Current Issue : Article / Volume 3, Issue 1
- Case Report | DOI:
- https://doi.org/10.58489/2836-497X/019
Twin reverse arterial perfusion sequence
- pole De Maternite De Lâhoptital Mere Enfant Harouchi, Morocco
Laaliaoui Aymen
Laaliaoui Aymen, Zine Elabbidine Agoug, Jallal Mohamed, Lemrissi Amine, Bouhya Said. (2024). Twin reverse arterial perfusion sequence. Archives of Gynaecology and Women Health. 3(1); DOI: 10.58489/2836-497X/019
© 2024 Laaliaoui Aymen, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 17-12-2023
- Accepted Date: 01-03-2024
- Published Date: 12-03-2024
Abstract
Twin Reverse Arterial Perfusion Sequence (TRAP) is a rare condition specific to identical twins. Overall, TRAP affects around 1% of all monozygotic twins, with 75% occurring in monochorionic-biamniotic twins and the remaining 25% in monoamniotic twins. TRAP is characterized by a normal-appearing pump twin supporting a dysmorphic acardiac twin through aberrant arterio-arterial anastomoses in the shared single placenta.
Introduction
Twin Reverse Arterial Perfusion Sequence (TRAP) is a rare condition specific to identical twins. Overall, TRAP affects around 1% of all monozygotic twins, with 75% occurring in monochorionic-biamniotic twins and the remaining 25% in monoamniotic twins. TRAP is characterized by a normal-appearing pump twin supporting a dysmorphic acardiac twin through aberrant arterio-arterial anastomoses in the shared single placenta.
Physiopathologie
Pathophysiologically, it is due to the development of arterio-arterial vascular anastomoses between the umbilical arteries of monozygotic twins early in embryogenesis that cause this particular condition [1]. In the TRAP sequence, blood enters the acardiac twin via the umbilical artery and leaves the acardiac twin via the umbilical vein. The directions of blood flow are totally opposite to normal physiological placenta-fetal circulation. This is why we call it the twin reverse arterial perfusion sequence (TRAP). Because one twin lacks a well-formed cardiac structure, it is like a parasite and totally dependent on the other twin for the complete blood supply. And the acardiac twin loses the direct vascular connection with the placental villi.
Without early detection, close follow-up and timely intervention, mortality rates for donor twins reach 50-70% [2].
Case report
A 32-year-old woman IIIG/IP consulted our institution for premature rupture of membranes in a twin pregnancy estimated at 37 weeks of amenorrhea.
This parturient had a history of spontaneous miscarriage at 02 months in an undetermined context.
Her current pregnancy was poorly monitored, with a single prenatal consultation in a public health facility at 10 weeks' amenorrhea, after which the patient was lost to follow-up.
She subsequently presented to us with premature rupture of the membranes after 06 hours of admission, with the following clinical findings: conscious parturient, normal-coloured conjunctiva, no apparent physical abnormalities, height: 1.65 cm, weight: 70 kg, blood pressure arterial blood pressure was 12/07 mm hg, maternal pulse 87 beats per minute temperature 36.3 c
A urine dipstick was performed with no abnormalities.
Examination under speculum revealed a closed cervix, water broken 6 hours previously, with clear, odourless amniotic fluid.
Examination of the pelvis and perineum was without anomaly, uterus relaxed, no bleeding or uterine contractions
Examination of the fetal heart rate revealed a single focus with a basic rhythm of around 130 beats per minute.
Obstetrical ultrasound: twin pregnancy with cardiac activity present for the first twin, who was in cephalic presentation with a biparietal diameter of 9.39 cm and femoral length of 6.30 cm.
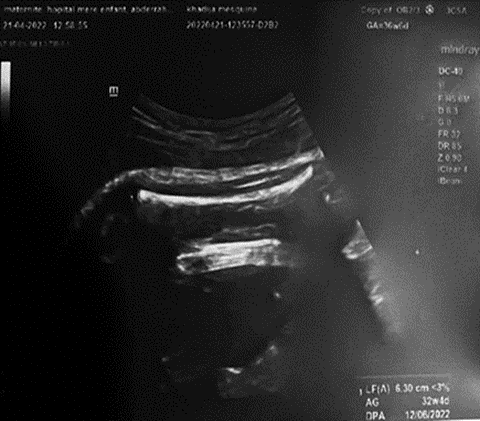
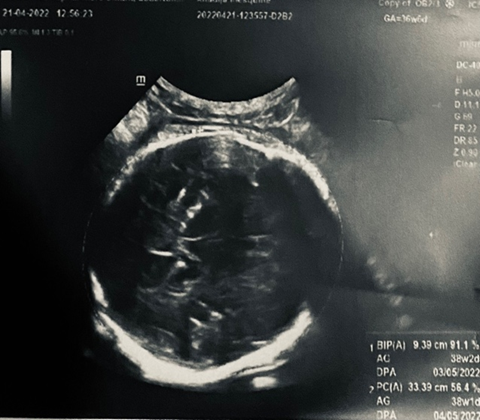


A biologic infectious workup was performed for premature rupture of membranes, which was negative.
The obstetrical staff decided to induce labor.
Delivery was uneventful, with episiotomy of the first cephalic male twin with a birth weight of 2350g apgar 10/10.
The second twin was a 700g polymalformed MFIU of indeterminate sex (TRAP).
