Archive : Article / Volume 3, Issue 2
- RESEARCH ARTICLE | DOI:
- https://doi.org/10.58489/2836-2322/031
Synthesis and Antimicrobial Evaluation of Some Schiff Base Derivatives
1Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria.
2Department of Haematology and Blood Transfusion Science, Faculty of Basic Medical Science, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria.
3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Benin, Benin City, Nigeria.
Samuel J. Bunu*
Edebi N. Vaikosen, Samuel J. Bunu, Oyeintonbara Miediegha, Uchechi P. Chilaka, Chibuzor E. Echendu, Cyril O. Usifoh, (2024). Synthesis and Anti-Microbial Evaluation of Some Schiff Base Derivatives. Pharmacy and Drug Development. 3(2); DOI: 10.58489/2836-2322/031
© 2024 Samuel J. Bunu, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 29-04-2024
- Accepted Date: 30-04-2024
- Published Date: 30-04-2024
Schiff bases, Antibacterial, Antifungal, Minimum Inhibitory Concentration, Minimum Bactericidal Concentration.
Abstract
Schiff bases are the nitrogen analogs of ketones or aldehydes, with the carbonyl functional group replaced by an imine or azomethine group. They are formed by the condensation reaction of a primary amine and a carbonyl group, and they are widely employed in industry and have a variety of biological activities. Four Schiff base derivatives were successfully synthesized from benzaldehyde (PC1), anisaldehyde (PC2), 4-nitrobenzaldehyde (PC 2), and cinnamaldehyde (PC4) and tested for their antibacterial capabilities were evaluated against bacteria and fungi. The Schiff base had a high percentage yield of 98.28% (PC1), 95.7% (PC2), 91.6% (PC3), and 98% (PC4), with melting points ranging from 178 -187°C, 183-191°C, 160-164°C, and 195-205°C respectively. All Schiff bases showed activity against Escherichia coli with a Minimum Inhibitory Concentration (MIC) of 62.5ug/ml (PC1, PC4) and 250ug/ml (PC2, PC3). The Minimum Bactericidal Concentrations (MBC) were 125ug/ml, 500ug/ml, and 250ug/ml for PC1, PC2 and PC4 respectively. PC3 showed no bactericidal activity against Escherichia coli. Schiff bases PC1, PC2, and PC3 showed activity against Staphylococcus aureus with MIC of 62.5ug/ml. The MBC obtained was 125ug/ml for PC1 and PC2, while 125ug/ml, and 250ug/ml for PC3. PC4 showed no bacteriostatic and bactericidal activity against Staphylococcus aureus. Schiff bases showed fungistatic activity against Candida albican with MIC of 250ug/ml, 62.5ug/ml, and 125ug/ml for PC1, PC2/PC3, and PC4 respectively. However, none showed fungicidal activity.
Introduction
Schiff bases are one of the most widely used organic compounds, as pigments and dyes, catalysts, organic synthesis intermediates, and polymer stabilizers, as well as a broad range of biological activities (Dhar and Taploo, 1982; Rehman et al., 2004; Sinha et al., 2008; Bayrak et al., 2009; Alphonse, et al., 2016). Schiff bases are produced via the interaction of primary amines with an aldehyde or ketone under specific experimental conditions (Schiff, 1864; Shanty et al., 2017). Azeotropic distillation is Schiff's traditional synthetic procedure of Schiff bases that condenses a carbonyl molecule and primary amine (Guo et al., 2007). Molecular sieves are subsequently utilized to entirely remove the water from the system (Zheng et al., 2008). The efficacy of these approaches was determined by the use of highly electrophilic carbonyl groups and highly nucleophilic amines (Chakraborti et al. 2004). Schiff bases are classed as bidentate, tridantate, tetradantate, or polydentate ligands, and they can form highly stable complexes with transition metal ions. When functional groups like as -NH2, -OH, or -SH are present, the resultant Schiff bases can function as mixed-donor ligands, partaking in bi-, tri-, tetra-, and even higher combination modes. These complexes were evaluated for DNA nuclease, antibacterial, and anticancer activities, and they showed encouraging results in the identification of new chemotherapeutic drugs (Varghese and Nair, 2010).
