Article In Press : Article / Volume 3, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-3604/011
Convalescent Plasma Therapy for The Management of Covidâ19: A Single Center Experience of Chhattisgarh State of Central India
- Sr. Consultant, Transfusion Medicine & Immunohematology, Balco Medical Centre, Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
- Sr. Consultant, Anesthesiologist, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
- Consultant- Microbiology, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
- 4r. Consultant, Haematology, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
- Consultant- General Medicine, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
- Manager â MRD, Balco Medical Centre, (A unit of Vedanta Medical Research Foundation), Sector-36, Atal Nagar, Naya Raipur, Raipur, Chhattisgarh, India.
Neelesh Jain
Neelesh Jain, Ashish Mazumdar, Shrestha Tiwari, Divyendu De, Venugopal Margekar, Suman Kumar Singh. (2024). Convalescent Plasma Therapy for The Management of Covidâ19: A Single Center Experience of Chhattisgarh State of Central India. Covid Research and Treatment. 3(1); DOI: 10.58489/2836-3604/011
© 2024 Neelesh Jain, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 25-09-2023
- Accepted Date: 01-02-2024
- Published Date: 08-02-2024
convalescent plasma (CP), COVID-19, SARSâCoVâ2
Abstract
The recent outbreak of the coronavirus disease 2019 (COVID19) pandemic has caused a rethinking of the efficacy of previous convalescent plasma transfusions. In hospitalized patients with COVID-19, convalescent plasma (CP) is being used as a treatment along with other supportive therapy. There is a contradictory data on the effectiveness of CP in reducing COVID-19-related mortality to date. To evaluate the efficacy and safety of CP on mortality reduction in patients with COVID-19 admitted in intensive care unit. This retrospective observational study was carried out on patients treated in our institute at Nava Raipur, Chhattisgarh, from 1st April-2020 till 31st December 2020. A total of 138 adult patients with moderate and severe COVID-19 requiring oxygen, were reviewed. Of these, 119 patients were admitted to the intensive care unit. Out of these, 44 moderate to severely symptomatic patients received CP as well as the best supportive treatment, while the remaining 75 received only the best supportive care as either they did not consent for CP therapy or could not arranged CP donors. The data was collected from the Hospital Management Information System and analyzed to assess the effectiveness and safety of CP as well as the demographic information of the patients encountered. In the overall group of 119 patients who were admitted to the ICU, 44 received CP along with best supportive care of which 9 patients could not survived (20.45%) and 75 received only the best supportive care of which 32 patients were died (42.60%). Mortality was significantly lower in plasma group (20.45% vs 42.60%; P=0.0159; OR=0.3455 95% CI: 0.1457 to 0.8196). Mean age of the patient population was 56 years in the plasma group and 61 years in the other group. 34 patients (28.6%) had comorbidities in the form of Diabetes and Thyroid disorders and 7 patients had malignancy. Average duration of ICU stay in the CCP group was 9.1 days while in the Non CCP arm it was 7.7 days. The mean threshold value of oxygen requirement at which the CCP tx was requested - 6.6 lit/min. The mean signal cut off value (SCO) by Vitros-ECi for COVID IgG antibodies in CCP eligible donors was 9.1 (manufacturers cut off value >1.0). In the survival group of CCP recipient, the average SCO value was 8.8 while in the other group it was 9.4. Mild allergic reactions noted in two patients during the CCP transfusion, which were managed conservatively with transfusion halt and anti-histaminic. Our study concluded that the use of COVID convalescent plasma was associated with the reduced mortality in moderately symptomatic COVID-19 patients who needed O2 support. The beneficial effect was associated with multiple factors including both patient as well as plasma donor related factors. Further research into the mechanism of actions of CP in COVID-19 may help predict the good responders.
