Archive : Article / Volume 1, Issue 2
Case Report | DOI: https://doi.org/10.58489/2836-2225/007
On the Mechanisms of Neuroendocrine Regulation of Fish Reproduction
Federal State Budgetary Educational Institution of Higher Education âSaint-Petersburg State Agrarian University, Russiaâ.
Correspondng Author: Garlov P.E
Citation: Garlov P.E., Dr. Sci. Biol, (2022). To Mechanisms of Neuroendocrine Regulation of Fish Reproduction. International Journal of Reproductive Research. 1(2). DOI: 10.58489/2836-2225/007
Copyright: © 2022 Garlov P.E., this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-11-10, Received Date: 2022-11-10, Published Date: 2022-12-23
Abstract Keywords: sturgeon and salmon fish, migration and spawning, neuroendocrine regulation of fish reproduction, hypothalamic-pituitary neurosecretory system of fish.
Abstract
The participation of the preoptic-postneurohypophysial neurosecretory system in fish breeding was shown by histomorphological, immunohistochemical and electronicmicroscopical researches. The activation of discharge of nonapeptide neurohormones into blood circulation from posterior neurohypophysis at the beginning of spawning, with the following decrease of functional activity of system after it are revealed in every lumpsum (simultaneous) spawning fishes idependently of its season (for example: Acipenser, Lota, Oncorhynchus). The similar reaction of neurosecretory system is observed morphologically also under the conditions of experimental stressâthe containing of the adult acipenseridae fish in hypertonic medium. The diphasic reaction of neurosecretory system conforming to stages of an alarm and a resistance of stress, is considered to be the reflection of participation of neurosecretory system in protective-adaptive reactions of an organism to a physiological stress arising at breeding period of lumpsum spawning polycyclic fishes. At monocyclic species at once after spawning there becomes the blockage of function of releasing of neurohormones from posterior neurohypophysis corresponding to supernatural inhibition of system at disstress. The functional role of nonapeptide neurohormones in breeding and the possibility of constructive usage of representation about the spawning as a physiological stress are discussed on the basis of analysis of morpho-functional mechanisms of participation of neurosecretory system in this process.
Results and discussion
By means of ecologo-histophysiological and experimental researches executed by the pathway of a direction of the professor N.L. Gerbilsky΄s school (Gerbilsky, 1956), the participation of hypothalamical preoptic-postneurohypophysial neurosecretory system (PNS) of fish in realization of all stages of breeding, irrespective its season, is established (Garlov, 1971, 2001; Polenov et. al., 1976). With application quantitative histomorphological and cytochemical methods of procedures, including cytospectrophotometry, immunocytochemistry and ultracytochemistry the dynamics in changes of functional activity of all departments of PNS is investigated during spawning at all of different-seasonal-breeding species of Acipenseridae, Salmonidae and Gadidae: the Russian sturgeon (Acipenser gueldenstaedtii Brandt), pink (humpback) salmon (Oncorhynchus gorbuscha Walbaum) and burbot (Lota lota Linne) (Fig. 1).
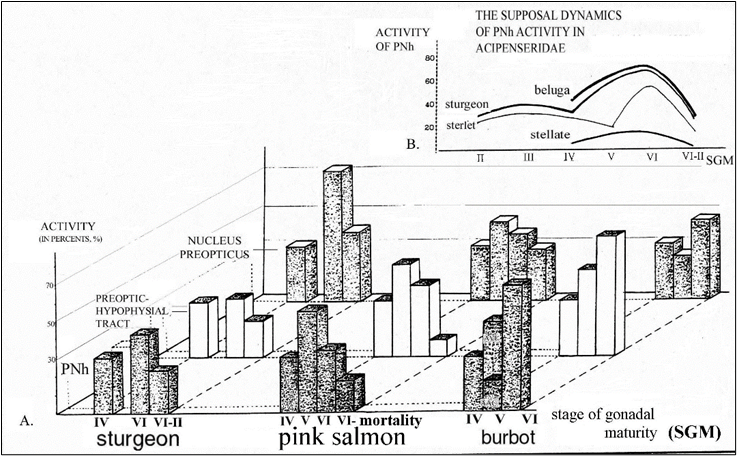
Figure 1. Dynamics of changes of functional activity of various departments of PNS in sturgeon, pink salmon and burbot at the process of spawning by data of the quantitative morphometry at levels of light and electron microscopy (after: Garlov, 2001). For comparison the data received in further works and in other species of the Acipenserid fishes are given (В). On a vertical - relative degree of functional activity. PNh - posterior neurohypophysis, PHT - preoptico-hypophysial neurosecretory tract, NPO - nucleus preopticus. On a horizontal - stages of gonadal maturity (SGM). The Roman figures under histogramm designate stages of gonadal maturity: IV - before spawning, V - at the beginning of spawning, VI - soon after spawning, VI-II - some later (about a month) after spawning, VI-DEATH - in a pink-salmon, before destruction after spawning.
All of the basic phylums of PNS constitution are submitted also at these kinds of fish (Polenov, Garlov, 1973; Fig. 2).