A variety of strategies have been utilized in the synthesis of Schiff base derivatives. The grindstone-green method involves the reaction of benzaldehyde derivatives and para-amino phenol in methanol and glacial acid solution, microwaved for 5 minutes and allowed to cool. The resultant combination is poured into cold water, and the solid product generated is filtered, refined by recrystallization from methanol, washed with methanol, and dried (Dayma et al., 2018). The classic method involves condensing benzaldehyde derivatives and para-aminophenol in methanol and acetic acid medium for four hours while stirring at room temperature. TLC is used to monitor the development of the reaction (Ebeshi et al., 2022; Bunu et al., 2023a, 2023b, 2024). Following completion, the product is filtered, dried, and recrystallized in methanol (Vellaiswamy et al., 2014). There are also ambient temperature and microwave options. The Microwave Method entails reacting benzaldehyde derivatives and para-aminophenol in methanol with certain drops of acetic acid in appropriate reaction media, then irradiating the mixture in a microwave at a power of 20% intensity (140 W) for 2 to 5 minutes. The resulting solution is allowed to cool before being poured into cold water. The separated solid is then filtered and recrystallized in ethanol (Yousif et al., 2013).
Schiff bases have been shown to have antibacterial, antifungal, and anticancer properties (Baluja et al., 2009; Varghese & Nair, 2010; Prakash & Adhikari, 2011). Schiff bases are effective antibacterial agents and metal complexation has been shown to improve their biological activity, with bacteria behaving differently. The antibacterial potentials of heterocyclic Schiff base derivatives, prepared by reacting aldehyde derivatives with para-aminophenol in hot methanol were tested to be promising (Shanty et al., 2017). The advent of microorganisms' resistance, particularly bacteria, to current antimicrobial drugs, has caused a renaissance in the quest for powerful antimicrobial agents (Awala et al., 2019; Bunu et al., 2020; Bunu et al., 2020a). It was reported that Schiff base derivatives with an electron-donating methoxy substituent had higher microbial inhibitory activity than compounds with an electron-withdrawing chloro- or nitro group (Aslam et al., 2016; Tolulope et al., 2017). The purpose of this study was to synthesize, physiochemically evaluate, and test the antibacterial characteristics of Schiff base formed from the reaction of benzaldehyde derivatives and para-aminophenol with S. aureus, E. coli, and C. albican clinical isolates.
Materials And Methods
Reagents and Equipment
All chemicals used were of analytical grade; para-aminophenol, benzaldehyde, 4-nitrobenzaldehyde, cinnamaldehyde, and anisaldehyde, a solvent such as Dimethyl lsulfoxide (DMSO), nutrient broth (200 g), Nutrient agar, muller Hinton agar (500 g), Macfarland standard, normal saline, methanol (MeOH), ethanol (EtOH), TLC plate, Autoclave. The antimicrobial screening was conducted with S. aureus, E. coli, and C. albican; obtained from the laboratory stock, and stored in the incubator.
Experimental Procedure: Microwave Method
An equivalent amount of 0.9 g of para-aminophenol was weighed, and 0.9 ml of benzaldehyde was determined using a measuring cylinder before being transferred to a beaker and dissolved in 25 ml methanol. Three drops of glacial acetic acid were added, microwaved for five minutes, and then allowed to cool. The resultant mixture was poured into a glass of cold water, and the solid product produced was filtered, recrystallized, washed with methanol, and dried. This resulted in PC1. The procedure was repeated with 0.9 ml of anisaldehyde (PC2), 1.1g of 4-nitrobenzaldehyde (PC3), and 0.9 ml cinnamaldehyde (PC4), respectively. Each of the synthesized Schiff bases was dissolved in hot methanol (25 ml) and stirred. As the solution got cool, crystals were formed, which were collected by vacuum filtration, then washed with methanol and allowed to dry.