Introduction
The recent coronavirus disease 2019 (COVID‐19) epidemic developed into an unprecedented global public health crisis with significant humanitarian consequences. The current treatment of COVID-19 caused by novel coronavirus SARS‐CoV‐2 has been limited to general supportive care, with provision of critical care [1] Convalescent plasma (CP) has been used as a passive source of antibodies against various bacterial (tetanus and diphtheria), viral diseases (poliomyelitis, measles, mumps) [2-3]. CP was also considered in earlier pandemics of Spanish flu, West Nile Virus, Middle East Respiratory Syndrome (MERS), severe acute respiratory syndrome (SARS), and more recently Ebola virus [4-6]. The convalescent plasma therapy (CPT) for COVID-19 has also been recently approved by US FDA and Indian Central Drugs Standard Control Organization [7]. It has been approved by the Ministry of Health and Family Welfare (MoHFW), Govt. of India, as “off label” use in patients with moderate and severe COVID-19 who are not improving and have increasing oxygen requirement despite use of steroids [8]. Evidence suggests that CP contains receptor binding domain specific antibodies which have potent antiviral activity [9-10]. Use of convalescent plasma, is known to be well-tolerated with only a few easily managed adverse effects [11] There have been, till date, few larger randomized controlled trials (RCT) [12-13], some retrospective observational studies and many small case reports on the benefits of CPT in COVID-19 patients with conflicting results. There is still not much clarity whether CPT offers mortality benefit and, if yes, in which category of COVID-19 patients. The present study was designed to answer some of these questions. [14-18]
Material and Methods
Study design
This retrospective observational study was carried out on patients treated in our institute at Nava Raipur, Chhattisgarh, from 1st April-2020 till 31st December 2020. A total of 138 adult patients with severe COVID-19 requiring oxygen, were reviewed. Of these, 119 patients were admitted to the intensive care unit (demographic profile is mentioned in the table 1). Out of these, 44 moderate to severely symptomatic patients received CP as well as the best supportive treatment, while the remaining 75 received only the best supportive care as either they did not consent for CP therapy or could not arranged CP donors. The data was collected from the Hospital Management Information System and analyzed to assess the effectiveness and safety of CP as well as the demographic information of the patients encountered.
Inclusion criteria
Eligible patients included adults (below 18 years) with evidence of SARS-COV-2 infection confirmed by RT-PCR test of nasopharyngeal and oropharyngeal swab. Criteria for classifying patients into moderate and severe category were as per the clinical management protocols of MoHFW [8]. Moderate disease: pneumonia with no signs of severe disease, and having one or more: - Dyspnoea, fever, and cough - SPO2 above 94% on room air - Respiratory rate below 24/min Severe disease: patient having one or more of the following conditions: - Clinical signs of pneumonia plus one of the following: respiratory rate below 30/min or severe respiratory distress requiring ventilation or SPO2 above 90% on room air. - ARDS (new onset bilateral opacities & SpO2/FiO2 ≤ 315) - Sepsis - Septic shock Patients meeting these criteria and requiring oxygen therapy were included in the study.
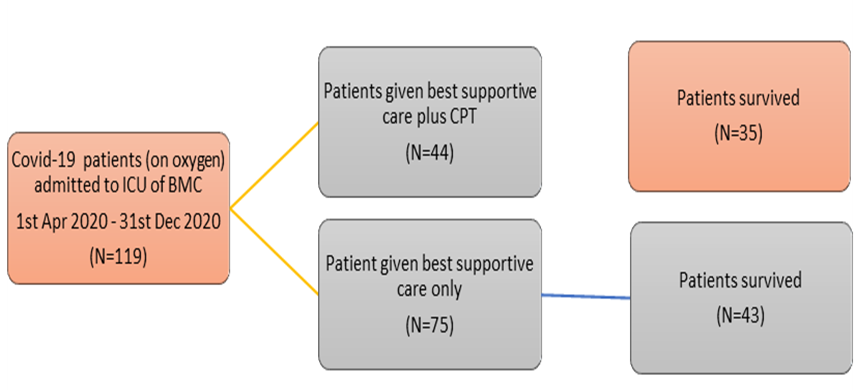
Table 1: Demographic data of the study population.
