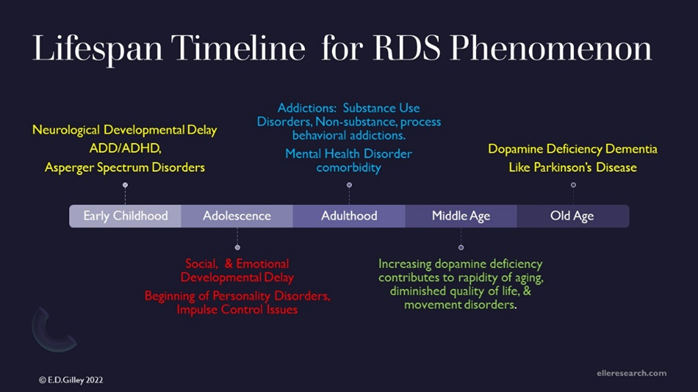
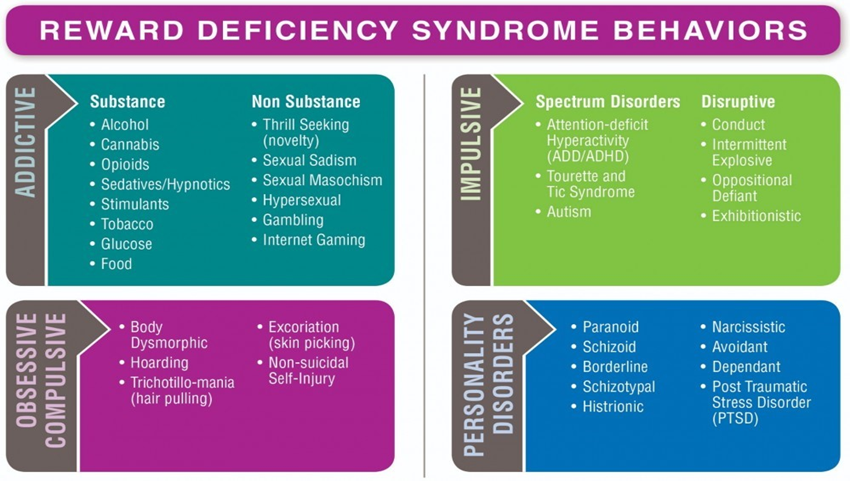
RDS is enlarged unifying theory, linking all dopaminergic deficiency addictions, both substance and behavior (Blum, Febo, Badgaiyan, Demetrovics, Simpatico, et al. 2017), with dopamine deficiency mental health disorders, which include obsessive compulsive, impulsive and personality disorders (Blum, & Braverman, 2000). Since Reward Deficiency Syndrome phenotypes run in families (Blum, Chen, Oscar-Berman, Chen, Lubar et al. 2011), the Elle Foundation recommends genetic testing not only for the children of addicts, but for all family members (Blum, Steinberg, Gondre-Lewis, Simpatico, Ceccanti, Steinberg, 2021). See Figure 2- Reward Deficiency Syndrome Behaviors, curtesy of GeneusHealth.com.
The Elle Foundation 100 series investigate RDS family genetics, and found concurring support for the efficacy of RDS Solutions ™, GARS ™, RDS treatment plans, RDS Solution Focused Brief Intervention therapies, for both the addict and family members. Treating the underlying genetic challenge of low dopamine availability is essential to improved quality of life and to lasting recovery.
The RDS paradigm shifts focus beyond the 1940-1950’s Minnesota Model of Twelves Step substance use disorder treatment. It combines elements from the 1980’s Harm Reduction Model and the 2000’s Neurodevelopmental Model. RDS has 4 phenotypes, including the following behaviors: Addictive, Obsessive-Compulsive, Impulsive and Personality Disorder In this era of genomic medicine, the state of the art of substance uses disorder and mental health disorder treatment in the 2020’s involves a Phase Two RDS treatment, which begins with psychiatric genomics (Blum, Cadet, Thanos, Baron, Mishrekar, Brewer, Bowirrat, Febo, & Gold, 2020).
RDS Solutions ™ are already on the market, available, now, in real time. For the addicted who are completing substance use disorder treatment in the traditional twelve step modality, RDS Phase Two Treatment is advised immediately after completion of substance use disorder inpatient. A brain checkup (Braverman, Dennen, Gold, Bowirrat, Gupta, Baron, et al 2022) and dopamine homeostasis treatment plan increase the odds of a successful, lasting recovery.
Methods
In review, for discussion the Elle Foundation Research Institute’s 101 Case Study of our proband, provides longitudinal study of a substance use disorder resistant patient (Balconi, 2015; Prendergast, Podus, Chang, & Urada, 2002), who suffers from a complexity of mental health disorder co-morbidity (Gilley, 2022a). EF Case Series Study 102, compares biological sisters’ genetics, addictive behavioral expressions (Blum, Cadet, Thanos, Baron, Mishrekar, Brewerm, Bowirrat, Febo, & Gold, 2020) and other mental health issues, depression and obesity (Gilley, 2022, b).
Case Series Study 103, compares genetic predisposition of eight RDS family members, who have reported life experience of many types of Reward Deficiency Syndrome behaviors. This family reports inclusion of the following: multiple poly substance uses disorders, ranging from alcohol, cocaine, and nicotine; Behavioral Process Addictions, such as eating disorders (Beitscher-Campbell, 2016), shopping disorders, obsessive cleaning, and handwashing, and sexual impulse disorders. The family reports neurocognitive developmental issues such as Asperger Spectrum, and attention deficit hyperactivity disorder especially in second and third generations. Obesity (Blum, Gold, Llanos-Gomez, Jalali, Thanos, Bowirrat, 2021) and depression (Blum, Hauser, Agan, Giordano, Frantantonio, Badgaiyan, & Febo, 2015). are reported by a majority of the sample (Blum, Thanos, & Gold, 2014).
