Archive : Article / Volume 1, Issue 3
- Research Article | DOI:
- https://doi.org/10.58489/2836-2322/012
Study of vinpocetine adsorption on the surface of liposomes obtained from soya lecitine
Federal State Budget Educational Institution of Higher Education "Voronezh State University," Russia, 394036, Voronezh, Russia.
Yu. A. Polkovnikova
Yu. A. Polkovnikova, (2022). Study of vinpocetine adsorption on the surface of liposomes obtained from soya lecitine. Journal of Pharmacy and Drug Development. 1(3). DOI: 10.58489/2836-2322/012
© 2022 Yu. A. Polkovnikova, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 30-11-2022
- Accepted Date: 12-12-2022
- Published Date: 22-12-2022
vinpocetine adsorption, liposome, soya lecitine
Abstract
Liposomes are one of the best studied types of nanoparticles that are considered as contemporary and efficient means for delivery of various medicinal agents widely applied in the clinical practice. Taking into account the features of transportation, translocation through histological barriers, cellular membranes and metabolic transformations liposomal pharmaceutical drugs show unique properties, first of all connected with the distinctions in their pharmacokinetics.
Objective of the study: determination of adsorption characteristics for vinpocetine applied on the surface of liposomes obtained from soya lecitine.
Liposomes from soya lecitine were obtained by hydration/rehydration technique. In order to study characteristics of vinpocetine adsorption on the surface of liposomes technique of equilibrium dialysis was utilized. Sol of iron hydroxide was used as a comparison adsorbent. Separation of the dispersive medium from sol colloid particles was performed with the use of column chromatography.
Comparative investigations of vinpocetine adsorption were performed both on comparison adsorbent and on the liposomes obtained from soya lecitine. Rather efficient adsorption of vinpocetine on the liposomes at their low concentrations was observed.
Introduction
Nanocarriers used for the drug delivery to the organs and tissues differ by their size, shape and composite materials [1]. The properties of each nanoparticle are defined by the extent of its payload with a drug, stability, the rate of the drug release and the presence of a ligand for the directed transportation [2,3].
In order to get the required therapeutic effect pharmaceutical substance encapsulated into the vesicles should be available to the targeted cells [4]. At this point liposomes differ from the other controlled delivery systems where the biologically active compounds are released either in the blood plasma or just in the place of injection. Then the captured preparation can be selectively accumulated within the affected part of an organism due to the passive (unintended) or active targeting [5].
Liposomes are spherical vesicle structures composed of a uni- or multilamellar lipid bilayer surrounding internal aqueous compartments and a relatively impermeable outer lipophilic phospholipid bilayer. Liposomes have gained considerable attention as drug delivery carriers because they are biocompatible, nontoxic, can deliver both hydrophilic and lipophilic drug molecules, protect their cargo from degradation by plasma enzymes, and transport their load across biological membranes and the blood brain barrier [6].
When using liposomes as an independent preparation or as a carrier for medicinal agents it is required to study the effect of liposomes on the cells in human organism. The determining factor in this case can be as the chemical composition of liposomes as their size [7].
Vinpocetine is a vasoactive and nootropic preparation that proves to be semisynthetic derivative of the common periwinkle plant alkaloid. Vinpocetine is referred to the type of compounds that are practically insoluble in water [8].
Objective of the investigation: determination of characteristics for adsorption of vinpocetine on the surface of liposomes obtained from soya lecitine.
Materials and methods
The study of vinpocetine adsorption characteristics on the surface of liposomes
Characteristics of vinpocetine adsorption on the surface of liposomes were studied by the equilibrium dialysis technique [9]. The choice of this method is stipulated by the fact that quantitative analysis of the equilibrium vinpocetine concentration in the dispersive medium necessary for the determination of the adsorption value is complicated by the presence of dispersive phase - liposomes. Semipermeable membrane with diameter of pores that is sufficient for the penetration of vinpocetine molecules but leakless for liposomes ensures obtaining of vinpocetine solution with the concentration close to that one vinpocetine in the liposomes dispersive medium. Solution prepared in such a way can be analyzed quantitatively with the use of spectrophotometry.