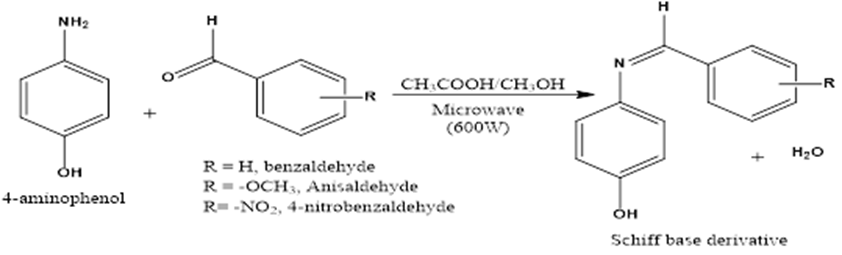
Figure 1: General Reaction Scheme in the synthesis of Schiff derivatives (Thaker & Barvalia, 2011).
Physicochemical Analysis: Solubility Test and Melting Point Determination
About 10 mg of each synthesized Schiff base was weighed and dissolved in 5 ml of methanol, acetone, DMSO, ethanol, and water in test tubes, then gently swirled with a glass stirring rod. Observations were made and recorded appropriately. The melting point of the Schiff base (solid) was evaluated by inserting a tiny amount of the sample into the capillary tube, which was tapped into the base of the capillary tube. The thermometer was inserted into the melting point equipment, which was turned on. As the Schiff base sample was heated, the melting process was attentively watched through the viewfinder (magnifying glass).
Physicochemical Analysis: Thin Layer Chromatography
The plates for TLC were cut precisely, with an origin at 0.8cm and a solvent front 9cm away from the origin. A saturated chamber with a solvent system consisting of 5ml n-hexane and 5ml ethyl acetate in a 1:1 ratio was designed. The plate was then inserted into the chamber. After 15 minutes, the chromatoplates were taken out as the solvent reached the marked front. The plate was left to dry before being examined under an iodine tank, and any visible spots were documented with a pointed pencil. The retardation factor was determined.
Antimicrobial Screening: Preparation of bacteria culture media
About 1.6g of Mac Conkey agar was dissolved in 45ml of distilled water respectively. The resultant mixture was autoclaved for 15 minutes at a temperature of 121°c. 15ml of the mixture was poured into three different Petri dishes, allowed to cool and set, then kept in the microwave to dry for 15min at a temperature of 45-60 °c. This procedure was repeated for 2.9g of sabouraud dextrose agar and 1.6g of cled agar respectively. The microorganisms were isolated aseptically from an agar plate and streaked on a sterile mac-Conkey agar plate (culture media) (Cooper, 1963).
Gram – Staining Technique
A loopful of bacteria was smeared over a sterile slide, followed by normal saline. The slide was cross-flamed three times, dried, then saturated with crystal violet staining reagent, and rinsed for two seconds. Mordant was added, waited 1 minute, and then rinsed for 2 seconds with a mild and indirect stream of tap water. After adding drops of acetone, wait 15 seconds until the decolorizing agent traveling through the slide was clear. Safranin drops were flooded onto the slide, left for 30 seconds to 1 minute, and then rinsed with a mild and indirect stream of tap water until no color emerged. It was allowed to dry and examined under oil immersion with a microscope.
Susceptibility Testing for Microorganisms with Agar Diffusion Method
A standardized bacteria suspension from an overnight incubation of a test organism was placed into sterilized Muller Hinton Agar plates (the medium had already been prepared). The microbial solution was poured twice over the entire surface to ensure uniform dispersion and confluent development, and any excess fluid was placed into a beaker containing sodium HCl. The inoculum on the Muller Hinton agar plates was left to set. A sterile corn borer is then inserted into the agar to create four holes in each agar plate, and the agar plugs are retrieved using a sterile loop and disposed of in a strong disinfection solution. To close each hole, use a sterile pipette made by Pasteur and two droplets of molten agar. The cups (holes) were then filled with various concentrations (500ug/ml, 250ug/ml, 125ug/ml, and 62.5ug/ml from a stock solution with a concentration of 1000ug/ml) and left to stand for 1 hour for sufficient pre-diffusion of the Schiff bases sample. This approach was utilized to test each Schiff base sample PC1, PC2, PC3, and PC4 against each of the test organisms (E. coli, S. aureus, and C. albican) individually and incubated at 37℃ for 24 hours. The zones of inhibition were measured to the nearest millimeter (mm) and documented.
