Current Issue : Article / Volume 3, Issue 2
- Research Article | DOI:
- https://doi.org/10.58489/2836-3582/015
Myelodysplastic syndrome/ Myeloproliferative disorder, Nos.
1Department of Cancer, Northwell Health Cancer Institute & Donald and Barbara Zucker School of Medicine at Hofstra, Lake Success, NY, USA.
Maria Jacqueline Nieto*
Margaret Locke, Maria Jacqueline Nieto. (2024). Myelodysplastic syndrome/ Myeloproliferative disorder, Nos. Journal of Hematology and Disorders. 3(2); DOI: 10.58489/2836-3582/015
© 2024 Maria Jacqueline Nieto, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 01-09-2024
- Accepted Date: 16-09-2024
- Published Date: 17-09-2024
Abstract
Background: Population-based research is limited for myelodysplastic syndrome/ myeloproliferative neoplasm, not otherwise specified (MDS/MPN-NOS) which comprises a heterogenous group of patients that do not fit into the other established MDS/MPN subgroups.
Methods: We identified patients from the Surveillance, Epidemiology, and End Results (SEER) database with a diagnosis of MDS/MPN-U logged from 2010-2020. Age-standardized incidence rates (ASIR) and incidence rate ratios (IRR) were calculated using eight age subgroups. Subgroup analyses were performed for race and gender. Join point regression analyses were performed to model trends in ASIRs, and annual percent change (APC) was calculated.
Results: A total of 2447 patients over age 50 were included in analysis. Incidence of MDS/MPN-U increased gradually between 2010-2016 (APC 12.90) then decreased between 2016-2020 (APC -19.56). ASIR was significantly higher in males compared to females in several age groups (age 65-69, 80-84, 85+).
Conclusion: The underlying molecular complexity of these diseases remains a significant challenge. It is critical to identify MDS/MPN- specific therapeutic response criteria and suitable end points correlated with survival and AML risk for transformation to acute myeloid leukemia (AML) uniform assessment of treatment benefit.
Introduction
Disorders with overlapping myelodysplastic and myeloproliferative features have been described and reclassified for several decades. The French-American-British (FAB) cooperative group introduced a classification system in 1976 that subdivided myelodysplastic syndrome (MDS) into two types: refractory anemia with excess blasts (RAEB) and chronic myelomonocytic leukemia (CMML) (1). This was revised in 1982 when FAB updated their classification of MDS into 5 subtypes, including CMML (2). The existence of MDS and myeloproliferative neoplasm (MPN) overlap syndromes was recognized in the World Health Organization (WHO)- classification in 2001 when CMML was moved into this newly founded category of myeloid malignancies (3). In addition to CMML, the new group included atypical chronic myeloid leukemia (aCML), juvenile myelomonocytic leukemia (JMML), and unclassifiable MDS/MPN (MDS/MPN-U). In the 2016 WHO classification, myelodysplastic/myeloproliferative overlap group was defined, which included CMML, JMML, aCML negative for BCR/ABL1 mutation, MDS/MPD with ringed sideroblasts and thrombocytosis (MDS/MPN-RS-T), and MDS/MPN-U (4).
MDS/MPN-U was later re-termed MDS/MPN, not otherwise specified (MDS/MPN-NOS) in the 2022 revision of the ICC and WHO classifications. Differences exist between the 2016 WHO classification, the 2022 International Consensus Classification (ICC) (5), and WHO 2022 5th edition (6), but they recognize the increasing relevance of mutations in the diagnosis and prognostic stratification of MDS/MPNs. Diagnosis is supported by the presence of mutations in addition to cytogenetic abnormalities as evidence of clonality.
MDS/MPN-NOS, accounting for less than 5% of all myeloid malignancies, is quite possibly the most heterogeneous subgroup of MDS/MPN and includes patients who lack the defining characteristics of the other MDS/MPN subtypes (7,8). Some patients with MDS/MPN-NOS may be phenotypically similar to those with aCML but lack the isolated granulocytic dysplasia that characterizes aCML. Instead, they may have basophilia and megakaryocytic hyperplasia accompanied by intense bone marrow fibrosis. Most patients present with both the “MDS-like” symptoms of ineffective hematopoiesis including fatigue, infections, and bleeding as well as the more “MPN-like” symptoms of proliferative hematopoiesis such as weight loss, night sweats, and increased risk of thromboembolism.