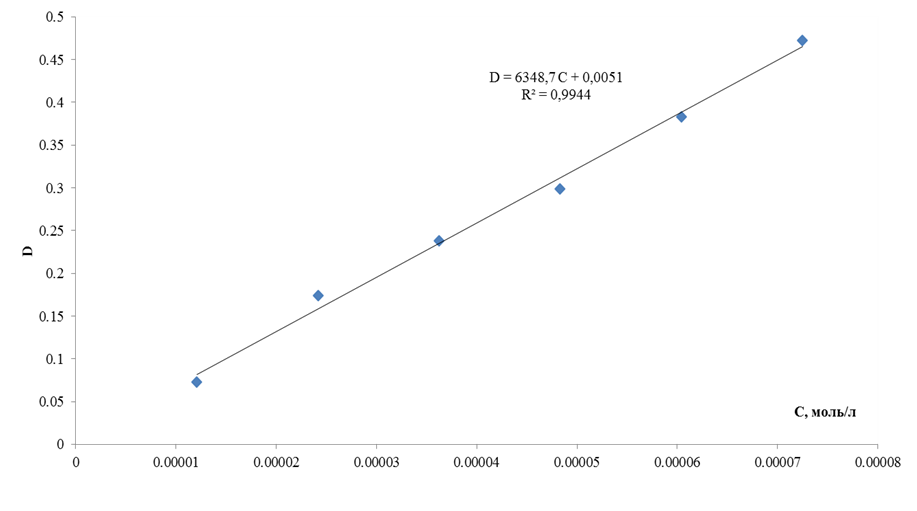
In order to determine the linearity of the applied photometric analysis technique a calibration graph was plotted within the range of concentrations and optical densities required for the performed study at the wavelength of 313 nm (figure 1).
<!-- /* Font Definitions */ @font-face {font-family:"Cambria Math"; panose-1:2 4 5 3 5 4 6 3 2 4; mso-font-charset:0; mso-generic-font-family:roman; mso-font-pitch:variable; mso-font-signature:-536869121 1107305727 33554432 0 415 0;} @font-face {font-family:Calibri; panose-1:2 15 5 2 2 2 4 3 2 4; mso-font-charset:0; mso-generic-font-family:swiss; mso-font-pitch:variable; mso-font-signature:-469750017 -1073732485 9 0 511 0;} /* Style Definitions */ p.MsoNormal, li.MsoNormal, div.MsoNormal {mso-style-unhide:no; mso-style-qformat:yes; mso-style-parent:""; margin-top:0in; margin-right:0in; margin-bottom:8.0pt; margin-left:0in; line-height:107%; mso-pagination:widow-orphan; font-size:11.0pt; font-family:"Calibri",sans-serif; mso-fareast-font-family:Calibri; mso-bidi-font-family:"Times New Roman"; mso-ansi-language:RU;} .MsoChpDefault {mso-style-type:export-only; mso-default-props:yes; font-family:"Calibri",sans-serif; mso-ascii-font-family:Calibri; mso-fareast-font-family:Calibri; mso-hansi-font-family:Calibri; mso-ansi-language:RU; mso-fareast-language:RU;} @page WordSection1 {size:8.5in 11.0in; margin:1.0in 1.0in 1.0in 1.0in; mso-header-margin:.5in; mso-footer-margin:.5in; mso-paper-source:0;} div.WordSection1 {page:WordSection1;} -->
FIGURE 1. Calibration plot for the quantitative determination of vinpocetine in the medium of 0,01 M of aqueous solution of the hydrochloric acid by spectrophotometry technique at the wavelength of 313 nm
In this study the values of vinpocetine adsorption on liposoimes at the different concentrations of vinpocetine were determined as well as the constants in Freundlich and Langmuir equations.
To solve this problem colloid solution of liposomes from soya lecitine was prepared and then its mass fraction was determined. After that the process standard sample (PSS) of vinpocetine was prepared and calibration graph was plotted and molar absorption coefficient of vinpocetine was determined. Optimal technique of equilibrium dialysis was chosen, equilibrium dialysis for different concentrations of vinpocetine was performed and analysis of the obtained results was executed.