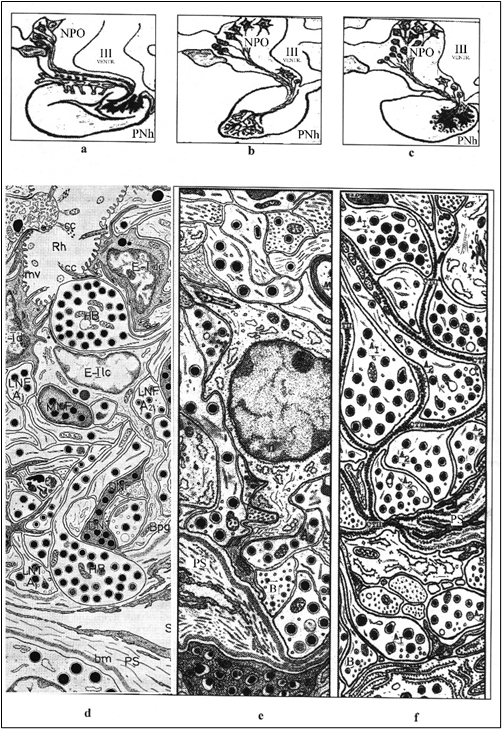
Figure 2. A scheme of the main features of a structure of PNS at the investigated kinds of the Chondrosteen and Teleosteen fishes (a - species Acipenser, b - species Oncorhynchus, c - species Lota), conforming to a level of their taxons position.
Ultrastructural organization of PNh, schemes: d - sturgeon, e -pink-salmon, f - burbot (fragments, after: Garlov, 2001). Alongside with axo-vasal neurosecretory contacts expressed at all of kinds, at the sturgeon are expressed axo-ventricular, and at the burbot - axo-adenar neurosecretory contacts.
Conditional notations: А1, А2, B - species (forms) of neurosecretory fibers, light neurosecretory fibers (LNF), dark neurosecretory fibers (DNF) myelinated neurosecretory fibers (MNF), its' swellings and neurosecretory terminals (NT), bm - basal membrane of pericapillary space, mv - microvilly, cc - cinocillias, Rh - cavity of recessus hypophyseus of the III ventriculi, Bpg - basement protrusion of glial cell (pituicyte), NPO - nucleus preopticus, P - pituicyte, PS - pericappilary space of sinusoidal capillary, PNh - posterior neurohypophysis, S- sinusoid capillary, Е - ependimal cells (tanicytes) in Acipenser, light cell (lc) and dark cell (dc), HB - Herring body.
The diphasic reaction of PNS, conforming to alarm and resistance stages of stress, is established at them in spawning process which is most brightly expressed in posterior neurohypophysis (PNh) (Fig. 3 a-d.).
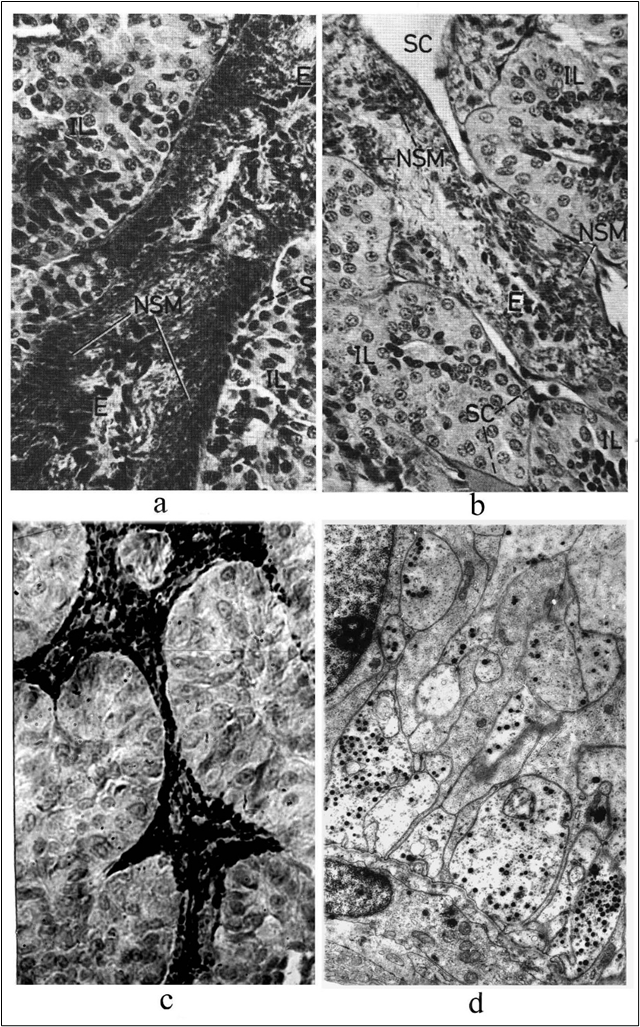
Figure 3. A preparates of state of PNh of sturgeon (a, b, d) and pink salmon (c) at various stages of spawning. A light microscopy (a, b): colouring paraldehyde - phuchsyn after Homori–Gabe and azan method after Heidengaine. The immunohistochemical reaction (c) to vasotocin (reaction of revealing of the unmarked antibodies after Sternberger and Joseph). An electronic microscopy (d): glutaraldehyde after Sabatini with Osmium tetroxide after Caulfield and having imbedded in Epon-812, mainly.
a - before spawning (IV the stage of gonadal maturity - SGM) roots of PNh of females of the sturgeon contains a plenty of Gomori-positive neurosecretory material, that shows an inactive state of PNS; b - soon after spawning (VI SGM) roots of PNh are discharged from a neurosecretory stuff as a result of mass removing of neurosecretory products into blood stream (ocul.: х7, obj: х20); c - soon after spawning roots of PNh of pink salmon are overflowed by a neurosecretory stuff immuno-positive (vasoactive) material on a preparation to a vasotocin (х10, х20); d - neurosecretory terminals of the distal departments of the roots PNh soon after spawning in sturgeon contain the small amount of elementary neurosecretory granuls and of synaptical vesicles (magnification: х4500).