GARS tests were administered as part of Elle Foundation Case Series 103, for a Reward Deficiency Syndrome family, who suffers from both addictions and mental disorders (El-Guebaly, 2004; Price, M. (2008). Gene analysis found disruption of serotonin and dopamine neurotransmitter channels, in addition to other genetic variances which as associated with other neurotransmitter imbalances (Downs, Chen, Chen, Waite, Braverman, Kerner, 2009; Uhl, & Liu, 2006).
Table 1 – Elle Foundation Case Study Series 103 Family Genomic Risk Alleles
| ID | COMT | DAT1 | DRD1 | DRD2 | DRD3 | DRD4 | DRD4R | GABRB3 | 5HHT | MAOA | OPRM1 |
| 101 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 |
| 102 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 0 |
| 103 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 0 |
| 104 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| 105 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 106 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| 107 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 |
| 108 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 |
| Total | 7 | 0 | 7 | 1 | 0 | 10 | 2 | 3 | 12 | 10 | 0 |
The Elle Foundation Case Series, #104 focuses upon two grandsons of our proband, age 2 and 15. The first part of CS104 focuses upon creating awareness of neurogenetic challenge, to provide information to care givers, parents and guardians to treat low dopamine availability, for prevention of RDS addictive behavioral manifestation. The second part of CS104 is a twenty-year longitudinal study in prevention which is currently ongoing (Blum, Steinberg, Gondre-Lewis, Simpatico, Ceccanti, Steinberg, 2021).
Generally, participants #105 and #106’s GARS analysis showed underlying neurogenetic risk regarding low dopamine availability, whether from too few dopamine receptors, increased reuptake or increased metabolic function in the BRC. Both 105 and 106, half siblings, birthed by the same mother, participant 104, share DRD1 variance, but differ in other polymorphic variance which predisposes risk for future neurodevelopmental issues.
Since neither our proband #101, nor her daughter, participant #104, have inherited single dopamine receptor D1 gene mutation variance, it is assumed that this allele risk variance, within Participants #105 and #106’s genome, was inherited from their perspective fathers, showing support for the tenet that RDS family members often marry other Reward Deficiency Syndrome challenged individuals. The family matriarch, their great grandmother, participant #103, does share this mutation. The DrD1 variant is the only shared GARS allele risk between these two half siblings.
According to information provided by Geneus Health Laboratory in San Antonio, Texas, and the Kenneth Blum Institute of Behavior and Neurogenetics, in Austin, Texas, this allele increases risk for alcohol and nicotine substance use disorders, and non-substance behavioral process novelty seeking disorder. More than 3705 peer reviewed studies on DrD1 have been published, in 20 databases within the National Institutes of Health, National Library of Medicine. It is believed that twenty-five percent of the population carry at least one copy of this risk variant allele (Blum, McLaughlin, Bowirrat, Modestino, Baron, Llanos Gomez, et al, 2022).
Under optimal conditions, this gene encodes the most abundant dopamine receptor in the central nervous system for proper brain function. The clinical impact of this risk allele variance is reduced gene expression, ultimately lowering the number of D1 dopamine receptors, contributing to reduced, or low dopamine availability and thus disrupting the balance between D1 and D2 receptor activity in the brain. Beyond the DRD1, each of the participants has a unique individual risk that differs from the other.
Participant #105, is a 15-year-old male, who is academically gifted, making as in accelerated classes, and is already, displaying an awarded work ethic. #105 has variance in COMT, DRD4 repeat, GABRB3, and MAOA genes. The subject has two copies of the Catechol-O-Methyltransferase, COMT risk allele, G (Rs4680) which indicates increased risk for alcohol, cannabis, glucose, nicotine, opioids and stimulant substance used disorders. This risk allele is also correlational to attention deficit hyperactivity disorder, anxiety, internet gaming, obsessive compulsive disorder, oppositional defiant, panic disorder and pathological aggression. More than 2400 peer reviewed research studies have been done on COMT. There are 21,931 references to COMT mentioned in the National Institutes of Health, National Library of Medicine, databases.
The Monoamine Oxidase A, or MAOA gene variance is not a single nucleotide but rather a tandem repeat, which means segments of DNA insertions and or deletions disrupt normal conditions. The MAOA has been associated with, correlational to alcohol, food, nicotine and opioid substance use disorders, and the non-substance behaviors associated with ADHD, harm avoidance and novelty seeking. There are 8342 references to MAOA within the NIH, National Library of Medicine databases.
The DRD4 gene has two allele risk variants: the C variant, which is a single nucleotide polymorphism, and variable tandem number repeats and DNA segment insertions and/or deletions. Both risk alleles: the C (rsl 800955) and the DRD4 Repeat 7R (long variant rs761010487) are associated with increased risk for alcohol, cannabis, glucose (Blumenthal, & Gold, 2010), nicotine, and opioid substance use disorders. These risk alleles are also associated with nonsubstance behavioral manifestation of Reward Deficiency Syndrome, including, ADHD, conduct disorder, hypersexuality, novelty seeking/thrill seeking, and pathological aggression. There are 6482 references to DRD4 within the NIH, National Library of Medicine databases.
