Current Issue : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/.
Clarion and Protuberant-Papillary Thyroid Carcinoma
Histopathologist, AB Diagnostics, India.
Correspondng Author: Bajaj A
Citation: Bajaj A (2022). Clarion and Protuberant-Papillary Thyroid Carcinoma. Journal of Cancer Research and Cell Development. 1(1). DOI: 10.58489/JCRCD.001
Copyright: © 2022 Bajaj A, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-09-08, Received Date: 2022-09-08, Published Date: 2022-10-31
Abstract Keywords: papillary carcinoma, tall cell, micro-carcinoma, follicular, oxyphilic, diffuse sclerosing, cribriform morular, solid or insular variant.
Abstract
Papillary thyroid carcinoma is a malignant thyroid neoplasm exhibiting histological subtypes as classic papillary carcinoma, tall cell, micro-carcinoma, follicular, oxyphilic, diffuse sclerosing, cribriform morular, solid or insular variant. Tumefaction enunciates distinctive nuclear features as altered nuclear magnitude and outline, nuclear enlargement or elongation, nuclear overlapping and ground glass nuclei. Genomic fusions of BRAF, RET, PPARG, NTRK1, NTRK3, ALK, LTK, MET, FGFR2 or THADA genes may be observed. Initial disease representation emerges as 67% singular thyroid nodules, 13% thyroid nodules combined with cervical lymph node metastasis and 20% neoplasms confined singularly to lymph nodes.
Introduction
Papillary thyroid carcinoma is a frequently denominated malignancy of thyroid gland with common histological subtypes as classic, micro-carcinoma and follicular variant. Neoplasm demonstrates BRAFV600E genetic mutation, especially encountered with classic and tall cell variant. Papillary carcinoma enunciates distinctive nuclear features as altered nuclear magnitude and outline, nuclear enlargement or elongation, nuclear overlapping and ground glass nuclei. Typically, nuclear chromatin exhibits clearing and margination. Besides, irregular nuclear membrane, non-uniform nuclear contour, nuclear grooves and nuclear pseudo-inclusions are characteristically encountered [1,2].
Contingent to amalgamation of manifestations such as neoplastic architecture, pattern of tumour evolution, cytological features, tumour magnitude and encapsulation, World Health Organization designates papillary thyroid carcinoma into distinct variants as prototypic classic papillary carcinoma, tall cell, micro-carcinoma, follicular, oxyphilic, diffuse sclerosing, cribriform morular, solid, insular and certain exceptionally discerned variants [1,2].
Papillary carcinoma exemplifies a female predilection with female to male proportion of ~3:1. Tumefaction may appear as occult, primarily discerned upon autopsy or multi-centric with distinct regional lymph node metastases. Occult neoplasms may be associated with diverse thyroid disorders and exhibit a male predominance [1,2].
Papillary carcinoma predominantly implicates thyroid gland although tumefaction may emerge within sites as thyroglossal duct, lingual thyroid or ectopic thyroid tissue as encountered with struma ovaria [1,2].
Factors contributing to occurrence of papillary carcinoma appear as exposure to ionizing radiation > 20 years for maladies such as acne, tonsillitis, tinea capitis and enlarged thymus or following exposure to nuclear explosions as associated with Chernobyl or Marshall Islands. Conditions such as Hashimoto’s thyroiditis or familial adenomatous polyposis may be associated with papillary carcinoma, especially cribriform morular variant. Familial or sporadic neoplasms demonstrate identical prognostic outcomes [1,2].
Commonly, BRAFV600E genetic mutation is associated with classic or tall cell variant of papillary carcinoma besides minimally differentiated thyroid neoplasms. Additionally, RAS (HRAS, NRAS or KRAS) genomic mutations are discerned, especially within follicular variant of papillary carcinoma. RAS genomic mutation is exemplified in well differentiated thyroid neoplasms [1,2]. Genomic fusions of BRAF, RET, PPARG, NTRK1, NTRK3, ALK, LTK, MET, FGFR2 or THADA genes may be detected wherein RET genetic fusion is frequently delineated [1,2].