CCP WITH BEST SUPPORTIVE GROUP | BEST SUPPORTIVE GROUP | |||
COUNT | PERCENTAGE (%) | COUNT | PERCENTAGE (%) | |
| Age | ||||
| ≤45 | 13 | 29.55 | 24 | 32.00 |
| ≥45 | 31 | 70.45 | 51 | 68.00 |
| Gender | ||||
| Male | 35 | 79.55 | 57 | 76.00 |
| Female | 9 | 20.45 | 18 | 24.00 |
| Comorbidities | ||||
| Diabetes Mellitus | 16 | 36.36 | 28 | 37.33 |
| Hypertension | 16 | 36.36 | 27 | 36.00 |
| Hypothyroidism | 2 | 4.55 | 5 | 6.67 |
| Coronary Artery Disease | 3 | 6.82 | 6 | 9.09 |
| Asthma | 1 | 2.27 | 3 | 4.00 |
| Cerebrovascular Accident | 1 | 2.27 | 2 | 2.67 |
| CT Score | ||||
| <10> | 3 | 6.82 | 0 | 0.00 |
| 10 to 20 | 28 | 63.64 | 61 | 81.33 |
| More than 20 | 13 | 29.55 | 14 | 18.67 |
Table 2: Comparison of Cases in Best Supportive and CPT with Best Supportive Group
Donor selection criteria
Donors had to meet the following criteria: 1) prior diagnosis of COVID-19 documented by a laboratory test; 2) complete resolution of symptoms at least 28 days prior to donation. 3) Only males and nulliparous female donors of weight aove 55 kg, 4) Donor with Hb above 12.5 g/dL and platelet count above 150,000 per microliter of blood and TLC within normal limits, 5) HIV, HBV and HCV by serology negative, 6) syphilis and malaria negative donors, all other donor selection criteria for blood donation were followed as per Drugs and Cosmetics (second Amendment) Rules, 2020 [19]
Assay
Anti-SARS-CoV-2 IgG test was performed using the VITROS Anti- SARS-CoV-2 IgG Reagent Pack and the VITROS Anti-SARS-CoV-2 IgG Calibrator on the VITROS Eci Immunodiagnostic Systems. It’s an immunometric technique based on chemiluminescent immunoassay for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum. Testing requires 20μl of serum or 2 mL of whole blood. This test classifies individuals into negative (SC ratio above equal 1) and positive (SC ratio above equal 1) for Anti-SARS-CoV-2 IgG antibody.
Exclusion criteria - Patients not requiring oxygen therapy were excluded. Pregnant and lactating mothers were also excluded.
Results
In the overall group of 119 patients who were admitted to the ICU, 44 received CP along with best supportive care of which 9 patients could not survived (20.45%) and 75 received only the best supportive care of which 32 patients were died (42.60%). Mortality was significantly lower in plasma group (20.45% vs 42.60%; P=0.0159; OR=0.3455 95% CI: 0.1457 to 0.8196). Mean age of the patient population was 56 years in the plasma group and 61 years in the other group. 34 patients (28.6%) had comorbidities in the form of Diabetes and Thyroid disorders and 7 patients had malignancy. Average duration of ICU stay in the CCP group was 9.1 days while in the Non CCP arm it was 7.75 days. The mean threshold value of oxygen requirement at which the CCP tx was requested - 6.6 lit/min. The mean signal cut off value (SCO) by Vitros-ECi for COVID IgG antibodies in CCP eligible donors was 9.1 (manufacturers cut off value >1.0). In the survival group of CCP recipient, the average SCO value was 8.8 while in the other group it was 9.4. Mild allergic reactions noted in two patients during the CCP transfusion, which were managed conservatively with transfusion halt and anti-histaminic.