Participant #105 inherited the DRD4 tandem number repeat insertions/deletions, as well as the Dinucleotide Repeat, GABRB3 gene. The GABRB3 gene is associated with alcoholism and Post Traumatic Stress Disorder. There are currently 2018 references to GABRB3 in the NIH, National Library of Medicine databases. Early genetic research showed the GABRA2 gene to be associated with early trauma, and increased risk for PTSD, and developmental addictions later in life (Enoch, 2010; Uhart, Weerts, McCaul, Guo, Yan, Kranzier, et al 2012).
There is concern for #105 because he too, experienced early trauma from being in an adverse environment (Anda et al, 2005), created by a young addicted teenage mother, and experienced panic as he was removed from her custody to family intervention. Unfortunately, #104, his mother was experiencing her own psychopathology from having been raised by a mother in active addiction, our proband. Addiction, or Reward Deficiency Syndrome, as is about to be known in the layperson world, is a generational disease. This family pattern has repeated to the third generation. Special psychotherapy for reintroducing the adolescent’s now psychologically healthy mother back into his life is appropriate, but may not be an option until he reaches the age of majority, or chooses to engage his mother (Sanders, & Mazzucchelli, 2022). Positive psychology and cognitive behavioral therapy are beneficial in helping a client progress from victim mode to creator mode, by learning to take responsibility for all life experiences, thoughts, and actions.
For many reasons a strained, alienated parent-child relationship between participant 104 and 105, as well as a strained relationship between 104, the biological mother, and 105’s guardian great aunt, participant #102, has developed (Flanagan, 2014). Healing the primary relationship between mother and child, unhealthy attachment and/or potential Attachment Disorders, including Avoidant, Ambivalent, and Disordered may be imperative to psychological wellbeing and successful transition into adulthood. Early psychic wounds often lay beneath the surface, of conscious awareness but yet fuel behavioral and relationships patterns such as perfectionism, and people pleasing, which is characteristic in those who felt abandoned and/or betrayed.
There is ongoing generational evidence of Attachment Disorder in the RDS family. Our proband reports an unsuccessful attachment with her mother. The adult relationship between proband and her mother could be characterized as less than optimal, needy, and sometimes passive aggressive. Participant 104, who is the daughter of our proband, has experienced both an Avoidant and/or Ambivalent Attachment Disorder. Participant #105 most likely has some form of undiagnosed attachment disorder, meaning an unhealthy attachment style with his absent biological parents, both of whom, were disenfranchised by the courts for active addiction, and intentionally kept away due to their lack of psychological soundness. Participant #105, who is both logical and rational, has the Myers Briggs personality type of Introverted, Sensing, Thinking, Judging (ISTJ). He is already working full time in a summer job, and has been selected, by his supervisor, to receive managerial training, when he reaches legal age.
As 105 matures from adolescence into early adulthood, he will be presented psychotherapeutic opportunities available through this longitudinal study. At a future date, he will undergo more intensive, more scientific personality testing (Gilley, 2021a), to utilize his strengths, to maximize his potential, and to possibly become informed of any potential social, or neurocognitive challenges which might surface along the RDS lifespan developmental track. Due to this unique neurogenetic predisposition risk, increased awareness and special precautions on the part of his caregivers, include avoidance of introduction of medicinal use opioids, for potential future pain or surgical situations, as there is increased risk for the development of opioid substance use disorder. His primary guardian is already on the watch for internet gaming compulsion.
His great aunt, Participant #102, the focus of Case Study 102, is his primary guardian. She focuses upon the wellness practices of proper nutrition, exercise (Archer et al, 2017), establishing healthy boundaries, fostering spiritual/religious development (Roy, Bowirrat, Smith, Braverman, Jalili, Badgaiyan, 2021), and has introduced social, artistic and musical opportunities for his nurturance. She has made psychotherapy possible with a child therapist, any time, he wished over the past decade. She has reviewed his GARS analysis including recommended nutrients to meet his genomic challenges.
Participant 106, is 2 years old and lives in the home of his married parents, and for all purposes appears to be thriving. His range of vocabulary is behind the normal two year, but he excels in many other areas, humor, driving motorized toy vehicles, using tools, identifying construction vehicles. #106 has a GARS risk score of 4, the lowest in this family sample. He has the DRD4 risk allele C variant, a single nucleotide discussed above. As a brief reminder this polymorphism created increased risk for developing future alcohol, cannabis (Gilley, 2018b), glucose, nicotine and opioid substance use disorders. This allele increases the risk for Attention Deficit Hyperactivity Disorder (Volkow, Wang, Kollins, Wigal, Newcorn, Teland, et al 2009), novelty seeking and pathological aggression.
#106 also inherited two copies of the S risk variant of the 5HTT-LINKWS polymorphic region of variable tandem number repeats, insertions, and/or deletions. These variant increases risk for alcohol, cannabis, cocaine, glucose, nicotine, and opioid substance use disorders. The variant increases risk for nonsubstance RDS behavioral expressions, such as ADHD, pathological gambling and PTSD (Blum, Gondre-Lewis, Modestino, Lott, Baron, Siwicki, et al 2019). This is the human serotonin transporter gene, which is known to be associated with mood disorders, like depression and/or bipolar.