TERT promoter mutation is frequently associated with aggressive histological variants demonstrating an inferior prognosis, as tall cell variant of papillary carcinoma [1,2]. Papillary carcinoma manifests as a painless thyroid nodule or tumefaction. Cervical lymph nodes may be palpable. Tumour nodule is cold on thyroid scan. Initial disease representation emerges as 67% singular thyroid nodules, 13% thyroid nodules combined with cervical lymph node metastasis and 20% neoplasms confined singularly to lymph nodes. Incriminated regional lymph nodes may appear as miniature with uniform consistency and clinically non discernible [1,2]. Upon gross examination, a solid, white, firm, encapsulated or infiltrative, multifocal tumefaction is observed. Focal cystic degeneration, fibrosis or calcification may occur. Superficial extra-thyroidal tumour extension may be discerned [1,2].
Frozen section is commonly employed to determine the presence of parathyroid tissue or regional lymph node metastasis [1,2]. Cytological aspirate is cellular with dense or attenuated fragments and comprised of mono-layered nests and cellular aggregates admixed with three dimensional papillary configurations with prominent fibro-vascular cores. Besides, multi-layered syncytial fragments or branched sheets of neoplastic cells may occur. Psammoma bodies may be enunciated. Tumour cells demonstrate enlarged, overlapping nuclei with irregular nuclear outline, intra-nuclear cytoplasmic inclusions, nuclear grooves and pale, finely dispersed chromatin [1,2]. Upon microscopy, papillary carcinoma exhibits distinctive nuclear features as ~altered nuclear outline and magnitude with nuclear enlargement, elongation and overlapping [1,2]. ~nuclear chromatin is optically clear and demonstrates chromatin margination, glassy or ground glass nuclei with configuration of Orphan Annie nuclei [1,2]. ~nuclear membrane and nuclear contour is irregular with the occurrence of nuclear grooves and nuclear pseudo-inclusions, indicative of cytoplasmic invaginations [1,2]. Papillary carcinoma enunciates distinctive subtypes as ~classic variant comprised of complex, branching, randomly arranged papillae with discernible fibro-vascular cores [1,2]. Neoplasms composed of an amalgamation of papillary and follicular architecture are designated as classic papillary carcinoma, on account of distinctive possibility of lymph node metastasis [1,2]. ~follicular variant exemplifies neoplastic cells configuring macro-follicles or micro-follicles with centric accumulation of colloid [1,2].
~cribriform morular variant delineates cribriform architecture intermingled with squamous epithelial morules. Tumour cells depict columnar or cigar shaped nuclei. Supra-nuclear or sub-nuclear vacuoles may be prominent [1,2]. ~diffuse sclerosing variant is a diffuse neoplasm incriminating a singular thyroid lobe. Focal fibrosis or squamous metaplasia is observed. Psammoma bodies are frequent. Regional lymph node metastasis may ensue. The variant is commonly discerned in young subjects with preceding chronic lymphocytic thyroiditis [1,2].
~Warthin-like variant is constituted of oncocytic tumour cells intermingled with reactive lymphoid stroma and accompanies preceding chronic lymphocytic thyroiditis. ~solid variant exemplifies a neoplasm with solid or an insular pattern of tumour configuration [1,2]. Amalgamation of diverse tumour articulations as papillary or follicular within a singular tumefaction is frequent [1,2]. Papillary carcinoma exhibits distinctive cytological features as ~tall cell where cellular height is around 3x of cellular width. Cellular margin is distinct. Tumour cells configure stretched, elongated or ‘tram-track’ papillae. Abundant, eosinophilic cellular cytoplasm with accumulated mitochondria is associated with frequent nuclear pseudo-inclusions. For adequate characterization, tall cell variant requires metamorphosis of minimally 30% of neoplastic cells into tall cells [1,2]. ~columnar cell where tumour cells demonstrate cigar shaped nuclei and nuclear pseudo-stratification. Columnar cell variant displays metamorphosis of minimally 30% of neoplastic cells into columnar cells [1,2].
~hobnail variant is comprised of tumour cells with prominent nucleoli and enhanced nuclear to cytoplasmic ratio nuclei. Nuclei appear to protrude away from stalk into glandular lumen [1,2]. ~oncolytic variant is constituted of tumour cells incorporated with abundant, eosinophilic cytoplasm [1,2]. Exceptionally, variants such as spindle-shaped cell or clear cell papillary carcinoma may be encountered [1,2]. Intraluminal colloid is dense and inspissated. Laminated micro-calcifications, denominated as psammoma bodies are frequently observed in classic, tall cell, hobnail or diffuse sclerosing variants of papillary carcinoma. Psammoma bodies are configured within hyalinised core or papillary stalk [1,2
Singular occurrence of psammoma bodies within regional lymph nodes is indicative of metastasis from primary papillary thyroid carcinoma. Majority of neoplasms are infiltrative although encapsulated or well demarcated lesions are encountered, especially within follicular variant. Tumour stroma appears as fibrotic, calcified or ossified. Fibrotic tumour stroma is preponderantly delineated in fibromatosis or fasciitis-like variant [1,2]. Primary or metastatic papillary carcinoma frequently depicts cystic alterations [1,2].