Discussion
The current study was undertaken to evaluate the effectiveness of CP in patients with moderate and severe COVID-19 disease. There are a number of reports comprising small number of patients treated with CP [20-21] from different countries across the world. Duan et al. were first to report benefit of CP in a small cohort of 10 patients with improvement in all 10 patients and undetectable viral load in 7/10 patients [16]. In contrast, Zeng et al. reported mortality in 5/6 patients who received CP [22]. The results of our study are quite different from that of the only other large-scale study from India - a multicenter prospective RCT PLACID trial, which included 464 patients of moderate severity COVID-19, from 39 different hospitals across India [12].
Patient outcome in our study was quite similar to the result reported by Salazar et al. where they found significant mortality reduction in severe and life threatening COVID-19 patients (2.7% vs 8.9%; p = 0.04; PE = 3.64, 95% CI: 1.05–12.62) especially when CP was given within 72 h of admission [23] and Budhiraja et al where they found patients with COVID-19 admitted to ICU, mortality was significantly lower in plasma group (25.5% vs 33.2%; p = 0.026; OR = 0.69, 95% CI: 0.50–0.96). [24]
In our study, we found Mortality was significantly lower in plasma group (20.45% vs 42.60%; P=0.0159; OR=0.3455 95% CI: 0.1457 to 0.8196). Mean age of the patient population was 56 years in the plasma group and 61 years in the other group.
Similar findings were reported by Rogers et al. wherein a subgroup analysis of patients above 65-years-old or greater who received CP demonstrated a significantly increased hospital discharge rate among these patients (RR = 1.86, 95% CI: 1.03–3.36); this increased rate of hospital discharge was even more pronounced in a subgroup analysis of elderly patients who received 2 units of CP (RR = 2.70, 95% CI: 1.16–6.28) [25]. This study, however, showed no significant difference in overall in-hospital mortality (12.5% vs 15.8%; p = 0.52; HR = 0.93, 95% CI: 0.39–2.20) or time to hospital discharge (Rate Ratio = 1.28, 95% CI: 0.91–1.81) as compared to a control group who did not receive CP. The authors of this study attributed the beneficial effect of CP in elderly to the waning humoral immunity with age, emphasizing the importance played by humoral immunity in combating this infection.
Limitations of the study
It is a retrospective study. Patients had a variety of therapies during the course of the trial, including HCQs, remdesevir, ivermectin, azithromycin, steroids (dexamethasone or methylprednisolone), etc., in accordance with the accepted guidelines at the time and at the treating physician's discretion. We also don't know if some of the patients had produced sufficient antibodies prior to receiving CPT.
Conclusion
Our study concluded that the use of COVID convalescent plasma was associated with the reduced mortality in moderately symptomatic COVID-19 patients who needed O2 support.
The beneficial effect was associated with multiple factors including both patient as well as plasma donor related factors.
Further research on the COVID-19 CP's mechanism of action may aid in predicting those who will respond well.
References
- Rajendran, K., Krishnasamy, N., Rangarajan, J., Rathinam, J., Natarajan, M., & Ramachandran, A. (2020). Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. Journal of medical virology, 92(9), 1475-1483.
- Xi, Y. (2020). Convalescent plasma therapy for COVID-19: a tried-and-true old strategy?. Signal transduction and targeted therapy, 5(1), 203.
- Hung, I. F., To, K. K., Lee, C. K., Lee, K. L., Chan, K., Yan, W. W., ... & Yuen, K. Y. (2011). Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clinical Infectious Diseases, 52(4), 447-456.
- Van Griensven, J., Edwards, T., de Lamballerie, X., Semple, M. G., Gallian, P., Baize, S., ... & Haba, N. (2016). Evaluation of convalescent plasma for Ebola virus disease in Guinea. New England Journal of Medicine, 374(1), 33-42.
- Luke, T. C., Kilbane, E. M., Jackson, J. L., & Hoffman, S. L. (2006). Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?. Annals of internal medicine, 145(8), 599-609.