His parents have been informed of nutrients recommended for proper brain function, which may help with his neurodevelopment (Rapp, Hamilton, Blum, & Thanos, 2022). They have been made aware of the expressed dangers of a sugar filled diet, with his genomic issues, as this may interfere with early neurocognitive development, by creating neural inflammation (Blum, Steinberg, Gondre-Lewis, Simpatico, Ceccanti, Steinberg, B. 2021. Care-givers are on the watch for observation of early signs of ADD/ADHD (Gold, Blum, Oscar-Berman, & Braverman, 2014; Archer et al, 2011), mood/emotional outbursts, which are to be expected in all toddlers, especially those with language delay (Estevez, Jauregui, Sanchez-Marcos, Lopez-Gonzalez, & Griffiths, 2017).
Summary
This RDS family is activity engaged in creating wellbeing (Gilley, 2017), and have made remarkable progress to foster healthy environments for their children (Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, et al 1998). Genetic Addiction Risk Severity (GARS) testing has given them an advantage other family to not have. The Elle Foundation Case Series 104 begins with the genomic testing for optimal brain functions, and to prevent potential manifestation of dopaminergic deficiency and/or disruption issues of the lifespan. This original research sets precedent for children of addicts and mental health disorder everywhere. The best defense is a good offense. We have the resources to stop addiction before it begins. The future is now (Blum, Steinberg, Gondre-Lewis, Baron, Modestino, Badgaiyan, et al 2021)!!
Over the course of the second part of this study, all family members, not just participants #105, and #106 will be provided the opportunity to participate in Reward Deficiency Syndrome Family Systems Therapy (RDS-FST), through the Dragon Slayer Ministry Pilot Study which is currently underway. The Elle Foundation research participants are welcome to participant in all Reward Deficiency Syndrome Solutions ™, such as the 1) GARS test (Blum, Han, Hauser, Downs, Giordano, et al. 2013; Vereczkei, Barta, Magi, Farkas, Eisinger, Kiraly, Belik, Griffiths, et al 2022); 2) Precision Addiction Medicine (PAM) (Blum, Kazmi, Modestino, Downs, Bagchi, Baron, et al 2021); 3) Nutrative Amino Acid Therapy, (NAAT) and Pro-dopamine regulation (Blum, Modestino, Gondre Lewis, Baron, Steinburg, Panayotis, Downs, Siwicki, Lott, Braverman, Moran, Miller, Fried, & Badgaiyan, R. 2018), created by Dr. Kenneth Blum over the 60 year sojourn (Blum & Badgaiyan, 2021), which established the veracity of his body of work.
Participants are also encouraged to participate in the RDS psychotherapies created by the author, original Elle Foundation founder, Elizabeth Gilley. RDS paradigm psycho-education (RDS-PPE) (Ekhtari, Rezapour, Aupperle, & Paulis, 2018), and RDS-Solution Focused Brief Intervention (RDS-SFBI), (Baron, 2018; Gilley, 2019) therapies are appropriate, and prescribed as part of the RDS treatment plan protocol (Gilley, 2018e).
In this genomic era of addiction medicine (Blum & Badgaiyan, 2015), homo sapien has many more resources than last century’s foundational Minnesota Model, which birthed the substance use disorder, addiction recovery twelve step industry and helped foster AA/NA meetings around the globe. Today, in 2022, in addition to twelve step treatments, there are RDS tests and measurements, the RDSQ-29 (Kotyuk, 2022) and the RDS Severity of Symptom (SOS) scale. There are nutraceutical therapies (Blum, Braverman, Carbajal, et al. 2011), genome informed pharmaceutical therapies (Stahl, Pradko, Haight, Modell, Rockett, Learned-Coughlin, 2004; Alguacil & Gonzalez-Martin, 2015), and epigenomic interventions (Blum, Chen, Chen, Braverman, Jeinking, Blum, et al (2008). There are Reward Deficiency Syndrome psychological therapies such as RDS-Paradigm Psychological Education (PPE), RDS-Solution Focused Brief Intervention (SFBI), which assists the client in learning to achieve dopamine homeostasis, and RDS Family Systems Therapy (FST) which helps families get on the same page, about addiction, prevention, and proactive prevention treatment for RDS over the lifespan.
References
- Alguacil L, Gonzalez-Martin C (2015). Target identification and validation in brain reward dysfunction. Drug Discovery Today 20: 347-352.
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders, 5th ed.
- Anda, R., Felitti, V. Bremner, J., Walker, J., Whitfield, C., Perry, B., Dube, S., & Giles, W. (2005).The enduring effects of abuse and related adverse experiences of childhood: A Convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci, 256(3), 174-186.
- Archer T., Badgaiyan. R. & Blum, K. (2017). Physical exercise interventions for drug addictive disorders. J Reward Defic Syndr Addict Sci, 3(1), 17-20. https://www. ncbi.nlm.nih.gov/labs/pmc/articles/PMC5640325/ 5.
- https://unisciencepub.com/storage/2022/02/Reconceptualizing-Addiction-Integrating-the-Sciences-of-Addiction-and-Reward.pdf
- Balconi, M., Finocchiari, R. (2015) Decisional impairments in cocaine addiction, reward bias, and cortical oscillation “unbalance.” Neuropsychiatric Disease and Treatment 11: 777-786.
- Baron, D., Blum. K., Chen, A., Gold, M., Badgaiyan, R. (2018) Conceptualizing addiction from an osteopathic perspective: Dopamine homeostasis. Journal A, Osteopath Assoc 118: 115- 118.
- Beitscher-Campbell, H., Blum, K., Febo, M., Madigan, M., Giordano, J., Badgaiyan, R. et al (2016). Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. Journal of Behavioral Addictions, 5(3), 533-541.