Ultrastructural examination exhibits significantly indented nuclear membrane, multi-lobed nuclei, nuclear pseudo-inclusions and aggregates of enlarged, inter-chromatin granules. Nucleoli depict micro-fibrillary cortex with segregation of nucleolar components. Dense nucleolar micro-spherules imbued with RNA may be exemplified [1,2].
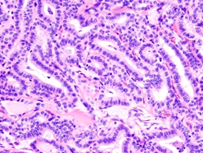
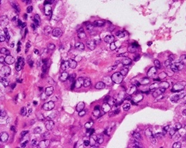
TNM staging of papillary carcinoma thyroid is denominated as
•TX: Primary tumour cannot be assessed.
•T0: No evidence of primary tumour.
•T1: Tumefaction ≤2 centimetres and confined to thyroid.
•T1a: Tumefaction is ≤1 centimetre and confined to thyroid.
•T1b: Tumefaction is between 1 centimetre to 2 centimetres and confined to thyroid.
•T2: Tumefaction is between 2 centimetres to 4 centimetres and confined to thyroid.
•T3: Tumefaction is > 4 centimetres or extension beyond thyroid commences.
•T4a: Tumefaction is moderately advanced, extends beyond thyroid and infiltrates adjacent tissues
As larynx, trachea, oesophagus or vagus nerve.
•T4b: Tumefaction is significantly advanced and infiltrates vertebral column or adjacent, enlarged blood vessels [2,3].
•NX: Regional lymph nodes cannot be assessed.
•N0: No regional lymph node metastasis.
•N1: Regional lymph node metastasis present.
•N1a: Tumour extends to thyroid circumscribing lymph nodes as pretracheal, paratracheal or prelaryngeal nodes.
•N1b: Tumefaction extends to cervical, retropharyngeal or superior mediastinal lymph nodes [2,3]. •MX: Distant metastasis cannot be assessed.
•M0: Distant metastasis absent.
•M1: Distant metastasis into diverse viscera, distant lymph nodes or bones may occur [2,3].
Staging of papillary carcinoma pertains to specific age groups and is designated
as ●Incriminated individuals <55>
•stage I (any T, any N, M0): Tumour magnitude or regional lymph node metastasis is variable although distant metastasis is absent.
•stage II (any T, any N, M1): Tumour magnitude or regional lymph node metastasis is variable. Distant metastasis is observed [2,3].
●Incriminated individuals > 55 years
•stage I (T1, N0, M0): Tumour is ≤ 2-centimetre magnitude and confined to thyroid. Regional lymph node or distant metastasis is absent.
•stage II (T2, N0, M0): Tumour is between 2 centimetres to 4 centimetres and confined to thyroid. Regional lymph node or distant metastasis is absent [2,3].
•stage III is comprised of ~T3, N0, M0: Tumour is > 4-centimetre magnitude with mild extension beyond thyroid. Regional lymph node or distant metastasis is absent [2,3]. ~T1 to T3, N1a, M0: Tumour magnitude is variable with mild extension beyond thyroid. Metastasis to regional nodes as pretracheal, paratracheal or prelaryngeal lymph nodes is observed. Metastasis to distant lymph nodes or diverse organs is absent [2,3].
•stage IV A is comprised of~T4a, any N, M0: Tumour magnitude is variable with extension into adjacent soft tissues. Regional lymph node metastasis may or may not occur. Distant metastasis is absent. ~ T1 to T3, N1b, M0: Tumour magnitude is variable with mild extension beyond thyroid. Metastasis into cervical, superior mediastinal or retropharyngeal lymph nodes is discerned. Distant metastasis is absent [2,3].
•stage IVB (T4b, any N, M0): Tumour magnitude is variable with tumour extension into vertebral column or adjacent, enlarged blood vessels. Regional lymph node metastasis may or may not occur. Distant metastasis is absent [2,3].