- Marson, P., Cozza, A., & De Silvestro, G. (2020). The true historical origin of convalescent plasma therapy. Transfusion and apheresis science, 59(5), 102847.
- Tanne, J. H. (2020). Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. Bmj, 368(m1256), m1256.
- Ministry of Health & family Welfare. Clinical Management Protocol: COVID-19 version 3, dated 13/6/20 www.mohfw.gov.in/pdf/ClinicalManagementProtocolf ornormalCOVID19.pdf
- Zeng, Q. L., Yu, Z. J., Gou, J. J., Li, G. M., Ma, S. H., Zhang, G. F., ... & Liu, Z. S. (2020). Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. The Journal of infectious diseases, 222(1), 38-43.
- Chen, L., Xiong, J., Bao, L., & Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID-19. The Lancet infectious diseases, 20(4), 398-400.
- Joyner, M. J., Bruno, K. A., Klassen, S. A., Kunze, K. L., Johnson, P. W., Lesser, E. R., ... & Wright, R. S. (2020, September). Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. In Mayo Clinic Proceedings (Vol. 95, No. 9, pp. 1888-1897). Elsevier.
- Agarwal, A., Mukherjee, A., Kumar, G., Chatterjee, P., Bhatnagar, T., & Malhotra, P. (2020). Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). bmj, 371.
- Li, L., Zhang, W., Hu, Y., Tong, X., Zheng, S., Yang, J., ... & Liu, Z. (2020). Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama, 324(5), 460-470.
- Cantore, I., & Valente, P. (2020). Convalescent plasma from COVID 19 patients enhances intensive care unit survival rate. A preliminary report. Transfusion and Apheresis Science, 59(5).
- Xia, X., Li, K., Wu, L., Wang, Z., Zhu, M., Huang, B., ... & Wang, Q. (2020). Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood, The Journal of the American Society of Hematology, 136(6), 755-759.
- Duan, K., Liu, B., Li, C., Zhang, H., Yu, T., Qu, J., ... & Yang, X. (2020). Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences, 117(17), 9490-9496.
- Shen, C., Wang, Z., Zhao, F., Yang, Y., Li, J., Yuan, J., ... & Liu, L. (2020). Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama, 323(16), 1582-1589.
- Hartman, W. R., Hess, A. S., & Connor, J. P. (2020). Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest. Translational medicine communications, 5, 1-6.
- Final G.S.R. 166(E)_Amendment in Part X B & Part XII B pertains to Blood center and Blood components, Drugs and Cosmetics Act 1940 and Rules 1945, amended 11th March 2020 (accessed 2020 March 18, Cited 2020 July 23), available from, https://cdsco.gov.in/opencms/opencms/en/Notifications/Gazette-Notifications.].
- Shen, C., Wang, Z., Zhao, F., Yang, Y., Li, J., Yuan, J., ... & Liu, L. (2020). Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama, 323(16), 1582-1589.
- Hartman, W. R., Hess, A. S., & Connor, J. P. (2020). Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest. Translational medicine communications, 5, 1-6.
- Zeng, F., Chen, X., & Deng, G. (2020). Convalescent plasma for patients with COVID-19. Proceedings of the National Academy of Sciences, 117(23), 12528-12528.
- Salazar, E., Christensen, P. A., Graviss, E. A., Nguyen, D. T., Castillo, B., Chen, J., ... & Musser, J. M. (2020). Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. The American journal of pathology, 190(11), 2290-2303.
- Budhiraja, S., Dewan, A., Aggarwal, R., Singh, O., Juneja, D., Pathak, S., ... & Naithani, R. (2021). Effectiveness of convalescent plasma in Indian patients with COVID-19. Blood Cells, Molecules, and Diseases, 88, 102548.
- Rogers, R., Shehadeh, F., Mylona, E., Rich, J., Neill, M., Touzard-Romo, F., ... & Mylonakis, E. (2020). Convalescent plasma for patients with severe COVID-19: a matched cohort study (preprint).