- Blum, K., & Badgaiyan, R. (2015). Reward Deficiency Syndrome (RDS): Entering the genomics and neuroscience era of addiction medicine. Journal of Reward Deficiency Syndrome Addiction Science, 1(1): 1-2.
- Blum, K., & Badgaiyan, R. (2021). Translational and molecular cytoarchitectural genetic guided therapy to induce Dopamine Homeostatic Neuro-signaling in Reward Deficiency and associated drug and behavioral addiction seeking: A 60 year sojourn the future is now. EC Psycho Psychiatr, 10(8):1-4/
- Blum K, Badgaiyan R, Agan G, Frantantonio J, Simpatico R, et al. (2015). Molecular genetic testing in reward deficiency syndrome (RDS): Facts and fiction. Journal of Reward Defic Syndr Addict Sci 1: 65-68.
- Blum, K., Baron, D., Lott, L., Ponce, V., Siwicki, D., et al. (2019) In search of Reward Deficiency Syndrome (RDS)-Free Controls: The Holy Grail in genetic addiction risk testing. Current Psychopharmacology 8: 1-15.
- Blum, K., Baron, D., McLaughlin, T., & Gold, M. (2020). Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS). Journal of Neurological Sciences, 411: 116733. https://doi.org/10.11016/j.jns.2020.116733 14.
- Blum, K., Bowirrat, A., Braverman, E., Baron, D., Cadet, J., Kasmi, et al (2021). Reward, Deficiency Syndrome (RDS): A cytoarchitectural common neurobiological trait of all addictions. International Journal of Environmental Research and Public Health, 18(11529):1-31.
- Blum, K., Bowirrat, A., Gondre-Lewis, M., Gold, M. et al (2021). Exploration of epigenetic state hyperdopaminergia (surfeit) and genetic trait hypodopaminergia (deficit) during adolescent brai development.Researchgate.net/publication/349342073.
- Blum, K., Steinberg, B., Gondre-Lewis, M, Simpatico, T., Ceccanti, M., Steinberg, B. (2021). Exploration of epigenetic state hyperdopaminergia (Surfeit) and Genetic Trait Hypodopaminergia (Deficit) during adolescent brain development. Curr Psychopharmacol. Doi: 10.10.2174/2211556010666210215155509
- Blum, K., Braverman, E. (2000). Reward Deficiency Syndrome (RDS): a biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. Journal of Psychoactive Drugs, 32:1-112 (Supplement).
- Blum K, Braverman E, Carbajal J, et al. (2011). Hypothesizing synergy between acupuncture/auriotherapy and natural activation of mesolimbic dopaminergic pathways: Putative natural treatment modalities for the reduction of drug hunger and relapse. Integrative Omics and Applied Biotechnology Letters, 1: 1- 14.
- Blum, K., Cadet, J., Thanos, P., Baron, D., Mishrekar, A., Brewerm R., Bowirrat, A., Febo, M. & Gold, M. (2020). Neurogenetics of alcohol use disorder a subset of reward deficiency syndrome: Candidate genes to be or not to be? In E. Tsermpini, M. Alda, & G. Patrinos (Eds.), Psychiatric Genomics, p. 105-147. Academic Press.
- Blum, K., Chen, A., Chen, T., Braverman, E., Jeinking, J., Blum, S. et al (2008). Activation instead of blocking mesolimbic dopaminergic reward circuitry is preferred modality in the long-term treatment of reward deficiency syndrome (RDS): A commentary. Theoretical Biology and Medical Modeling, 12: 5-24
- Blum, K., Chen, T., Downs, W., Bowirrat, A., Waite, R., Braverman, E. et al (2009).Neurogenetics of dopaminergic receptor super-sensitivity in activation of brain reward circuitry and relapse: Proposing deprivation-amplification relapse therapy (DART). Postgrad Med, 121(6):176-196.
- Blum, K., Chen, A., Giordano, J., Borstein, J., Chen, T. et al. (2012) The addictive brain: All roads lead to dopamine. Journal of Psychoactive Drugs 44: 134-143.
- Blum, K., Chen T., Morse, S., Giordano, J., Chen, A., Thompson, J. et al (2010). Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and poly-drug abusers, utilizing putative dopamine D2 agonist therapy: Part 2. Postgraduate Medicine, 122:214-26. [PubMed:21084796]
- Blum K, Chen A, Oscar-Berman M, Chen M, Lubar J, et al. (2011) Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: Selecting appropriate phenotypes for reward dependence behaviors. Int J Environ Res Public Health 8: 4425-4459.
- Blum, K., Febo, M, Badgaiyan, R., Demetrovics, Z., Simpatico, T., et al. (2017). Common Neurogenetic diagnosis and Meso Limbic manipulation of hypo-dopaminergic function in reward deficiency syndrome (RDS): Changing the recovery landscape. Current Neuropharmacology, 15:184-194.
- Blum, K., Febo, M., McLaughin, T., Cronje, F., Han, D., et al. (2014) Hatching the behavioral addiction egg: Reward deficiency syndrome solution system (RDSS), as a function of dopaminergic, brain functional connectivity linking all addiction under a common rubric. Journal of Behavioral Addictions, 3: 149-156.
- Blum, K., Fried, L., Madigan, M., Giordano, J., Modestino, E., Steinbergy, B. et al (2017). Critical Analysis of White House Anti-Drug Plan. Glob J Addict Rehabil Med, 1(4).