•stage IVC (any T, any N, M1): Tumour magnitude is variable and extension beyond thyroid may or may not occur. Regional lymph node metastasis may or may not be discerned. Distant metastasis is present [2,3].
Papillary carcinoma is immune reactive to cytokeratin AE1/AE3, CK7, TTF1, thyroglobulin or PAX8. Immune reactivity to BRAFV600E and NRAS is observed. Employment of immune markers as CK19, galectin3 or HBME1 for tumour categorization remains debatable. Immune reactivity to CDX2 or β-catenin is variable. Ki-67 labelling index is <5>10% appear aggressive with enhanced tumour reoccurrence [3,4]. Papillary carcinoma is immune non-reactive to CK20 or calcitonin.
Papillary carcinoma requires segregation from conditions such as severe chronic lymphocytic thyroiditis with reactive nuclear changes, papillary foci of Graves’s disease or papillary hyperplasia of thyroid, medullary thyroid carcinoma, hyalinising trabecular tumour and hyperplastic ultimobranchial body rests or solid cell nests [3,4]. Additionally, segregation is necessitated from reactive alterations induced by fine needle aspiration, adenomatoid nodule, diffuse thyroid hyperplasia, dyshormonogenetic goitre, follicular adenoma, follicular thyroid carcinoma or metastatic neoplasms of distant primaries.
Contingent to classic cytological features, papillary carcinoma can be appropriately detected upon preoperative fine needle aspiration cytology [3,4]. Molecular evaluation of BRAFV600E genetic mutation upon cytological aspirate may aid preoperative tumour discernment [3,4]. Surgical resection specimens can be appropriately diagnosed contingent to nuclear features as altered nuclear magnitude and outline, non-uniform nuclear membrane or chromatin configuration [3,4]. Additionally, neoplastic subtyping is achieved with combined assessment of morphological features as tumour architecture denominated as solid, classic, follicular or cribriform morular, cytological features as oncolytic, tall cell, hobnail or columnar cell, tumour magnitude as micro-carcinoma and infiltrative neoplasms or tumour encapsulation as encountered with encapsulated papillary variant, encapsulated follicular variant or infiltrative follicular variant [3,4].
Therapeutic strategies are contingent to neoplastic risk stratification and are designated as
•high risk tumefaction which is treated with total thyroidectomy and postoperative radioactive iodine [3,4].
•intermediate risk neoplasms can be managed with subtotal or total thyroidectomy. Postoperative radioactive iodine therapy is contemplated to be an individual requirement [3,4]. •low risk tumours as intra-thyroidal encapsulated follicular variant or papillary carcinoma devoid of aggressive morphological features configuring intermediate risk or high-risk segments can be adequately treated with singular lobectomy [3,4].
•very low risk neoplasms as papillary micro-carcinoma devoid of clinically discernible metastasis, localized tumour invasion or cytological expression of aggressive manifestations can be subjected to cogent, active surveillance as an alternative to active surgical intervention. Papillary carcinoma is associated with an overall superior prognosis. Disease specific survival of incriminated subjects <20> 5 incriminated lymph nodes and an enlarged metastatic focus < 3>
•high risk tumefaction depicting gross, extra-thyroidal tumour extension into strap muscles or beyond, inadequate tumour resection, distant metastasis and regional lymph node metastasis (N1) with an enlarged metastatic focus ≥ 3-centimetre magnitude [3,4]. Initial tumour discernment within elderly subjects ≥ 55 years is associated with unfavourable prognosis. Tumour progression into poorly differentiated or anaplastic thyroid carcinoma enunciates an inferior prognosis [3,4].
Conclusion
Papillary carcinoma is immune reactive to cytokeratin AE1/AE3, CK7, TTF1, thyroglobulin or PAX8. Ki-67 labelling index is <5>
References
- Limaiem F, Rehman A et al. (2022), Papillary Thyroid Carcinoma. Stat Pearls International, Treasure Island, Florida.
- Baloch ZW, Asa SL et al. (2022), Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. ;33(1):27-63.
- Hunsaker JC, Hoffman G. (2022), Papillary Thyroid Carcinoma: An Autobiographical Case Report. Cureus. ;14(2): e22559.
- Choi JB, Lee SG et al. (2019), Oncologic outcomes in patients with 1-cm to 4-cm differentiated thyroid carcinoma according to extent of thyroidectomy. Head Neck. ;41(1):56-63.
- Image 1 Courtesy: Wikimedia commons
- Image 2 Courtesy: Radiopaedia.com