Results
Percentage yield, Melting point, and Solubility profile
Table 1: Percentage yield, melting point, and Solubility profile of the synthesized Schiff bases.
S/N | SchiffBase | PercentageYield (%) | Melting Point(℃) | Solubility | |||
| DMSO | EtOH | Acetone | Distilled Water | ||||
PC1 | 98.28 | 178 -187 | soluble | insoluble | Partially soluble | insoluble | |
| PC2 | 95.7 | 183 -191 | soluble | insoluble | Partially soluble | insoluble | |
| PC3 | 91.6 | 160 -164 | soluble | insoluble | Partially soluble | insoluble | |
| PC4 | 98.0 | 195 - 205 | soluble | insoluble | Partially soluble | insoluble | |
KEY: Percentage Yield = actual yield/theoretical yield *100%. All the synthesized Schiff base compounds obtained had an excellent yield which means that a lot of the reactant chemicals used, successfully reacted to form the product. DMSO = Dimethyl sulfoxide, EtOH -ethanol.
Thin Layer Chromatography
Table 2. TLC Rf-value for synthesized Schiff base compounds
| Schiff base samples | Solvent System (n-Hex: ethyl acetate) | Spot | Rf- Value |
| Benzaldehyde in MeOH | 1:1 | 1 | 0.84 |
| Anisaldehyde in MeOH | 1:1 | 1 | 0.89 |
| 4-Nitrobenzaldehyde in MeOH | 1:1 | 1 | 0.50 |
| Cinnamaldehyde in MeOH | 1:1 | 1 | 0.89 |
| PAP in MeOH | 1:1 | 1 | 0.57 |
| PC1 in DMSO | 1:1 | 1 | 0.86 |
| PC2 in DMSO | 1:1 | 1 | 0.81 |
| PC3 in DMSO. | 1:1 | 1 | 0.70 |
| PC4 in DMSO | 1:1 | 1 | 0.81 |
KEY: Retention factor (Rf-value) = Distance moved by solute / Distance moved by solvent (9cm). A paper chromatogram is used to distinguish between pure and impure substances. From the result above, the various synthesized Schiff base samples showed a single spot which implies that the samples are pure. Compounds with high Rf values are less polar compounds, whereas those with low Rf values are said to be more polar. MeOH – methanol.
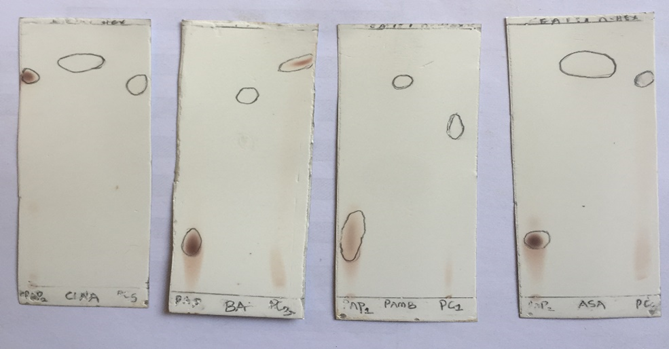
Figure 2: TLC spots for the different synthesized Schiff bases, para-aminophenol, and the derivatives of benzaldehyde. PAP=para-aminophenol, CINA= cinnamaldehyde, 4NB= 4-Nitrobenzaldehyde, BA= benzaldehyde, ANI= anisaldehyde, PC = PAP + Each derivative of benzaldehyde respectively (Schiff base).