- Blum, K., Gold, M., Llanos-Gomez, L., Jalali, R., Thanos, P., Bowwirrat, A. (2021). Hypothesizing nutrigenomic-based precision anti-obesity treatment and prophylaxis: Should we be targeting sarcopenia induced brain dysfunction? Int J. Environ. Res. Public Health, 18 (9774):1-18.
- Blum, K., Gondre-Lewis, M., Modestino, E., Lott, L., Baron, D., Siwicki, D., et al (2019). Understanding the scientific basis of Post-Traumatic Stress Disorder (PTSD): Precision behavioral management overrides stigmatization. Mol Neurobio, 56(11):7836-7850/
- Blum, K., Han, D., Hauser, M., Downs, B., Giordano, J., et al. (2013). Neurogenetic impairments of Brain Reward Circuitry links to Reward Deficiency Syndrome (RDS) as evidenced by Genetic Addiction Risk Score (GARS): A case study. IIOABJ, 4: 4-9.
- Blum, K., Hauser, M., Agan, G., Giordano, J., Frantantonio, J., Badgaiyan, R. & Febo, M. (2015). Understanding the importance of dopaminergic deficit in Reward Deficiency Syndrome (RDS): Redeeming joy overcoming “darkness” in recovery. Psychology, 6, 435-439.
- Blum, K., Kazmi, S., Modestino, E., Downs, W., Bagchi, D., Baron, D., et al (2021). A novel precision approach to overcome the Addiction Pandemic by incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration. Journal of Personalized Medicine, 11(212):1-18.
- Blum, K., McLaughlin, T., Bowirrat, A., Modestino, E., Baron, D., Llanos Gomez, L. et al,(2022). Reward Deficiency Syndrome (RDS) surprisingly is evolutionary and foundeverywhere: Is it “blowin’ in the wind”? Journal of Personalized Medicine, 12, 321.
- Blum, K., Modestino, E., Gondre Lewis, M., Baron, D., Steinburg, B., Panayotis, T., Downs, W.,Siwicki, D, Lott, L., Braverman, E., Moran, M., Miller, D., Fried, L., & Badgaiyan, R. (2018). Pro-dopamine regulator (KB220) a fifty-year sojourn to combat Reward Deficiency Syndrome (RDS): Evidence Based Bibliography (Annotated). CPQ Neurol Psychol, 1(2).
- Blum, K., Oscar-Berman, M., Demetrovics, Z., Barh, D., & Gold, M. (2014). Genetic Addiction Risk Score (GARS): Molecular neurogenetic evidence for predisposition to Reward Deficiency Syndrome (RDS). Mol Neurobio, 50(3), 765-796.
- Blum, K., Raza, A., Schultz, T., Jalali, R., Green, R., Brewer, R., et al (2021). Should we embrace the incorporation of genetically guided “dopamine homeostasis” in the treatment of Reward Deficiency Syndrome (RDS) as a frontline therapeutic modality? Acta Sci Neurol, 4(2):17-24. J Osteopath Med. https://doi.org/10.1515/jom-2021-0026. 37.
- Blum, K., Steinberg, B., Gondre-Lewis, M., Baron, D., Modestino, E., Badgaiyan, R., et al (2021). A review of DNA risk alleles to determine epigenetic repair of mRNA expression to prove therapeutic effectiveness in Reward Deficiency Syndrome (RDS): Embracing“Precision Behavioral Management.” Psychol Res Behav Manag, 14, 2115-2134.
- Blum, K., Thanos, P. & Gold, M. (2014). Dopamine and glucose, obesity and reward deficiency syndrome. Frontiers in Psychology, 5, Article 919:1-11
- Blumenthal, D. & Gold, M. (2010). Neurobiology of food addiction. Curr Opin Clin Nutr Metab Care, 13(4):359-65.
- Braverman, E., Dennen, C., Gold, M., Bowirrat, A., Gupta, A., Baron, S., et al (2022). Proposing a “Brain Health Checkup (BHC)” as a global potential “Standard of Care” to overcome Reward Dysregulation in Primary Care Medicine: Coupling genetic risk testing and induction of “Dopamine Homeostasis.” International Journal of Environmental Research and Public Health, 19, 5480.
- CASA Columbia (2012). Addiction medicine: Closing the gap between science and practice Columbia University Press. 573. Retrieved from https://books google.co.in/books/about/Addiction_Medicine. html?id=hCIhnQAACAAJ&redir_esc=y 42.
- Dackis, C. & Gold, M. (1985). New concepts in cocaine addiction: The dopamine depletion hypothesis. Neuroscience and Biobehavioral Reviews, 9(3):469-477.
- Dick, D. & Agrawal, A. (2008). The genetics of alcohol and other drug dependence. Alcohol Research & Health, 31(2), 111-118.
- Diana, M. (2011). the dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2(64), 5. DOI: 10.3389/fpsyt.2011.00064
- Downs, B., Chen, A., Chen, T., Waite, R., Braverman, E., Kerner, M (2009). Nutrigenomic targeting of carbohydrate craving behavior: Can we manage obesity and aberrant craving behavior with neurochemical pathway manipulation by immunological compatible substances (nutrients) using a Genetic Positioning System (GPS) map? Med Hypotheses, 73, 427-434.
- Edwards, D., Roy, 3rd, AK, Boyett, B., Badgaiyan, R., Thanos, P., Baron, D., et al (2020).Addiction by any other name is still addiction: Embracing molecularneurogenetic/epigenetic basis of reward deficiency syndrome. J Addict Sci, 6(1):1-4.