At springly-breeding females of sturgeon it is expressed by an activation of release of the nonapeptide neurohormones (NpNh) from PNh into general blood circulation soon after spawning and by the following subsequent decrease of PNS activity up to pre-spawning level (Fig. 1А; Polenov et. al., 1976).
At winterly-spawning burbot the consecutive reaction of PNS is established: 1) at the beginning of spawning there is an activation of NpNh release from PNh in range of the so-called: “axo-adenar” neurosecretory contacts (Polenov et. al., 1986) with simultaneous decrease of neurosecretory activity both in Nucleus praeopticus hypothalamus and neurosecretory terminals of their axons in PNh in range of “axo-vasal” neurosecretory contacts and 2) just after spawning the bright activation already of all departments of PNS occurs. These two consecutive pathes of the extrusion of NpNh (“trans-adenohypophysial and para-adenohypophysial”) are characteristic for stress reaction also, that may be an optimal natural model for the analysis of this state (Garlov, Polenov, 1996).
At monocyclic autumnly-spawning pink salmon a bright activation of all departments PNS is revealed at the beginning of spawning, particularly in a state of the mass release of NpNh from PNh. The following decrease of the activity of all departments PNS occurs just after spawning, the functional blockade of PNh, particularly blockind the extrusion of NpNh, growthing to the death, to the moment, that is proved first of all. Thus, by a quantitative electronic microscopical evaluation of a degree of functional activity of neurosecretory terminals in PNh it is established, that such a blockage descends as a result of infringement of their exstrusional cycle, as against known for polycyclic fishes (Garlov, Polenov, 1996; a Fig. 3c).
These researches were advanced in many subsequent works. So, the expressed activation of various departments PNS, showing in exhausting all of its departments from a neurosecretory stuff, activation of neuroglial elements and hyperemia of nucleus preopticus and PNh ranges, is established to the present time at many kinds of teleostei with lumpsum spawning, but: “at breeding season”, or “during breeding” as a whole, without the count of stages of gonadal maturity (Peter, 1986). For example, it is described the activation of neurosecretory cells of nucleus preopticus (pars magnocellularis), or PNh at springly-spawning Cyprinidae, Clupeidae and Gobiidae, e.g.: wild carp (Cyprinus carpio L.), goldfish (Carassius auratus L.), toad goby (Mesogobius batrachocephalus Pallas), mud carp (Cirrhinus mrigala L.) (Kaul, Vollrath, 1974; Moiseeva, 1975; Moitra, Medya, 1980; Polenov et al., 1986); autumnly-spawning polycyclic Salmonidae: Atlantic salmon (Salmo salar L.), kundscha (Salvelinus leucomaenus pluvius (Hilgendorf) and rainbow trout (Salmo irideus L.) (Arvy et al., 1959; Honma, Tamura, 1965; Barannikova, 1975; Terlou et al., 1978); sea tropical fishes: barb (Puntius sarana Ham.), Indian carp (Labeo calbasu Ham.), mud eel (Amphipnous cuchia Ham.), gilthead bream (Sparus auratus L.) (Prasado-Rao, 1970; Tripathi, Pandey, 1986; Ramadan et al., 1988), and also Dipnoi: Indian featherback (Notopterus chitala Ham) (Pracash et al., 1984). Besides and in earlier works it was repeatedly specified a possible activation of various departments PNS at a breeding period of some viviparious forms, for example representatives Chondrichthyes: small-spotted dogfish (Scyliorhinus canicula L.), thorny skate (Raja radiata Don.) (Mellinger, 1963; Meurling, 1970) and Osteichthyes: killifish (Fundulus heteroclitus L.) and viviparus blenny (Zoarces viviparus L.) (Sokol, 1961; Oztan, 1966). The precise enough pictures of release of neurohormones from PNh into blood stream at the beginning of spawning are established by electronic microscopy only at goldfish (Kaul, Vollrath, 1974) and, to a lesser degree, at wild carp (Polenov et al., 1986). The data on functional blockage of PNh after breeding, well coordinated to our results at pink salmon, are received at capelin males (Mallotus villosus Muller.), which majority perishes in this season (Oganesyan, 1986). And, at last, preliminary results of the further development of our works on trade kinds of Acipenseridae fish have shown, that of a various degree the activation of PNh at the spawning season descends practically at all investigated species, for example beluga (Huso huso l.), starred (stellate) sturgeon (A. stellatus Pallas) and sterlet (A. ruthenus L.) (Fig. 1B).