Preparation of the liposomes samples prepared from soya lecitine
To obtain liposomes from soya lecitine hydration/re-hydration was applied. Soya lecitine solution (Sigma) in ethyl alcohol was evaporated in the rotor evaporator at the temperature of 45ºC and pressure of –0,085 MPa. Then 0,01 M solution of hydrochloric acid (pH=2,0) was added. For obtaining of liposomes solutions were subjected to irradiation with ultrasound disintegrator for 15 minutes. Next, liposomes were filtered through glass filter with diameter of pores 16 m.
Preparation of the process standard sample (PSS) of vinpocetine
An accurate sample of 12 mg of vinpocetine was solved in 0,01 M aqueous solution of hydrochloric acid in the graduated flask of 50 ml volume and was brought up to the label with a solvent.
Equilibrium dialysis was performed with the use of dialysis vials Easy Dial–L having semipermeable membrane and its passing characteristic was of 14 kDa. For the determination of vinpocetine adsorption value by liposomes the main experiment was made (dialyzer A), where adsorption was observed, reference experiment (dialyzer B) where dialysis took place but there was no liposomes, and the experiment for the measurement of content of the free lecitine in the dispersive medium (dialyzer C). 12 ml of liposome solution and dialysis vial were placed into dialyzer A. Note, that 3 ml of vinpocetine solution with the concentration C0 was placed into the dialyzer vial. 12 ml of 0,01 M solution of hydrochloric acid and dialysis vial were placed inside dialyzer B. After that 3 ml of vinpocetine solution with the concentration of C0 was placed into dialyzer vial. And 12 ml of liposome solution and the dialyzer vial were placed into dialyzer C, while in the dialyzer vial 3 ml of 0,01 M solution of hydrochloric acid was placed as well. Dialysis was performed in thermostat at the temperature of 37 ºC for 12 hours. After that using spectrophotometer LOMO SF-56 optical density of solutions was measured in the dialyzer vials previously placed in the dialyzers A, B and C at the wavelength of 313 nm (DA, DB and DC, respectively). For the determination of vinpocetine concentration in the dialyzer A the difference of optical densities D = DА – DВ was applied.
Results
Study of the adsorption characteristics of vinpocetine on the surface of liposomes
Taking into account vinpocetine solubility in the acidic medium as a dispersive medium for liposomes 0,01 M solution of hydrochloric acid was chosen as such a medium. Vinpocetibe is also soluble in ethanol, but during liposome interaction with ethanol their coagulation occurs.
Order of plotting calibration graph and determination of absorptivity for vinpocetine in 0,01 M medium of aqueous solution of hydrochloric acid
During the study of adsorption characteristics of vinpocetine on liposomes the quantitative determination of vinpocetine in 0,01 M aqueous solution of hydrochloric acid was performed by spectrophotometry technique. To do so vinpocetine spectrophotometry spectrum was measured for 0,01 M of vinpocetine in the aqueous solution of hydrochloric acid.
For 0,01 M of aqueous solution of hydrochloric acid ultraviolet spectrum of vinpocetine is characterized by the peaks of optical density at the wavelengths of 268 and 313 nm. The choice of the maximum in the optical density used for the quantitative determination of vinpocetine was made basing on the possible influence of the free lecitine used as a dispersive medium for liposomes capable to penetrate through semipermeable membrane [20]. To check this idea a test dialysis was performed where 3 ml of solution with liposomes were placed into dialysis vial while 12 ml of 0,01 M of the aqueous solution with hydrochloric acid were placed into a dialyzer. Dialysis was performed for 12 hours at 37º C. Then ultraviolet absorption spectrum of solution in dialyzer was measured. For the analysis of the quantitative content of vinpocetine the peak corresponding to 313 nm maximum of absorption was chosen since in this spectral range the contribution of lecitine in the dispersive medium for liposomes in the optical density proves to be minimal.
In order to determine the linearity of the applied photometric analysis technique a calibration graph was plotted within the range of concentrations and optical densities required for the performed study at the wavelength of 313 nm.