Table 4: Antimicrobial activity of Schiff base derivatives
| Samples ID | Conc.(ug/ml) | Zone of Inhibition (mm). | ||
| E. coli | S. aureus | C. albican | ||
| PC1 | 500 | 12 | 13 | 9 |
| 250 | 11 | 13 | 9 | |
| 125 | 9 | 10 | R | |
| 62.5 | 7 | 8 | R | |
| PC2 | 500 | 10 | 10 | 7 |
| 250 | 8 | 10 | 7 | |
| 125 | R | 9 | 7 | |
| 62.5 | R | 8 | 7 | |
| PC3 | 500 | 7 | 15 | 8 |
| 250 | 7 | 14 | 8 | |
| 125 | R | 7 | 8 | |
| 62.5 | R | 7 | R | |
| PC4 | 500 | R | R | 13 |
| 250 | 9 | R | 11 | |
| 125 | 8 | R | 10 | |
| 62.5 | 7 | R | 8 | |
| Positive Control (DMSO water and organism) | - | S | S | S |
| Negative Control (DMSO water only) | - | R | R | R |
Key: mm=millimeter, S =Susceptible, R= Resistance.
Table 5: The MIC and MBC of Schiff derivatives on test clinical isolates
| Samples ID | Micro-organism (clinical isolates) | MIC (µg/ml) | MBC (µg/ml) |
| PC1 | E. coli | 62.5 | 125 |
| S. aureus | 62.5 | 125 | |
| C. albican | 250 | - | |
| PC2 | E. coli | 250 | 500 |
| S. aureus | 62.5 | 125 | |
| C. albican | 62.5 | - | |
| PC3 | E. coli | 250 | - |
| S. aureua | 62.5 | 250 | |
| C. albican | 125 | - | |
| PC4 | E. coli | 62.5 | 250 |
| S. aureus | - | - | |
| C. albican | 62.5 | - |
Key: MIC - Minimum inhibitory concentration, MBC - Minimum Bactericidal concentration.
Proposed Schiff base formation Reaction Mechanism
A proton is abstracted from the acetic acid catalyst, leaving the acetate ion. One the double bond between carbonyl carbon and oxygen opens up to form the benzaldehyde carbocation, which is a rich site for a nucleophilic attack by the amino group of the 4-aminophenol. The quaternary nitrogen compound formed rearranges to eliminate water from a quasi-Schiff base. This is followed by the abstraction of a proton by the acetate ion to form the Schiff base.
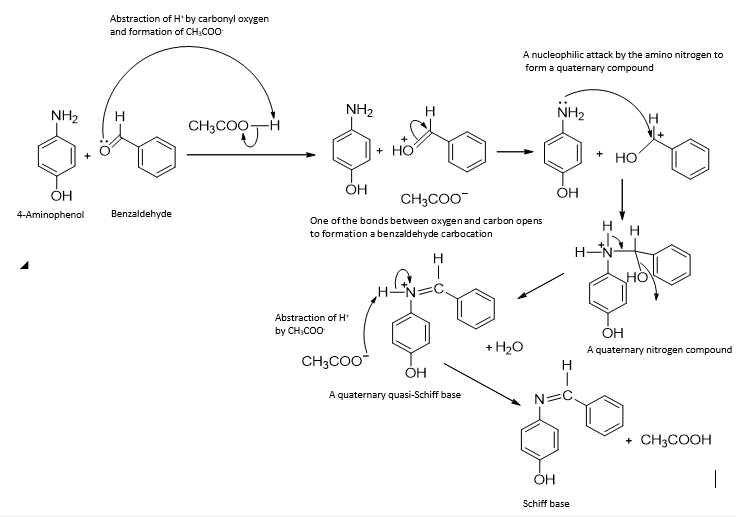
Figure 3. The proposed reaction mechanism for the acid-catalyzed synthesis of Schiff base from 4-aminophenol and benzaldehyde
Discussion
Four Schiff base derivatives were successfully synthesized from the reaction of para-aminophenol and benzaldehyde with its derivatives (PC1, PC2, PC3, and PC4) had a very high percentage yield of 98.28%, 95.7%, 91.6%, and 98% respectively, indicating that a lot of the reactant chemicals used, successfully reacted to form the Schiff bases compounds. The melting point range of Schiff base PC1, PC2, PC3 and PC4 are 178 -187°C, 183-191°C, 160-164°C, and 195-205°C respectively. The result obtained from TLC using a solvent system of 5ml n-hexane and 5ml ethyl acetate in a ratio of 1:1 showed a single spot for each Schiff base sample used respectively, which implies that the samples are pure. The compounds with high Rf-value are less polar, whereas those with low Rf-value are said to be more polar. The antimicrobial susceptibility test carried out using the Schiff base (PC1) showed activity against E. coli at zones of inhibition of 7mm-12mm, with a Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of 62.5ug/ml and 125ug/ml respectively. It also showed activity against S. aureus at zones of inhibition of 8-13mm with MIC and MBC of 62.5ug/ml and 125ug/ml respectively. When used against C. albican, a zone of inhibition of 9mm with MIC of 250 ug/ml was obtained, and it showed no Minimum Fungicidal Concentration (MFC).