So, at spawning process in many fishes, irrespective of a season of a breeding, the activation of PNS, showing as exhausting from a neurosecretory stuff, takes place. It is accompanied by an activation of neuroglial cells and hyperemia of all departments of this system, but only at kinds with precisely expressed lumpsum spawning, as, for example, at investigated there Russian sturgeon, burbot, pink salmon (Garlov, 2001). Thus, dynamics of functional activity fluctuation of neurosecretory cells in dorsal, mostly differetiated magnocellular part of a praeoptic nucleus (Nucleus Preopticus pars magnocellularis), is similar almost at all investigated species of fish (Fig. 1А). Before spawning (the so-called in fish industry: IV stage of gonadal maturity - SGM) the neurosecretory cells are already in a rather awake functional state, and at the beginning of spawning (V SGM) there is intensifying of synthesis of neurosecretory products and their transport from pericaryons into axons. After spawning and later (VI and VII-II SGM) the gradual decrease of activity of these processes up to pre-spawning level is observed (Polenov et al., 1974; Garlov, Polenov, 1996).
We assume, that NpNh firstly participate in initiation of spawning behaviour, founding on these data and also on that they invoke "spawning reflex" in fishes (Pickford, Strecker, 1977; Demski, Sloan, 1985; Peter, 1986). The initiation of spawning behaviour before breeding in fishes is provided with the removing of NpNh, mainly of arginin-vasotocin (AVT) into cerebral-spinal fluid ("liquor") of a III-rd cerebral cavity, where they combined with gonadoliberin (LH-RH), render the neurotropic effect upon the behavioural centers of central nerve system (CNS), apparently, in septal, hyppocampal and amigdal ranges (Demski, Sloan, 1985; Knight, Knight, 1996; Garlov, 2001). NpNh are discharged from neurosecretory cells of a praeoptic nucleus in the range of dendro-ventricular and somato-ventricular neurosecretory contacts, and from neurosecretory terminals in the range of axo-ventricular neurosecretory contacts of PNh and also of the homologue of eminencia mediana of hypothalamus in Chondrichthyes, Crossopterygii, Chondrostei, Holostei, Dipnoi (Garlov, Polenov, 1971; Polenov et al., 1972, 1976). In Teleosteen fishes NpNh can penetrate also into liqour through intercellular clefts from range of axo-vasal contacts in PNh (Garlov, Polenov, 1996). These pathes of removing of NpNh are expressed morphologically in mass exhausting of neurosecretory cells, in particular in a reduction of the special forms of mass accumulation of neurosecretory products specialized for realization of a breeding, e.g. from droplets of neurosecrete and Herring bodies in different departments of PNS (Garlov, Polenov, 1971; Garlov, 2001; Fig. 2d, 3). At the majority of fish, the spawning behaviour is kept almost up to the end of breeding, to what apparently is partly connected both initial and subsequent activations of PNS (Godwin et al., 1996; Ota et al., 1996; Wong 1997).
It is important, that the development (and conservation) of spawning dress also is biologically interconnected and synchronic with spawning behaviour by means of the stimulating influence of NpNh on function of the melanotropocytes in an intermediate lobe of the adenohypophysis ("mesoadenohypophysis"), with which PNh compounds a neurointermediate complex of a pituitary body (Fig. 2, 3).
Morphologically such a participation is expressed most brightly in PNh of burbot at the beginning and just after spawning (V, VI SGM) as a progressive evacuation of NpNh from neurosecretory terminals in the range of axo-adenar neurosecretory contacts (Fig. 1А). The leading role in this process, probably, also is carried out by vasotocin, as a synergist of corticoliberin (CRH), stimulating the release of the hormones of opioidal series at stress, in particular, adrenocorticotropic (ACTH) and melanocyte-stimulating (a-MSH) hormones, i.e., invoking a corticoliberin-like effect as a whole (Donaldson, 1981).
The activation of PNS is expressed maximum at the beginning of spawning (Fig. 1А), as at the moment of an ovulation and sperm release, in connection with the requirement of NpNh, stimulating contraction of smooth muscle elements in gonads (Oztan, 1966; Peter, 1986; Garlov, Polenov, 1996). The leading role of isotocin (IT) is shown in these processes, having of 10-rates greater the uterotonic activity, than of vasotocin (Pickford, Strecker, 1977; Peter, 1986). We assume, that the main target of NpNh action at ovulation are the thecal cells of follicles of an ovary (Fig. 4), in which all attributes of smooth-muscle elements (3 forms of myofilaments, sarcoplasmic reticulum, dense bodies on a plasmalemma) are brightly expressed at acipenserid fishes (Polenov et al., 1976; Garlov, Polenov, 1996).
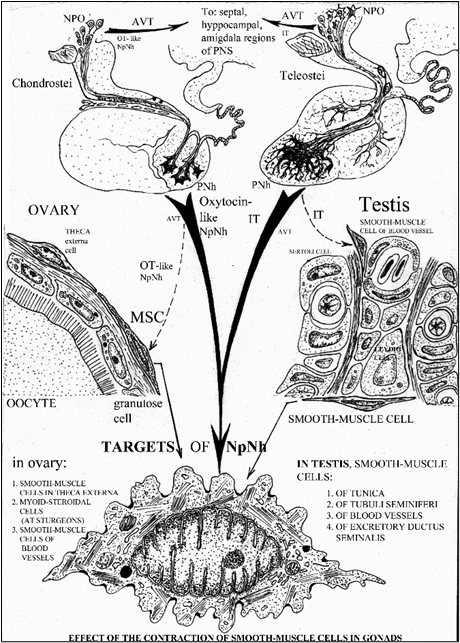
Figure 4. The scheme of prospective pathes of the neuroendocrine (NpNh) regulation of the function of smooth-muscle elements in gonads of fishes at spawning. Conditional notations: AVT - arginin-8-vasotocin, CNS – central nerve system, IT - isotocin, MSC – myoid-secretory cell, NH - neurohormones, NLT - nucleus lateralis tuberis, NpNh – nonapeptide neurohormones, NPO - nucleus preopticus, OT – oxytocin, PNh - posterior neurohypophysis.