- Ekhtari, H., Rezapour, T., Aupperle, R., & Paulis, M., (2018). Neuroscience-informed psychoeducation for addiction medicine: A neurocognitive perspective. Prog Brain Res, 235, 239-264.
- El-Guebaly, N. (2004). Concurrent substance-related disorders and mental illness: The North American experience. World Psychiatry, 3(3):182-187.
- Enoch, M., Hodgekinson, C, Yuan, Q., Shen, P., Goldman, D. & Ray, A. (2010). The influence of GABRA2, childhood trauma and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry, 67(1), 20-27.
- Estevez, A., Jauregui, P., Sanchez-Marcos, I., Lopez-Gonzalez, H., & Griffiths, M. (2017).Attachment and emotion regulation in substance addictions and behavioral addictions Journal of Behavioral Addictions, 6(4), 534-544.
- Felitti, V., Anda, R., Nordenberg, D., Williamson, S., Spitz, A., Edwards, V. et al (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. Am J Prev Med,14(40), 245-258.
- Flanagan, O. (2014). The shame of addiction. Frontiers in Psychiatry, 4:120. Doi: 10.3389/fpsyt-2013.00120
- Gilley, E. D. (2018e). A proposed treatment plan model for Reward Deficiency Syndrome: To Help in restructuring the Addiction Recovery Industry. European Journal of Biomedical and Pharmaceutical Sciences, 5(11), 84-90. Retrieved from https://storage.googleapis.com/journaluploads/ejbps/article_issue/volume_5_november_ issue_11/1540964883.pdf 54.
- Gilley, E. D. (2019). Reward Deficiency Syndrome Solution Focused Brief Therapy to begin integrating the Sciences of Addiction and Reward Deficiency Syndrome (RDS). Journal of Reward Deficiency Syndrome and Addiction Science, 5(1), 1-6. https://doi.org/10.17756/ jrdas.2018- 042 125.
- Gilley, E. D. (2017). Integrating the Science of Addiction and the Science of Wellbeing. Journal of Alcoholism and Drug Dependence, 5(4), 275-281. DOI: 10.4172/2329-6488.1000275
- Gilley, E. D. (2018a). The Evolution of Addiction Treatment: The disease is Reward Deficiency Syndrome (RDS) and Addiction is its symptom. European Journal of Biomedical and Pharmaceutical Sciences, 5(1), 161- 166. Retrieved from https://storage.googleapis.com/ journal uploads/ejbps/article_issue/volume_5_january_ issue_1/1514635399.pdf
- Gilley, E. D. (2018, b). The truth is usually somewhere in the middle: Introduction to the debate on medical marijuana. Journal of Addiction Research, 2(1), 1-4.
- Gilley, E. D. (2021b). Integrating the Science of Addiction and the Science of Wellbeing: 5 years update for the GAB21. The 2nd Edition, Global Conference on Addiction Medicine, Behavioral Health and Psychiatry, 5(4), 65. DOI: 10.4172/2329-6488.1000275
- Gilley, E. D. (2021c). Re-conceptualizing Addiction: Integrating the Sciences of addiction medicine and Reward Deficiency Syndrome. The 2nd Edition: Global Conference on Addiction Medicine, Behavioral Health and Psychiatry, October 23, 2021, p 66. 1
- https://unisciencepub.com/storage/2022/02/Reconceptualizing-Addiction-Integrating-the-Sciences-of-Addiction-and-Reward.pdf
- https://fortuneonline.org/articles/pthe-new-science-of-attention-deficit-hyperactivity-disorder-news-from-the-cutting-edge-of-research-sciencep.html
- Gilley, E. D. (2018c). The new science of attention deficit hyperactivity disorder: News from the cutting edge of research science. European Journal of Biomedical and Pharmaceutical Sciences, 5(9), 587-590.
- Gilley, E. D. (2022a). Reconceptualizing Addiction: Integrating the sciences of addiction and Reward Deficiency Syndrome, (RDS), Part 2: Case Report. Journal of Addictive Disorders and Mental Health, 2(1), 1-10.
- Gilley, E. D. (2022 b). Reward Deficiency Syndrome phase two addiction treatment, targets the unique needs of the individual’s brain: Project Reconceptualizing Addiction: The Elle Foundation Case Study 102 Case report J of Addict Dis & Ment Heal, 2(2), 1-9.
- Gilley, E. D. (2022 c). The Reward Deficiency Syndrome (RDS) paradigm – RDS is the phenotype: Addiction and mental disorder are endotypes: Elle Foundation Research Institute 100S Series, Family Generational Genomic Case Series Study #103. European Journal of Biomedical and Pharmaceutical Sciences, 9(8), 14p.
- Gilley, E. (2020). Reconceptualizing Addiction: Integrating the sciences of addiction and reward deficiency syndrome, Part 1. Journal of Addiction Research, 4(1):1-5.
- Gilley, E., Bowirrat, A., Gupta, A., Giordano, J, Dennen, C., Braverman, E., Badgaiyan, R.,McLaughin, T., Baron, D., & Blum, K., (2022). Precision Genomic Addiction Medicine as a Frontline Modality as function of inducing “Dopamine Homeostasis” in Reward Deficiency Syndrome (RDS): The future is Now. Current Pharmaceutical Biotechnology, next edition.