Although previously largely a diagnosis of exclusion, the 2022 diagnostic criteria for MDS/MPN-NOS have been refined to require the presence of cytopenias and absence of tyrosine kinase gene fusions. MDS/MPN with isolated isochrome i (17q) was also added as a provisional sub-entity under this category (5, 6). MDS/MPN-RS-T, previously a provisional entity within the MDS/MPN-U category, has since been reclassified based on presence of SF3B1 mutation (Per ICC, variant allele frequency (VAF) of SF3B1 should be ≥10%) as MDS/MPN with SF3B1 mutation and thrombocytosis (MDS/MPN-T-SF3B1) (5, 6).
With the evolving classifications that continue to refine this unique subset of patients, research into patients with MDS/MPN-NOS has been limited. Our aim was to evaluate patients with a confirmed diagnosis of MDS/MPN-U (MDS/MPN-NOS was only re-termed after 2022) and, to provide insights into the nature of this unique myeloid overlap syndrome as well as implications for treatment strategies.
Methods
Data was extracted using the Surveillance, Epidemiology, and End Results (SEER) database with 8 registries subleased in November 2022. The review included all patients aged 50-85 years with an MPD/MPN-U diagnosis logged in the SEER database from 2010 to 2020.
Age at diagnosis was formatted as 5-year age groups (50-54, 55-59, 60-64, 65-69, 70-74, 75-79, 80-84, 85+ years). Age-standardized incidence rates (ASIR) and incidence rate ratios (IRR) were calculated using these 8 age subgroups.
Statistical Analysis
To explore the potential heterogeneity in incidence rates, we performed subgroup analyses and computed ASIRs by age at diagnosis and gender. To explore the effects of age at diagnosis and gender on the potential heterogeneity in the ASIRs of different age at diagnosis and gender, we computed ASIR and IRR of race and gender categories within each age group. The relevant analyses were performed using SEER*Stat version 8.4.3.
To investigate the potential nonlinear trends in ASIRs over the period 2010 to 2020, we fitted Join point regressions to model the natural log transformed annual ASIRs. Join point regression starts with a straight line (0 join point), and tests whether more statistically significant join points need to be added to the model based on the Monte Carlo Permutation method. We allowed a maximum of 3 join points per model.
To evaluate the heterogeneity in trends, we repeated join point analysis by age at diagnosis and race. Join point analyses were implemented in the National Cancer Institute (NCI) Join point regression software version 5.0.2. Annual percent change (APC) confidence intervals were estimated by the parametric method.
Results
In total, 2,447 patients aged 50 to 85+ years diagnosed with MDS/MPN-U from 2010 to 2020 were included in these analyses. The majority of patients were white (1,942 white [79.36%], 183 Black [7.47%], and 265 other American Indian/AK native, Asian/Pacific Islander [10.82%], 57 Unknown [2.32%]).
Overall MDS/MPD Incidence Rates and Trends
Differences in ASIRs and IRRs grouped by race and ethnicity and gender are in Table 1. Incidence of MDS/MPN-U increased gradually between 2010-2016 (APC 12.90), and it was statistically significant. The incidence decreased between 2016-2020 (APC -19.56) (Figure 1).
The ASIR for African American patients age 60-64 was 2.0, which was higher than other races and it was statistically significant P=0.446. Patients with other races including American Indian/AK native, Asian/Pacific Islander, age 65-69, had an ASIR of 1.3 and it was statistically significant P=0.0211. The ASIR for African American patients, age 80-84 was 2.5, and it was statistically significant P=0.0066. For Other American Indian/AK native, Asian/Pacific Islander, the ASIR was 3.6 for age 80-84. For patients 85+, Other American Indian/AK native, Asian/Pacific Islander, ASIR was 4.2 and it was statistically significant P=0.0006.
The differences in MDS/MPN-U incidence rates by age group and gender showed that among male and female patients between age 65-69, the ASIR was higher for male patients (2.6) compared with females (1.6) and it was statistically significant (P=0.0232) for males and (P=0.0194) for females.