These cells are myoid-steroidproducing elements, apparently, as a key enzyme of a steroidogenesis - 3b-hydroxysteroiddehydrogenase (3b-HSD) is revealed in their cytoplasm clearly by an ultraimmunocytochemical method, though the main structural attributes of a steroidogenesis (smooth cytoplasmic reticulum, mytohondria with tubular-vesicular crists, lipid drops) are expressed at them rather poorly (Garlov, Mosyagina, 1998).
In spermary (testis) of fish such, but already multiple targets of NpNh action are smooth-muscle elements of seminal channels, sperm ducts, tunica and of large vessels (Fig. 4). All these elements are in a state of a synchronic activation at the moment of ovulation and sperm release at the beginning of spawning (Garlov, Mosyagina, 1998; Garlov, 2001). The stimulation of both of these processes on the part of a hypothalamus is carried out by neurohormonal integration (by the complex of gonadoliberines and NpNh, particularly) and by two pathways ("trans- and para-adenohypophysial pathways", after: Polenov et. al., 1976, 1986). The leading among them in sperm release is the para-adenohypophysial pathway of direct stimulating influence of NpNh of oxytocin series upon a spermary (Fig. 4) (Billard et al., 1981; Wendelaar Bonga, 1997; Garlov, 2001).
Taking into account a progressive hydrops of muscles during migration and breeding, especially at polycyclic of the salmons, meanwhile it is not less important also the participation of NpNh in maintenance of water-salt balance of an organism (Arvy et al., 1959; Robertson et al., 1961; Krayushkina, Moiseenko, 1983). And finally, at spawning process and especially after it, NpNh participate in realizing of the protective-adaptive reactions of an organism to a physiological stress, which comes during lumpsum spawning at many species especially at salmonid fishes, on our representation (Garlov, 1971, 2001; Garlov, Polenov, 1996). The beginning of an activation of PNS at spawning can be considered, on this plan, first of all as the main parameter of a primary stressful effect (Billard et al., 1981; Donaldson, 1981, 1990; Wendelaar Bonga 1997).
For checking of such a hypothesis about participation PNS in protective-adaptive reactions of an organism to a physiological stress we carried out a comparative analysis of morpho-functional mechanisms of an activation of PNS during spawning and under the experimental stress. The latter was rather adequate for through passage (anadromical) fishes, caused by contents of the adult sturgeons and stellates in hypertonic solution of cooking salt at 17, 22 and 32 ‰ salinity (Garlov, Polenov, 1971; Polenov, Garlov, 1974). The similar activation of PNS, expressed in mass release of NpNh into a blood stream in both situations is established (Fig. 5 A, B).
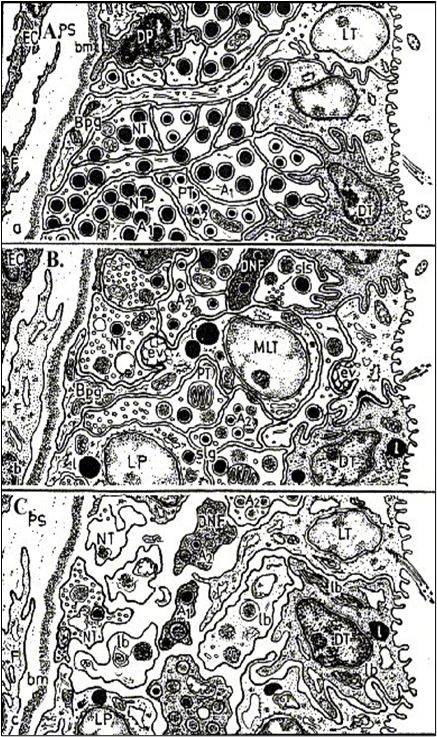
Figure 5. The scheme of the basic changes of ultrastructures of PNh in sturgeon at spawning season and after the contents in salt solution by concentration 32 ‰ (after: Polenov, Garlov, 1974). A - before spawning the neurosecretory terminals are fullfilled of elementary neurosecretory granuls and all of cell-tissue components of PNh are in an inactive functional state; B - soon after spawning, as well as in initial terms of hyperosmotic treatment (1,5 - 2 hours.), the contents of neurosecretory granuls in neurosecretory terminals is sharply reduced and the concentration of synaptic vesicles and residual granuls arises, and the glial cells and synusoidal capillares are activated too; C - by the end of the contents in hyperosmotic medium (to 6 - 10 hours, at a sublethal state of specimens) a lot of neurosecretory terminals are empty and even blasted, the contents of "dark" and degenerative neurosecretory elements arises. Conditional notations: А1, А2 - kinds of neurosecretory terminals with large and smaller neurosecretory granules, bm - basement membrane, Bpg - basement protrusion of glial cell, DNF - dark neurosecretory fiber, DP - dark pituicyte, DT - dark tanycyte, EC - endothelial cell, ev - empty vesicles, F - fibroblast, l - lipid droplet, lb - lamellar body, LP - light pituicyte, LT - light tanycyte, NT - neurosecretory terminal, PS - pericapillary space, sis - sinapsis, PT - pituicyte.