The choice of technique for the equilibrium dialysis in order to study the process of adsorption for vinpocetine on liposomes
The objective of the choice of technique for the equilibrium dialysis [20] was the search for conditions when the change of vinpocetine concentration due to its adsorption on liposomes would be maximum one. In the process of the choice of technique concentrations of vinpocetine in a dialyzer varied as well as the volumes of liquids by both sides of the dialyzer membrane.
O make the study of the effect of vinpocetine concentration on the process of its adsorption on the liposomes 7 dialyzers were prepared. In the dialyzers 1 – 5 были OSS solutions were placed according to the Table 6, with a volume of 12 ml. 12 ml of solution 5 from the Table 6 was placed into dialyzer 6. 12 ml of the aqueous solution of hydrochloric acid with a concentration of 0.01 M was placed in a dialyzer 7. Next, in all of the dialyzers dialysis vials of Easy Dial-L type with semi-permeable membranes and transmission characteristic of 14 kDa were placed. 2 ml of liposome solution were placed into dialysis vials of the dialyzers 1 – 5 and 7. And 2 ml of 0,01 M of the aqueous solution of hydrochloric acid was placed indialyzer 6. Dialysis was performed in a thermostat at 37ºC for 12 hours.Then, the optical density of solutions in the dialyzers was measured. Solutions in the dialyzers 3 – 6 were diluted with 0.01 M solution of the hydrochloric acid before measurements. Results of the measurements are presented in Table
| TABLE 1 Results of the equilibrium dialysis for vinpocetine solutions with different concentration in the presence of liposomes | ||||||
| № of dialyzer | Dilution during measure-ments of the optical density | Optical density at the wavelength of 313 nm | Optical density at the wavelength of 313 nm with the account of dilution and free lecitine | Optical density at the wavelength of 313 nm with the account of dilution for PSS of vinpocetine | Change of the optical density for vinpocetineas a result of adsorption | The presence of coagulation in the dialysis vial |
| 1 | 1 | 0,2353 | 0,2178 | 0,2582 | 0,0404 | Absence of coagulation |
| 2 | 1 | 0,4645 | 0,4470 | 0,5164 | 0,0694 | Littleflakysediment |
| 3 | 5,22 | 0,1910 | 0,9795 | 1,0328 | 0,0532 | Little flaky sediment |
| 4 | 5,21 | 0,3653 | 1,8857 | 2,0655 | 0,1798 | Almost complete coagulation, solution is close to transparent one |
| 5 | 10 | 0,4024 | 4,0065 | 4,1310 | 0,1245 | Complete coagulation, transparent solution |
| 6 | 10 | 0,4131 | 4,1310 | – | – | – |
| 7 | 1 | 0,0175 | 0,0000 | – | – | Absent |
Conditional optical density was calculated with the account of dilution of the solutions just before the spectrophotometry measurements. Optical density of solution from the dialyzer 7 was subtracted from the total optical density (implying optical density of the free lecitine).
According to the results of the executed dialysis it is possible to make a conclusion that concentration of vinpocetine with the optical density of solution more than 0,25 and this can result in coagulations of liposomes. If concentration of vinpocetine corresponds to the optical density of 0,25 then the change of the optical density under adsorption on liposomes was of 0,04.
In order to enhance the amount of the adsorbed vinpocetine the volume occupied by liposomes was increased up to 12 ml. And solution of liposomes was placed directly in a dialyzer while 2 ml of vinpocetine was placed into the dialysis vial (dialyzer A). Vinpocetine solution for this experiment was prepared by mixing of OSS solution of vinpocetine and 0,01 M of the aqueous solution of hydrochloric acid with a ratio of 1:1 (solution А). 12 ml of the aqueous solution of 0,01 M of hydrochloric acid was placed into the second dialyzer (dialyzer B) as well as a dialysis vial. 2 ml of solution A was measured and placed into the dialysis vial. 12 ml of liposomes solution was measured and placed into third dialyzer (dialyzer B) and then placed there dialysis vial with was filled with 2 ml of aqueous solution of 0,01 M of hydrochloric acid. The dialysis was performed in thermostat at the temperature of 37 ºC for 12 hours. No coagulation of liposomes was observed in the dialyzers A and B after 12 hours of the experiment.