Among male patients age 80-84, ASIR was higher among male patients than females. The ASIR was 8.2 for males and 5.2 for females, and it was statistically significant (P=0.0151 and P=0.0326, respectively).
Male patients 85+ had a ASIR of 10.4 (P=000.6) which was higher than that of females 85+ with ASIR of 6.2, and it was statistically significant P=0.0006 for males and (P=0.0116).
Trends in MPD/MDS (U) Incidence Among Men and Women Aged 50-85+ Years by Race, Sex, and Age, 2010-2020.
| Characteristics | Age adjusted incidence rate No per 100,000 | Incidence rate ratios | Ratio P value | Annual percentage change |
| Age 50-54 | ||||
| White | 0.6 | 0.9784 | 0.6013 | 2010-2020 APC 2.22 |
| Black | 0.8 | 1.4396 | 0.2153 | |
| Other | 0.3 | 0.6097 | 0.1566 | |
| Male | 0.6 | 0.9856 | 1.0000 | 2010-2016 APC 12.90 |
| Female | 0.6 | 1.0141 | 0.9897 | 2016-2020 APC -19.56 |
| Age 55-59 | ||||
| White | 0.7 | 0.8864 | 0.3835 | |
| Black | 1.0 | 1.2521 | 0.4340 | |
| Other | 1.0 | 1.2088 | 0.4381 | |
| Male | 0.9 | 1.0994 | 0.5363 | |
| Female | 0.7 | 0.9051 | 0.5430 | |
| Age 60-64 | ||||
| White | 1.2 | 0.9043 | 0.3694 | |
| Black | 2.0 # | 1.5455 | 0.0446 | |
| Other | 1.4 | 1.0635 | 0.8056 | |
| Male | 1.5 | 1.1733 | 0.1773 | |
| Female | 1.1 | 0.8392 | 0.1717 | |
| Age 65-69 | ||||
| White | 2.2 | 1.0335 | 0.7432 | |