However, the level in the activation of structures and ultrastructures of PNh in experiments was expressed more brightly, and even pathological changes of ultrastructures, especially in neurosecretory terminals are established at the maximal treatment (32 ‰) (Fig. 5 C). Thus it has confirmed a well-known conclusion about direct dependence a degree of a PNS activation on intensity and duration of treatment (Polenov, Garlov, 1974; Krayushkina, Moiseenko, 1983; Garlov, Polenov, 1996;). Therefore, the activation of PNS during spawning at the investigated species we also consider as the result of a natural physiological stress and approximately estimate the degree of its manifestation, as average of a sharp stress (Billard et al., 1981; Wendelaar Bonga, 1997). It is supposed, that plenties of NpNh at a stress reduce a degree of functional activity of secretory glands - NpNh targets after their hyperfunction, providing water-salt and metabolic homeostasis of an organism and, thus, preventing "to intrinsic combustion of an organism" (Polenov et al., 1976; Garlov, Polenov, 1996).
The data on decreases of the functional activity of an interrenal tissue and so the contents of corticosteroids in blood stream after spawning at sturgeon in comparison with pre-spawning state also will be well coordinated to it (Barannikova, 1975). The activity of the thyroid gland also is reduced after spawning at the sturgeon females (Garlov, Polenov, 1971, 1996; Polenov et al., 1976).
The retaining of metabolic equilibrium of an organism at this period can be provided substantially by a bright antigonadotropic effect of the NpNh action (Garlov et. al., 1987; Garlov, Polenov, 1988; Garlov, 2001). This effect is probably realized by inhibition of the gonadoliberin secretion and also by stimulation of the ACTH secretion (synergy with CRH, particularly), direct influence of NpNh upon endocrine and generative functions of gonads (Syrotkin et. al., 1987; Garlov, Masyagina, 1998).
Moreover, by the prolonged comparative tests of the action of preparations of the isolated anterior pituitary lobe, extracts of a whole pituitary body and also an isolated posterior pituitary lobe upon the process of sexual maturity of the sturgeon and stellate breeders it was shown, that the large doses of NpNh disturb the process of an ovulation, and their highly increased and superlimited doses invoke an especially expressed antigonadotropic effect (Garlov et. al., 1987; Garlov, Polenov, 1988; Garlov, 2001). As it was shown by a data of the hystophysiological analysis, the long delay of an ovulation and resorption of sexual products in law-salinity water an average to 5-7‰ (the so-called "critical salinity") also is the effect of the slightly increased contents of NpNh in blood of the acipenserid fishes (Garlov, 1990). Moreover, it was established by a laboratory experiment on mass trade object - Rutilus rutilus volgensis (L.), that NpNh really intensify the resistibility of an organism to such a chronic slight stress (Garlov, Polenov, 1988).
On the contrary, the blockage of the PNh function after spawning at monocyclic species of fish, apparently corresponds to a state of protective inhibition or "shock" in conditions of a superstrong stress (disstress). A well-known physiological state of hyperadrenocortycism, educing at the spawning process in monocyclic of the salmons of a kind Oncorhynchus (Robertson et al, 1961; Barannikova, 1975; Donaldson, 1981), probably, also is the cause of such a blockage of the NpNh discharge (Garlov, Polenov, 1996).
It is supposed, that in a basis of desintegration of neuroendocrine mutual relations the infringement of mechanisms of a self-regulation in hypothalamo-hypophysial-adrenocortical axis plays the main role (Robertson et al., 1961; Donaldson, 1981, 1990). Thus, the consecutive disfunctions of PNS and deenergizing of NpNh functions, as a result, play especially important, probably initiation role in an acceleration of processes of aging of an organism and fast destruction of fishes, i.e., in realization of monocyclia – the major specific adaptation of a kind Oncorhynchus (Garlov, Polenov, 1996; Garlov, 2001).
The character of participation of NpNh in protective-adaptive reactions of an organism to a stress at spawning is determined, apparently, by a coordination of mutual relation of a secretory cycle (of pericarions of neurosecretory cells in praeoptic nucleus) and of an extrusive cycle (of neurosecretory terminals in PNh), which is carried out by a principle "coordination - dissociation" and has an adaptive value accordingly - on the organismal, or (at monocyclic of kinds) on populational-specific level (Garlov, 2001). The possible pathes of NpNh influence and functional role of PNS in realizing of breeding process are given on the scheme (Fig. 6).