Schiff base (PC2) showed activity against E. coli at zones of inhibition of 8-10mm with MIC and MBC of 250ug/ml and 500ug/ml respectively. It also showed activity against S. aureus at zones of inhibition of 8-10mm with MIC and MBC of 62.5ug/ml and 125ug/ml respectively. When used against C. albican, a zone of inhibition of 7mm with MIC of 62.5ug/ml was obtained. It showed no Minimum Fungicidal Concentration (MFC).
Schiff base (PC3) showed activity against E. coli at the zone of inhibition of 7 mm, MIC of 250ug/ml with no MBC. It also showed activity against S. aureus at zones of inhibition of 7-15mm with MIC and MBC of 62.5ug/ml and 250ug/ml respectively. When used against C. albican, a zone of inhibition of 8mm with MIC of 125ug/ml was obtained. It showed no MFC. Schiff base (PC4) showed activity against E. coli at zones of inhibition of 7 - 9 mm with MIC and MBC of 62.5ug/ml and 250ug/ml respectively. It showed no action against S. aureus. When employed against C. albican, zones of inhibition ranging from 8 to 13mm were found, with a MIC of 62.5ug/ml and no measurable MFC. These findings are consistent with Yadav et al. (2019) study on the antibacterial activity of benzaldehyde derivatives of Schiff base, in which all of the tested compounds showed increased antibacterial activity against bacterial strains but no fungicidal activity against the tested fungi. Wang et al. (2018) discovered that various cinnamaldehyde Schiff bases have potent antibacterial properties. According to Hassan et al.'s (2018) examination of the antimicrobial activity of benzaldehyde Schiff base derivatives, antimicrobial activity increases with Schiff base concentration. With more in-silico and molecular studies, these chemicals can serve as potential intermediates in ant-infective drug discovery (Bunu et al., 2023; Bunu et al., 2024). The microbiological studies discovered that all of the highly active compounds showed good selectivity in their action against bacterial strains as compared to fungal test strains, and this selectivity appears to be related to the existence of particular chemical properties.
Conclusion
The Schiff base derivatives demonstrated bacteriostatic and bactericidal action against two (2) test pathogens, E. coli, and S. aureus, except for Schiff base PC4, which did not affect S. aureus. This provides some evidence that Schiff base derivatives can be utilized to treat disorders or infections caused by E. Coli and S. aureus. All four (4) test substances suppressed the growth of the fungus (C. albican) but had no fungicidal effect. The susceptibility test results also pave the path for future research in drug design, discovery, and development, in addition to other conventional antibacterial and antifungal agents.
Acknowledgment
The authors sincerely acknowledge The Tertiary Education Trust Fund (TETFund) for the financial support provided in conducting this research. We also appreciate the staff of the Department of Pharmaceutical and Medicinal Chemistry, Niger Delta University, and the Department of Pharmaceutical Chemistry, University of Benin, Nigeria.
References
- Alphonse, R., Varghese, A., & George, L. (2016). Synthesis, characterization and photophysical studies of a novel schiff base bearing 1, 2, 4-Triazole scaffold. Journal of Molecular Structure, 1113, 60-69.