| Black | 2.0 | 0.9618 | 0.9768 | |
| Other | 1.3# | 0.6117 | 0.0211 | |
| Male | 2.6# | 1.2568 | 0.0232 | |
| Female | 1.6# | 0.7696 | 0.0194 | |
| Age 70-74 | ||||
| White | 3.5 | 0.9965 | 0.9989 | |
| Black | 3.3 | 0.9390 | 0.8746 | |
| Other | 2.9 | 0.8164 | 0.2645 | |
| Male | 4.0 | 1.1262 | 0.2124 | |
| Female | 3.2 | 0.8911 | 0.2390 | |
| Age 75-79 | ||||
| White | 5.4 | 1.0290 | 0.7458 | |
| Black | 4.1 | 0.7795 | 0.3565 | |
| Other | 3.8 | 0.7323 | 0.0793 | |
| Male | 5.8 | 1.1047 | 0.3008 | |
| Female | 4.8 | 0.9156 | 0.3570 | |
| Age 80-84 | ||||
| White | 7.2 | 1.1083 | 0.2229 | |
| Black | 2.5# | 0.3868 | 0.0066 | |
| Other | 3.6# | 0.5600 | 0.0035 | |
| Male | 8.2# | 1.2691 | 0.0151 | |
| Female | 5.2# | 0.8072 | 0.0326 | |
| Age 85+ | ||||
| White | 8.2 | 1.0732 | 0.3383 | |
| Black | 6.4 | 0.8373 | 0.5601 | |
| Other | 4.2# | 0.5522 | 0.0006 | |
| Male | 10.4# | 1.3536 | 0.0006 | |
| Female | 6.2# | 0.8102 | 0.0116 |
# The rate ratio indicates that the rate is significantly different than the rate for (1975-2020) {p less than 0.05}
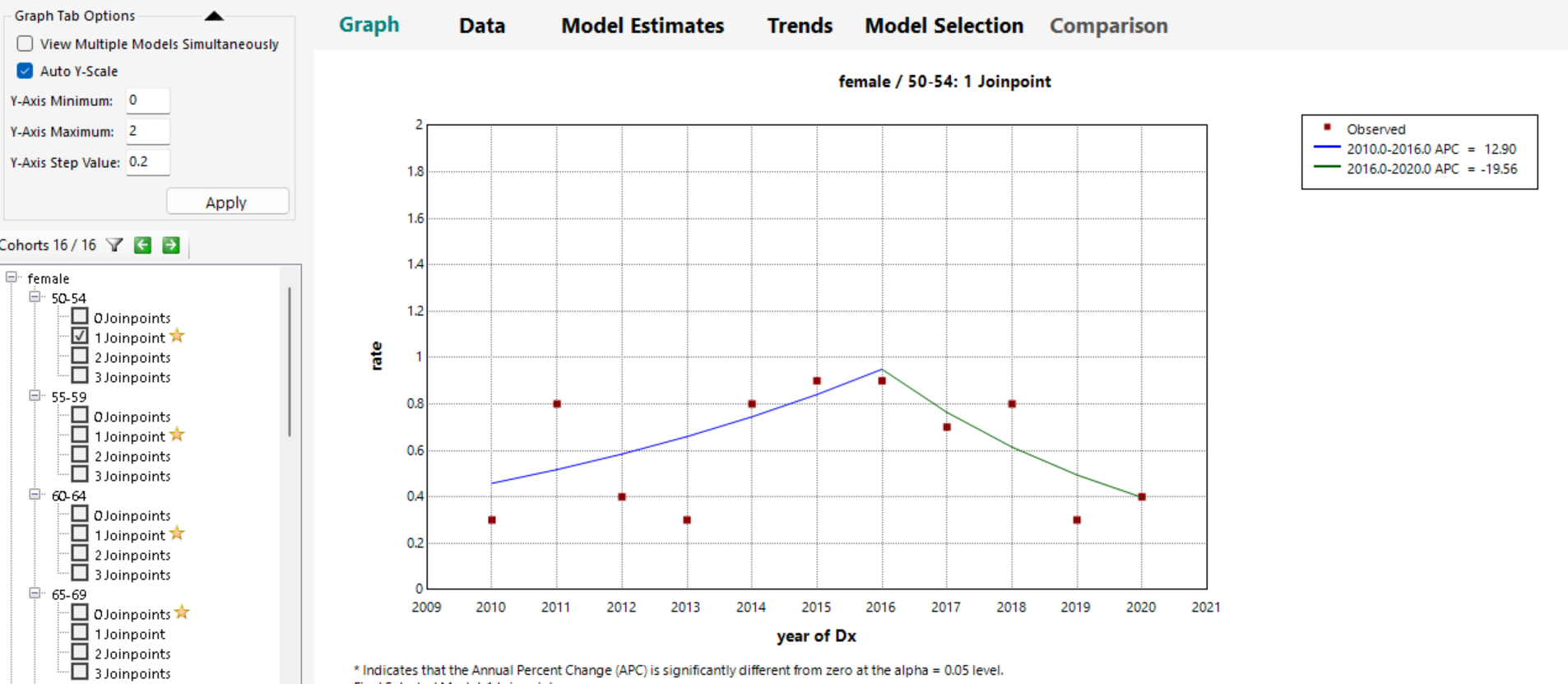
Fig: 1. Join point Analysis of Overall Incidence of Myelodysplastic/Myeloproliferative, NOS Among US Patients Aged 50 to 85+ Years, 2010-2020
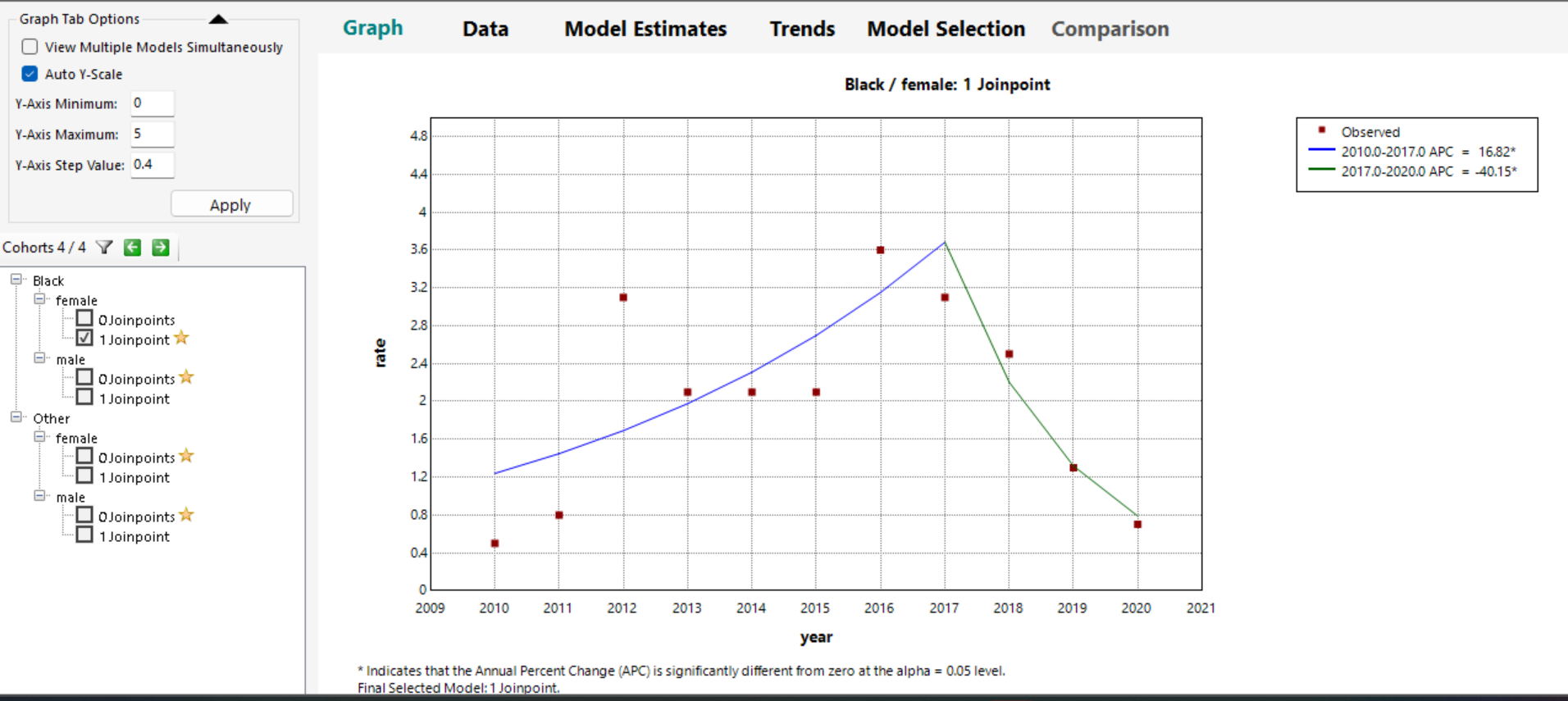
Fig 2. Join point Analysis of Overall Incidence of Myelodysplastic/Myeloproliferative, NOS Among US Patients Aged 50 to 85+ Years, 2010-2020
The Join point analysis between blacks and Other American Indian/AK native, Asian/Pacific Islander, showed one Join point among female black patients and it was statistically significant. Incidence increased gradually between 2010-2017 with an APC 16.82, slowly declined APC -40.15 between 2017-2020, and it was statistically significant (Figure 2).
Discussion
We described trends in MDS/MPN-U incidence rates among patients aged 50 to 85+ years, between 2010-2020. We observed an increase in incidence rates among patients aged 50-85+years between 2010 and 2016 APC 12.90 p= less than 0.05. Then, we observed a decline between 2016 and 2020 APC -19.56 p= less than 0.05. The join point analysis between whites and blacks showed a join point between females, ages 50-54 and it was statistically significant.
Population-based studies describing survival of MDS/MPD in the US are sparse. Our study focuses exclusively on Myelodysplastic/Myeloproliferative disorder MDS/MPN-U. A previous study by AS. Srour et al (9), evaluated the incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-2012. The Age-adjusted incidence rates for whites was 4.7, blacks 3.3, and incidence rate ratios of myelodysplastic/myeloproliferative neoplasms between blacks and whites (CI 0.61-0.79) p= less than 0.001.