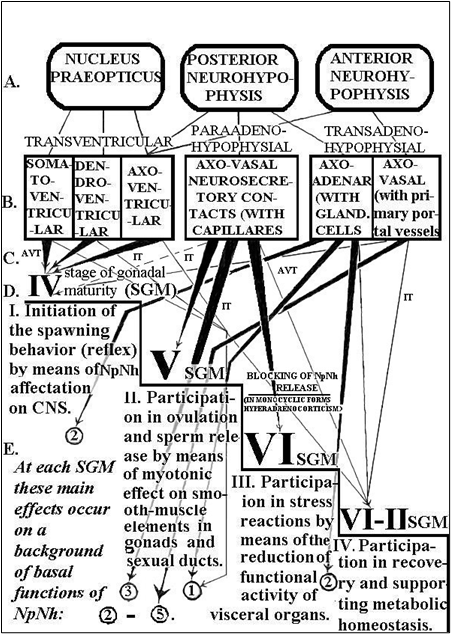
Figure 6. THE POSSIBLE TRACTS OF NONAPEPTIDE NEUROHORMONAL (NpNh) INFLUENCE AND THE FUNCTIONAL ROLE OF FISH PREOPTIC-POSTNEUROHYPOPHYSIAL NEUROSECRETORY SYSTEM (PNS) AT THE PERIOD OF SPAWNING.
Indications of structural and functional levels of NpNh release and effects (A – F): A – pathways of NpNh influence. B – forms of neurosecretory contacts (structural basis of “A”). C –the main functional significance of NpNh at every stage of gonadal maturity. (From IV till VI-II of SGM); so are marked (“¯”) the common tracts of NpNh release; so are marked: (“▼”) the main tracts of NpNh release, mostly expressed at the definite stage of gonadal maturity. D – the author’s explanation of the guessed main and basal functional roles of NpNh in spawning at every SGM.
E – the prolonged effects of NpNh at different SGM; so are marked: (“↑”). The basal functions and the main mechanisms of NpNh, common to the majority of fish species (in circles: 1-5): the development of spawning and paternal behavior by means of the affectation on central nerve system (septum, hyppocump, amigdala, particularly). Amplification of GnRH, steroid and sexual hormonal effects. The combined influence of their colaboration.
The becoming, development and conservation of conjugal staining by means of the affecting to melanotropocytes, accounting colocalization and synergism of NpNh with CRH and collaboration with monoamines. Regulation of the water-mineral equilibrium by meams of the affecting to kidney, tunica of vesica urinaria, Na-excreting cells in gills epithelium. Synergism of PNS (NpNh) with Caudal NS (Urotensins), mineralocorticoids, etc. The participation in supporting of the metabolic homeostasis by means of of the general and prolonged function of NpNh, affecting to systems of cells, tissues and organs, as a whole, by trans- and para-adenohypophysial tracts (osmoregulatory, vasopressure and reproductive functions, upon functional states of interrenal tissue, hepatocytes, etc.).
Constant influence upon all departments of the hypothalamo-hypophysial-gonadal axis, particularly:
A – on Central Nerve System by means of metabolism of MA, GnRH, upon caudal neurosecretory system functions. B – on hypophysis by stimulating the sensivity of the GTH cells to GnRH, on the secretion of several hypophysial tropic cells. C – on gonads by stimulation the smooth-muscle and follicular elements. Thus, it is shown, that the leading functional role of NpNh before spawning consists in initiation of spawning behaviour, at the beginning of spawning - in a stimulation of deducing of reproductive products in a perigastrium and external environment (in ovulation and sperm release), after spawning - in their participation in protective-adaptive reactions of an organism to a physiological stress - spawning (by their antigonadotropic action especially).
Conditional notations to all of figures: ANh - anterior neurohypophysis, AVT - arginin-vasotocin, CNS - central nervous system, CRH - corticotropin-releasing hormone (corticoliberin), GnRH - gonadotropin- releasing hormone (gonadoliberin), IT - isotocin, MA - monoamines, Nh - neurohormones, NPO - nucleus preopticus, NpNh - nonapeptide neurohormones, NS - neurosecretory, PNh - posterior neurohypophysis, SGM - stages of gonadal maturity (IV - VI-II SGM).
This scheme reflects, first of all, the process of gradual development of the participation of PNS in adaptations of an organism at different stages of gonadal maturity, as against the standard schemes of the static neuro-endocrine mutual relations in the phenomenon of a breeding (e.g. scheme in Fig. 4).
The antigonadotropic effect of NpNh influence appears to be the decisive for conservation metabolic equilibrium of an organism after spawning, as it allows to affect considerably on the character of exchange processes, by their possible "relaying" to a plastic exchange, that must be especially underlined. This mechanism apparently reflects a general principle of complex participation of NpNh in a breeding of vertebrates (combined uterotonic effect with metabolic) and it is possible to consider such a participation as the original key mechanism of a reversion or functional convertibility of exchange processes, absent at monocyclic forms (Garlov, 2002). Therefore, we consider, that the scheme in a Fig. 6, being dynamic, is perfectly constructive for the elaboration of bioengineering methods to control the reproductive process of fishes (Garlov et. al., 1987; Garlov, Polenov, 1988, Garlov, 1990).
Thus, the natural physiological stress arises in breeding process at many kinds of fishes apparently, that evidently also prove the preliminary results of the further development of researches PNS at Acipenseridae (Fig. 1B). Moreover, the degree of it expression can reach thus critical limits for an organism even at polycyclic forms, as the elimination of a part of specimens, especially of males, after first spawning already (for example salmon, capelin, some kinds of Gobiidae), is widly extended. Therefore, we consider possible to examine such stress as a state of physiological specific norm for the present specy and, in the cautious form we assume, that at maximal expression it may act as the factor of natural selection at polycyclic species, or as the major mechanism ensuring digenesis at monocyclic of kinds (Garlov, 2001).