- Aslam, M., Anis, I., Mehmood, R., Iqbal, L., Iqbal, S., Khan, I., ... & Perveen, S. (2016). Synthesis and biological activities of 2-aminophenol-based Schiff bases and their structure-activity relationship. Medicinal Chemistry Research, 25, 109-115.
- Awala EV, Bunu JS, Haruna B, & Oluwadiya JO, (2019). Synthesis, antimicrobial, and anti-inflammatory evaluation of epoxide and 4-methoxy- and 4,6-diphenyl-2-thiopyrimidine derivatives of chalcones. Scholars Academic Journal of Pharmacy. 8(8): 436-442.
- Baluja, S., Solanki, A., & Kachhadia, N. (2006). Evaluation of biological activities of some Schiff bases and metal complexes. Journal of the Iranian Chemical Society, 3, 312-317.
- Bayrak, H., Demirbas, A., Karaoglu, S. A., & Demirbas, N. (2009). Synthesis of some new 1, 2, 4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. European journal of medicinal chemistry, 44(3), 1057-1066.
- Bunu JS, Alfred-Ugbenbo D, Miediegha O, & Baba H (2023). Characterization and Molecular Docking of Cinnamic acid derivatives: potential inhibitors of cyclo-oxygenase enzymes. Innovare Journal of Life Sciences, 11 (2023); 41-46.
- Bunu J.S., Ere D., & Wilson O. D., (2020). Simple thin-layer chromatographic and UV-spectrophotometric analysis of Promethazine and its N-demethylation metabolites from biological fluids. International Journal of PharmTech Research. 13 (4): 316-324.
- Bunu, J. S., Victoria, A. E., & Deboh, E. D. (2020). Preparation and Antifungal Properties of Chalcone and Halogenated Derivatives. Saudi J Med Pharm Sci, 6(4), 379-389.
- Bunu JS, Aniako V, Karade VP, Vaikosen EN, & Ebeshi B.U., (2023a), Thin-Layer Chromatographic and UV-Spectrophotometric Analysis of Frequently Utilized Oral Macrolide Antibiotics, International Journal in Pharmaceutical Sciences, 1(9), 265-274.
- Bunu J.S., Kela−Eke S., & Ebeshi B. U., (2024). Titrimetric and thin layer chromatographic fingerprint analysis of captopril solid dosage form – an Angiotensin-Converting Enzyme Inhibitor. Drug Discovery, 18(41); 1-7.
- Bunu J.S., Okei O.J., Miediegha O., Ebeshi B.U., & Chukwuemerie O.L., (2023b). Assessment of Secondary Metabolites and Thin-Layer Chromatographic Analysis of Carica papaya (Caricaceae) Leaves Ethanolic Extract. Journal of Pharmaceutical Research International, 35(36); 21-28.
- Jacob, B. S., Baba, H., & Oluwadiya, J. O. (2020). Synthesis, Characterization, and evaluation of Anti-inflammatory and antimicrobial properties of some cinnamic acid derivatives. Nigerian Journal of Pharmaceutical Research, 16(1), 1-8.
- Vaikosen, E. N., Bunu, J. S., Rafiu, R. O., & Eze, C. V. (2024). UV-Spectroscopic Analysis and In vitro Equivalence Study of Generic Paracetamol Tablets under Biowaiver Conditions. Chemistry Research Journal, 9(2), 50-61.
- Chakraborti, A. K., Bhagat, S., & Rudrawar, S. (2004). Magnesium perchlorate as an efficient catalyst for the synthesis of imines and phenylhydrazones. Tetrahedron letters, 45(41), 7641-7644.
- Cooper, K. E. (1963). The theory of antibiotic inhibition zones. In Analytical microbiology (pp. 1-86). Academic press.
- Dayma, V., Sharma, P., Salvi, P., Rathore, M. K., & Baroliya, P. K. (2018). Comparative study of Schiff base using various synthesis methods and their theoretical prediction of activities. International Journal of Research in Advent Technology, 6(8).
- Dhar, D. N., & Taploo, C. L. (1982). Schiff bases and their applications. J Sci Ind Res, 41(8), 501-506.