According to the results of experiment with an increased volume of liposomes solution a technique was chosen for the further investigation which includes placing of 12 ml of the liposomes solution in the dialyzer. After that in order to study adsorption of vinpocetine on liposomes the authors applied vinpocetine concentrations that did not cause liposomes coagulation
Determination of adsorption value for vinpocetine on the liposomes by equilibrium dialysis.
In order to determine adsorption parameters of vinpocetine on the liposomes a series of vinpocetine solutions with different concentrations was prepared for the study. Their dialysis was made according to the technique presented above. Equilibrium concentrations of vinpocetine in the dialyzers A and B were calculated with the use of the molar absorption coefficient.
Next, a calculation of the value of vinpocetine adsorption value on the liposomes was performed in accordance with the results of determination for the equilibrium concentration in the dialyzer vials of the main and reference experiments.
Under increase if vinpocetine concentration the value of adsorption upraises as well and it attains maximum at the concentrations of about 0,030–0,035 mole/kg.
Results of determination of the characteristics for vinpocetine adsorption on the liposomes are presented in Table 2.
| TABLE 2. Results of determination for adsorption characteristics of vinpocetine adsorption on liposomes | ||
| Equation | Constant | Value |
| Freundlich equation | 1/n | 0,505919 ± 0,108038364 |
| k, mole/kg | 3,615507457 ± 1,163161619 | |
| Langmuir equation | A∞, mole/kg | 0,0122987 ± 0,005485 |
| b, mole/l | 4,08717*10–6 ± 6,74916*10–6 | |
Conclusion
1. Ultimate adsorption of vinpocetine on liposomes is considerably less than that one on the colloid particles of iron hydroxide (III) sol.
2. Constant b in Langmuir equation (the value of concentration when a half of the ultimate adsorption is attained) proves to be less as compared with that one characteristic for adsorption on the colloid particles of iron hydroxide (III) sol; it means quite efficient adsorption of vinpocetine by liposomes at low concentrations.
3. According to the results of performed investigations one can make a conclusion that incorporation of vinpocetine into liposomes by its addition to the ready-made colloid solution of liposomes is rather low-efficient.
References
- W. Brenner, F. Gschnait, A. Hautarzt, 29(7), 392-4 (1978).
- Y.A. Polkovnikova, A.A. Glushko, I.Y. Mikhailovskaya, Y.S. Karieva, (2017). Pharmacy & Pharmacology, 5(4), 344-367.
- A.W. Scholtz, A. Hahn, B Stefflova, D Medzhidieva, SV Ryazantsev, A Paschinin, N Kunelskaya, K Schumacher, G. Weisshaar, 39(11),1045-1056 (2019).
- S. Sethi, B. Mangla, S. Kamboj, V.A. Rana Int J Biol Macromol. 1(117), 350-36113 (2018).
- A.A. Shahba, A.R. Ahmed, F.K. Alanazi, K. Mohsin, S.I. Abdel-Rahman, AAPS PharmSciTech., 19(5), 2087-2102 (2018).
- H.O. Ammar, M. Ghorab, R. Kamel, A.H. Salama, Drug Deliv Transl Res. 6(3),195-209 (2016).
- G. Hou, J. Niu, F. Song, Z. Liu, S. Liu (2013), Studies on the interactions between ginsenosides and liposome by equilibrium dialysis combined with ultrahigh performance liquid chromatography-tandem mass spectrometry / Journal of Chromatography B. 923–924, 1–7.
- R. Dwiastuti, M. Radifar, S. Noegrohati, E.P. Istyastono, Indones. J. Chem. 16, 222 – 228 (2016).
- Yu. A. Polkovnikova, A. I. Slivkin, Pharmaceutical Chemistry Journal, 50(8), 553-555 (2016).
- Y.A. Polkovnikova, A.A. Glushko, Pharmacy & Pharmacology, 6(2), 197-210 (2018).11.