Our study showed a higher incidence rate among male patients, aged 80-85+. The incidence rates were also statistically significant for blacks and Other American Indian/AK native, Asian/Pacific Islander. The join point analysis for blacks and Other American Indian/AK native, Asian/Pacific Islander also showed an increased in incidence rate between 2010-2017 APC 16.82 p= less than 0.05 and a decline between 2017 and 2020 APC -40.15 p= less than 0.05. There was 1 join point among black females.
One of the limitations of the study is underreporting, which is likely improving now with the availability of clonal markers in the 21st century. A significant proportion of these cases have been shown to be associated with abnormal karyotypes. In a study by Man Gaonkar et al, trisomy 8, monosomy 7/ deletion 7q, deletion 20q were the most common cytogenetic abnormalities detected (10). ASXL1, SRSF2, SETBP1, JAK2V617F, NRAS and TET2 are the most common mutations reported to be associated with MDS/MPN-NOS (10). TP53 mutations are seen in about 6% of the cases and are associated with a higher frequency of monosomal karyotype (10). TP53 and CBL mutations are found to be associated with an inferior overall survival (10). In contrast to some other types of MDS/MPN neoplasms, ASXL1 mutations were not found to be predictive of overall survival (10). In another study, apart from ASXL1, TET2, JAK2 and SRSF2 mutations, EZH2, U2AF1 and RUNX1 mutations were also identified (10-12). CALR mutations were seen in 3% of the cases and correlated significantly with improved survival (13). ZRSR2 mutations were associated with a better overall survival while STAG2, CEBPA and EZH2 mutations were associated with an inferior overall survival.
MDS/MPN with i(17q) has been recognized as a new provisional sub-entity under the umbrella of MDS/MPN, NOS in the 2022 ICC classification (13). Morphologically, MDS/MPN with i(17q) has marked hypolobulation of the neutrophils. These neoplasms have been found to have an aggressive clinical course with a median survival of 11 months and most patients undergoing rapid progression to acute myeloid leukemia (14). These patients were also found to have a high frequency of SRSF2, SETBP1, ASXL1 and NRAS mutations (14). They are present in the younger age group (median age of 67 years), with lower platelet and absolute neutrophil counts, higher circulating blasts and higher frequency of SETBP1 and SRSF2 mutations which can coexist (13). It is interesting to note that in spite of loss of one allele of 17p, TP53 mutations were rare in the uninvolved TP53 allele (14). Further, cases with i(17q) were found to have a shorter medial overall survival and hence, recognition of this distinct entity has been suggested.
Although previously a “catch-all” diagnosis, the definition of MDS/MPN-NOS has been refined in recent years. This has helped to establish a MDS/MPN-NOS as its own unique clinical entity, and requires further investigation for characterization.
Diagnosis can be very challenging, because myelodysplastic/myeloproliferative disorder has pathologic overlapping features of myelodysplastic syndrome and myeloproliferative disorder. The diagnosis cannot be made only on morphology alone, the identification of these specific mutations in MDS/MPD-, NOS will help in further classification and will aid in the development of targeted therapy. The eligibility for future clinical studies should be based on clinical disease phenotype or proliferative versus non-proliferative features. In addition, clinical studies should target molecular pathways, these should ideally determine patient selection based upon tyrosine kinase signaling pathway mutations or those with transcription factor mutations. The underlying molecular complexity of these diseases remains a significant challenge. It is critical to identify MDS/MPN- specific therapeutic response criteria and suitable end points correlated with survival and AML risk for transformation to acute myeloid leukemia (AML) uniform assessment of treatment benefit.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: There is no conflict of interest.
Ethical Statement: The study was reviewed and approved by the institutional review boards (IRBs) of participating sites.
Abbreviations
(MDS/MPN-NOS) myelodysplastic syndrome/ myeloproliferative neoplasm, not otherwise specified
(ASIR) Age-standardized incidence rates
(IRR) incidence rate ratios
(APC) annual percent change
(AML) Acute myeloid leukemia
(RAEB) Refractory anemia with excess blasts
(CMML) Chronic myelomonocytic leukemia
(aCML) Atypical Chronic myeloid leukemia
(JMML) juvenile myelomonocytic leukemia
(MDS/MPD-U) Unclassifiable MDS/MPN
(MDS/MPN-RS-T) MDS/MPD with ringed sideroblasts and thrombocytosis.