Monochorionic monoamniotic placenta
Discussion
The TRAP sequence classification has undergone changes over time. Thus, initially, the malformed fetus
malformed fetus (the "perfused" twin) was considered acardiac. Initially, two groups of acardiac acardiac gemellarity groups: hemicardium and holocardius classification proposed by Malone & D'Alton [12].
The acardiac twin, or TRAP (twin-reversed arterial perfusion) sequence, or acardius acranius or acardiac mass remains a severe pathology of monochorionic twin pregnancies [1,2]. In fact, it is a major and rare form of transfused-transfused syndrome [3-6], manifested by the absence of development of cardiac structures, associated with a spectrum of developmental and reductional malformations in the fetus known as acardiac mass [1
The presence of an acardiac twin is exceptional, affecting one in 35,000 births [5,6] and 1% of monozygotic pregnancies [1].
This entity represents the second most common complication of vascular anastomoses in monochorionic placentas, after transfused-transfused syndrome [5]. The pathogenesis of this rare complication is controversial [2].
some authors believe that the average premium is primary cardiac dysmorphogenesis, and that placental vascular anastomoses are only necessary for the development of the acardiac fetus. Others believe that the anomaly in question is the presence of reversed vascular flow, which is secondarily responsible for cardiac atrophy. Indeed, the most favoured theory at present is based on the association of two obligatory conditions [7,8]: early onset of circulatory insufficiency (between 8 and 12 SA) in the future acardiac fetus, and the presence of placental veno-venous and arterio-arterial anastomoses [1,2].
The future acardiac can be anatomically normal or abnormal. It resembles a true parasite [7] which is perfused from the pump twin by the umbilical arteries in a retrograde flow [3] via arterio-arterial and venovenous chorionic plate anastomoses (Figure 3) [3]. This oxygen-poor blood leads to poor development of the head, heart and lower limbs [7], hence the name twinreversed arterial perfusion sequence (TRAP) [1,5]. This acardiac fetus is never viable, and complications concerning the pump fetus are frequent: prematurity, hydramnios, hydrops fetoplacental, heart failure (50% of cases) and in utero death, which occurs in 50% to 70% of cases [5].
On ultrasound, it appears as a teratomatous, edematous mass with disorganized anatomical elements, echogenic zones (disorganized bony structures) and anechogenic zones of a liquid nature (occluded digestive segments). The umbilical cord is short and very often contains a single artery [3]. It is only the visualization of an inverted pulsatile flow of the umbilical artery of the acardiac fetus with color Doppler that will confirm the diagnosis [1]. A careful search for associated structural malformations is indicated in cases of TRAP. Umbilical cord anomalies are quite common in both twins, with a single umbilical artery present in up to two-thirds of cases. In addition to serial ultrasound and color Doppler studies to establish the diagnosis of TRAP,Fetal DNA analysis and fetal echocardiography are also recommended Up to 10% of TRAPs will have associated chromosomal abnormalities and 5-10% will have other structural defects, congenital heart disease being the most common. Fetal echocardiography is also useful for assessing the pump twin's cardiac function and the need for intervention. Fetal MRI can be useful if associated anomalies are suspected, particularly those involving the pump twin's brain.
TRAP is often overlooked as a possible diagnosis when it is noted that a twin of an identical pair has no visible cardiac activity early in gestation. However, rather than the early death of a single twin, it becomes clear on subsequent imaging that both twins continue to grow despite the absence of cardiac activity in one of the twins.
Color flow mapping can help to distinguish TRAP from the death of a single twin, as there will be absent flow in the setting of intrauterine fetal death compared with demon-trable flow in an acardiac twin.
In very rare circumstances, a placental mass such as a teratoma may have sonographic features that resemble an amorphous acardiac twin. Both will have a perceptible flow on color Doppler imaging, but only the acardiac twin will have an umbilical cord in addition other differential diagnoses to evoke are fetal death in utero of a normal twin, anencephalic
or associated with a large cystic hygroma; placental or intra-amniotic tumour
Several types of prenatal treatment are available. Some are etiological, aimed at interrupting the circulation of the acardiac fetus, either by laser treatment of the anastomoses between the two twins, or by mono- or bipolar coagulation of the acardiac twin's umbilical cord, or by embolizing the cord with thrombogenic substances such as alcohol [5], or by percutaneous vascular destruction using radiofrequency [3, 6].
Symptomatic treatments such as indomethacin (due to its tocolytic effect and the reduction in amniotic fluid), amniodrainage in the event of hydramnios, or digitization may be used [5], Some authors have proposed a conservative attitude when the ratio of umbilical artery PIs of the two twins is less than 1.3 or the difference in resistance indexes is greater than 0.20 [5], while others are guided essentially by the ratio of abdominal circumference of the acardiac fetus to the pump fetus (Figure 4) [1, 5]. However, symptomatic treatment is generally indicated in cases of major hydramnios or cardiac failure of the pump twin [3].
Conclusion
Perinatal mortality in twin pumps is 35-50%, but early diagnosis of acardiac twins is essential for optimal management. We therefore draw practitioners' attention to the enormous value of early prenatal diagnosis, which can be enhanced by regular ultrasound follow-up, adjusting the course of action according to prognostic factors between simple follow-up and prenatal intervention. prognosis of the double pump and avoid accidental discovery of this entity during labor, as we have observed.