- Ebeshi UB, Bunu JS, Vaikosen NE, Kashimawo JA, Kpun HF, Okpareke D (2022). Physicochemical Analysis and Thin-Layer Chromatographic Fingerprinting of Some Beta-Lactam Antibiotics. International Journal of Pharmaceutical Research and Applications, 7 (3), 961-967.
- Guo, Z., Xing, R., Liu, S., Zhong, Z., Ji, X., Wang, L., & Li, P. (2007). Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydrate research, 342(10), 1329-1332.
- Hassan, M. A., Omer, A. M., Abbas, E., Baset, W. M., & Tamer, T. M. (2018). Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Scientific reports, 8(1), 11416.
- Prakash, A., & Adhikari, D. (2011). Application of Schiff bases and their metal complexes-A Review. Int. J. Chem. Tech. Res, 3(4), 1891-1896.
- Rehman, W., Baloch, M. K., Muhammad, B., Badshah, A., & Khan, K. M. (2004). Characteristic spectral studies and in vitro antifungal activity of some Schiff bases and their organotin (IV) complexes. Chinese Science Bulletin, 49, 119-122.
- Schiff, H. (1864). Mittheilungen aus dem Universitätslaboratorium in Pisa: eine neue Reihe organischer Basen. Justus Liebigs Annalen der Chemie, 131(1), 118-119.
- Shanty, A. A., Philip, J. E., Sneha, E. J., Kurup, M. R. P., Balachandran, S., & Mohanan, P. V. (2017). Synthesis, characterization and biological studies of Schiff bases derived from heterocyclic moiety. Bioorganic chemistry, 70, 67-73.
- Sinha, D., Tiwari, A. K., Singh, S., Shukla, G., Mishra, P., Chandra, H., & Mishra, A. K. (2008). Synthesis, characterization, and biological activity of Schiff base analogues of indole-3-carboxaldehyde. European journal of medicinal chemistry, 43(1), 160-165.
- Thaker, B. T., & Barvalia, R. S. (2011). Microwave-assisted synthesis and characterization of unsymmetrical tetradentate Schiff base complexes of VO (IV) and MoO (V). Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 84(1), 51-61.
- Fasina, T. M., Ejiah, F., Oloba-Whenu, O. A., Revaprasadu, N., & Familoni, O. B. (2017). Synthesis, characterization and structure activity relationship of schiff bases derived from 2-Aminophenol and substituted benzaldehydes.
- Dhavale, N., Patil, C. S., Tadke, V., & Vhankate, S. (2019). Synthesis, Characterization and Antibacterial Activity of Mixed Cobalt-Transition Metals Compounds. Journal of Current Pharma Research, 10(1), 3594-3604.
- Vellaiswamy, G. O. M. A. T. H. I., & Ramaswamy, S. E. L. V. A. M. E. E. N. A. (2014). Synthesis, spectral characterization and antimicrobial screening of novel Schiff bases from sulfa drugs. International Journal of Pharmacy and Pharmaceutical Sciences, 6(1), 487-91.
- Wang, H., Jiang, M., Sun, F., Li, S., Hse, C. Y., & Jin, C. (2018). Screening, synthesis, and QSAR research on cinnamaldehyde-amino acid Schiff base compounds as antibacterial agents. Molecules, 23(11), 3027.
- Yadav, P., Sarkar, A., & Kumar, A. (2019). Synthesis and biological activities of schiff bases and their derivatives: a review of recent work. Journal of Basic and Applied Engineering Research, 6(1), 62-65.
- Yousif, E., Majeed, A., Al-Sammarrae, K., Salih, N., Salimon, J., & Abdullah, B. (2017). Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arabian Journal of Chemistry, 10, S1639-S1644.
- Zheng, Y., Ma, K., Li, H., Li, J., He, J., Sun, X., ... & Ma, J. (2009). One pot synthesis of imines from aromatic nitro compounds with a novel Ni/SiO 2 magnetic catalyst. Catalysis letters, 128, 465-474.