(SEER) Surveillance, Epidemiology, and End Results
References
- Bennett, J. M., Catovsky, D., Daniel, M. T., Flandrin, G., Galton, D. A., Gralnick, H. R., & Sultan, C. (1976). Proposals for the classification of the acute leukaemias French‐American‐British (FAB) co‐operative group. British journal of haematology, 33(4), 451-458.
- Bennett, J. M., Catovsky, D., Daniel, M. T., Flandrin, G., Galton, D. A. G., Gralnick, H. R., & Sultan, C. (1982). Proposals for the classification of the myelodysplastic syndromes. British journal of haematology, 51(2), 189-199.
- Jaffe, E. S. (2001). World Health Organization classification of tumors. Pathology and genetics of tumors of hematopoietic and lymphoid tissues, 185-187.
- Arber, D. A., Orazi, A., Hasserjian, R., Thiele, J., Borowitz, M. J., Le Beau, M. M., ... & Vardiman, J. W. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, The Journal of the American Society of Hematology, 127(20), 2391-2405.
- Arber, D. A., Orazi, A., Hasserjian, R. P., Borowitz, M. J., Calvo, K. R., Kvasnicka, H. M., ... & Tefferi, A. (2022). International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood, The Journal of the American Society of Hematology, 140(11), 1200-1228.
- Khoury, J. D., Solary, E., Abla, O., Akkari, Y., Alaggio, R., Apperley, J. F., ... & Hochhaus, A. (2022). The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. leukemia, 36(7), 1703-1719.
- Cannella, L., Breccia, M., Latagliata, R., Frustaci, A., & Alimena, G. (2007). Clinical and prognostic features of patients with myelodysplastic/myeloproliferative syndrome categorized as unclassified (MDS/MPD-U) by WHO classification. Leukemia research, 32(3), 514-516.
- Wang, S. A., Hasserjian, R. P., Fox, P. S., Rogers, H. J., Geyer, J. T., Chabot-Richards, D., ... & Orazi, A. (2014). Atypical chronic myeloid leukemia is clinically distinct from unclassifiable myelodysplastic/myeloproliferative neoplasms. Blood, The Journal of the American Society of Hematology, 123(17), 2645-2651.
- Srour, S. A., Devesa, S. S., Morton, L. M., Check, D. P., Curtis, R. E., Linet, M. S., & Dores, G. M. (2016). Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–12. British journal of haematology, 174(3), 382-396.
- Mangaonkar, A. A., Lasho, T. L., Ketterling, R. P., Reichard, K. K., Gangat, N., Al-Kali, A., ... & Komrokji, R. (2022). Myelodysplastic/myeloproliferative neoplasms with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T): Mayo-Moffitt collaborative study of 158 patients. Blood cancer journal, 12(2), 26.
- Patnaik, M. M., & Lasho, T. L. (2020). Genomics of myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Hematology 2014, the American Society of Hematology Education Program Book, 2020(1), 450-459.
- Jeromin, S., Haferlach, T., Weissmann, S., Meggendorfer, M., Eder, C., Nadarajah, N., ... & Schnittger, S. (2015). Refractory anemia with ring sideroblasts and marked thrombocytosis cases harbor mutations in SF3B1 or other spliceosome genes accompanied by JAK2V617F and ASXL1 mutations. Haematologica, 100(4), e125.
- Kanagal-Shamanna, R., Orazi, A., Hasserjian, R. P., Arber, D. A., Reichard, K., Hsi, E. D., ... & Bueso-Ramos, C. (2022). Myelodysplastic/myeloproliferative neoplasms-unclassifiable with isolated isochromosome 17q represents a distinct clinico-biologic subset: a multi-institutional collaborative study from the Bone Marrow Pathology Group. Modern Pathology, 35(4), 470-479.
- Kanagal-Shamanna, R., Luthra, R., Yin, C. C., Patel, K. P., Takahashi, K., Lu, X., ... & Bueso-Ramos, C. E. (2016). Myeloid neoplasms with isolated isochromosome 17q demonstrate a high frequency of mutations in SETBP1, SRSF2, ASXL1 and NRAS. Oncotarget, 7(12), 14251.