- https://www.researchgate.net/profile/Elizabeth-Gilley/publication/361790105_Reward_Deficiency_Syndrome_phase_two_addiction_treatment_targets_the_unique_needs_of_the_individual's_brain_Project_Reconceptualizing_Addiction_The_Elle_Foundation_Case_Study_102/links/62c54b98721b9c41cc32a2b5/Reward-Deficiency-Syndrome-phase-two-addiction-treatment-targets-the-unique-needs-of-the-individuals-brain-Project-Reconceptualizing-Addiction-The-Elle-Foundation-Case-Study-102.pdf
- Gondre-Lewis, M., Bassey, R., & Blum, K. (2020). Preclinical models of Reward Deficiency Syndrome: A behavioral octopus. Neurosci Biobehav Rev, 115:164-188.
- Gupta, A., Bowirrat, A., Llanos Gomez, L., Baron, D., Elman, I., Giordano, J., et al (2022). Hypothesizing in the face of the opioid crisis coupling Genetic Addiction Risk Severity (GARS) testing with electrotherapeutic nonopioid modalities such as H-Wave could attenuate both pain and hedonic addictive behaviors. International Journal of Environmental Research and Public Health, 19, 552.
- Kotyuk. E., Urban, R., Hende, B., Richman, M., Magi, A., Kiraly, O. et al (2022). Development and validation of the Reward Deficiency Syndrome Questionnaire (RDSQ-29). Journal Psychopharmacol, Doi: 10. 1177/02698811211069102.
- Leyton M. (2014). What’s deficient in reward deficiency? J Psychiatric Neurosci, 39, 291-293.
- Leyton, M. (2017). Altered dopamine transmission as a familial risk trait for addictions. Current Opinion in Behavioral Sciences, 13:130-138.
- Mancheno, J., Navas-Leon, S., Fernandez-Calderon, F., Gutierrez, M., Sanchez-Garcia, M. et al (2021). Coordinated treatment between addiction and mental health services versus uncoordinated treatment for patients with dual diagnosis: Higher dropout rates but lower impairment functional disability. Actas Esp Psiquiatry, 49(2), 71-80.
- Moberg, C. & Humphreys, K. (2016). Exclusion criteria in treatment research on alcohol, tobacco, and illicit drug use disorders: A review and critical analysis. Drug and Alcohol Review, 36:378-388. Doi: 10.1111/dar.12438.
- Moran, M., Blum, K., Valdez Ponce, J., Lott, L., Gondre-Lewis, M., Badgaiyan, S. (2021). High genetic addiction risk score (GARS) in chronically prescribed severe chronic opioid probands attending multi-pain clinics: An open Clinical Pilot Trial
- Paquette, C., Daughters, S. & Witkiewitz, K. (2022). Expanding the continuum of substance use disorder treatment: Nonabstinence approaches. Clinical Psychology Review, 91. 102110
- Price, M. (2008). Genes matter in addiction. American Psychological Association, 39(6), 14.
- Prendergast, M., Podus, D., Chang, E., & Urada, D., (2002). The effectiveness of drug abusetreatment: A meta-analysis of comparison group studies. Drug and alcohol dependence, 67:53-72.
- Rapp, C., Hamilton, J., Blum, K., & Thanos, P. (2022). The long-term interaction of diet and dopamine D2 gene expression on brain microglial activation. Psychiatry Research: Neuroimaging, 320, 111430.
- Roy, A., Bowirrat, A., Smith, D., Braverman, E., Jalili, R., Badgaiyan, R. et L (2021). Neurobiology and spirituality in addiction recovery. Acta Sci Neurol, 4(9), 64-71.
- Sanders, M., & Mazzucchelli, T. (2022). Mechanisms of change in population-based parenting interventions for children and adolescents. Journal of Clinical Child & Adolescent Psychology. https://dio.org/10.1080/15374416.2022.205598.
- Stahl, S., Pradko, J., Haight, B., Modell, J., Rockett, C., Learned-Coughlin, S. (2004). A review of the neuropharmacology of Buproprion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion Journal of Clinical Psychiatry, 6(4):159-166.
- Tsermpini, E., Alda, M., & Patrinos, G. (Eds.) (2022). Psychiatric Genomics: Translational and Applied Genomics Series. Academic Press.
- Uhart, M., Weerts, E., McCaul, M., Guo, X., Yan, X., Kranzier, H. et al (2012). GABRA2 markers moderate the subjective effects of alcohol. Addict Bio, 18(2), 357-369.
- Uhl, G. & Liu, Q. (2006). NIDA researchers identify 89 genes implicated in addiction: At least 21 are likely to affect brain’s memory processes. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics), 141-B, 1-8.
- Uhl, G., Liu, Q., Walter, D., Hess, J., & Naiman, D. (2002). Researchers report first “Genome Scan” for drug abuse. American Journal of Human Genetics.
- Vaillancourt, K., Ernst, C., Mash, D., & Turecki, G. (2017). DNA methylation dynamics and cocaine in the brain: Progress and prospects. Genes, 8, 138.
- Vereczkei, A., Barta, C., Magi, A., Farkas, J., Eisinger, A., Kiraly, O., Belik, A., Griffiths, M. et al (2022). FOXN3 and GDNF polymorphisms as common genetic factors of substance use and addictive behaviors. Journal of Personalized Medicine, 12, 690.
- Volkow, N., Fowler, J., & Wang, G. (2002). Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behav Pharmacol, 13, 355-366.
- Volkow, N., Wang, G., Kollins, S., Wigal, T., Newcorn, J., Teland, F.et al (2009). Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA 301, 1084-1091.