The stress, which at all animals is characterized, first of all by disbolism of metabolic equilibrium towards catabolism, is accepted to consider as the result of the concrete stressfull agent influence, then to systematize and to characterize it upon the nature of such an influence (Billard et al., 1981; Donaldson, 1981, 1990; Wendelaar Bonga, 1997). Stress at spawning, however, at the present investigated species of fishes, also is characterized by bright catabolic changes, that is well-known.
As we believe, it is the result of complications of mutual relation of the major physiological processes in an organism, which have a diversed coursed (or even opposite) adaptive character.
The material-power resources of an organism especially at the period of a reproduction, really, provide, first of all, two (2) the main basic directions of the interconnected and cooperating biological processes, in which are realized, as we believe, the regularities of development of various levels of biological organization.
At the first (1), it is cyclic processes of functioning of an organism in conditions of his "individual" physiological norm, which corresponds to a stage of individual development. These processes, which are carried out in interests of the present concrete specimen and, as we believe, the regularities of biogenetic law mainly are realized in them. It is natural, that all these processes having the main adaptive value (or effects) at the level of an organism, are characterized by a state of metabolic equilibrium optimum (for the present stage of ontogenesis) equation of anabolic and catabolic processes ensuring constant restoration of material - power expenses of an organism.
At the second (2), in an adult organism it is occured the whole complex of cyclic reproductive processes (to which all stages of a lay of germinal foundation, differentiation and development of gonads, puberty and breeding are concerned), reflecting, first of all, function of an organism as self-reproductive system (Anohin, 1948).
These reproductive processes reflect functions of an organism as component of system of an aboveorganism level (e.g., the population and species) and have an aboveorganism adaptive value (for each of these systems). These 2 directions of processes have well-known distinctions in the character of metabolism of an organism - the processes of the first direction (1) are characterized by a plastic metabolism, the second (2) - by generative. The latters (2) are characterised by the increase of the infringements of metabolic equilibrium, caused also by a natural physiological load upon an organism, e.g., in connection with formation of reproductive system and metamorphosis, migrations, and breeding. In the ontogenesis all of these processes, in parallel proceeding and cooperating (1,2), are in dynamic equilibrium, conforming to a stage of a life cycle of specimen, and the filling up of the material - power expenses of an organism is characteristic for processes of "normal" functioning (1). However, at breeding season the equilibrium between these processes can be disturbed and the reproductive processes (2) may dominate. Such a dominance of processes of above organism adaptive character, practically only consuming material - power resources of an organism ("to export", as reproductive products and intensifying of a catabolism in connection with a breeding), can cause the conflict between these (- 1 and 2) processes in the form of the infringement of metabolic equilibrium and as the result - the exhaustion of material-power resources of an organism. We consider that stress, arising at breeding of the investigated species of fishes reflects this phenomenon of an organism level. So, the adaptive reaction of PNS, having a protective-adaptive character, also is directed to restore the metabolic equilibrium by the suppressing of a level of catabolic processes.
Thus, during breeding of fishes there is a phenomenon of predominance of processes of an above-organism adaptive character (connected with spawning process) over cyclic processes of a "normal" (or daily) functioning of an organism. "Such a predominance, possibly, is capable to cause the conflict in the form of the infringement of metabolic equilibrium and physiological stress, consequently, which degree of manifestation reflects a level of this predominance" (Garlov, 2001). And, at last, stress arising at breeding we consider to be the final link in consecutive gradual processes ensuring the phenomenon of a progressing decrease of eurybiotic (viability, in particular), developing during a puberty, migrations and breeding of fish (Gerbilsky, 1956; Hoar, Randall, 1969; Fontain, 1972; Ota et al., 1996; Garlov, 2001). As the further researches of this phenomenon are of great interest for us both in scientific and in practic (fish-farm biotechnique) aspects, we offer a brief working hypothesis about the neuroendocrine mechanisms of the such a decrease of fish viability at breeding.
First of all, NpNh play the important role in a determination of spawning and migratory behavior (Arvy et al., 1959; Fontain, 1972; Ota et al., 1996), exciting in CNS (in a complex with “sexual” hormones) the so-called: "Sexual Dominance". The initiation of spawning behaviour, caused by the NpNh influence on CNS (by vasotocin, mainly), is connected with an emotional stress especially expressed at males (Ota et al., 1996; Wong, 1997). Let's remind, that at breeding season NpNh probably promote ovulation and sperm release (mainly isotocin in Teleostei, or oxytocin-like NpNh in Acipenseridae), stimulating a contraction of smooth-muscle elements of gonads (Fig. 4) and their regulatory blood vessels (besides neuro-transporting mechanisms of regulation). NpNH also potentiate the action of “sexual” hormones (inhibit the release of Gn-RH and increase the sensitivity of gonadotropocytes to it), participate in a regulation of generative and endocrine functions of gonads, stimulate a secretion of ACTH and thyrotropin, prolactin-like hormone (Sirotkin et. al., 1987; Fig. 4, 6). The function of PNS in realization of such a "stress-spawning" is shown especially brightly in connection with the wide influence of NpNh upon a complex of visceral organs and tissues- the excretory system, smooth-muscle elements of blood vessels (in peripheric endocrine glands, digestive tract), store of fats and carbohydrates (Fig. 6). The degree of manifestation of such a reaction of PNS is in a direct dependence on the "intensity" of spawning course (and in a reverse dependance - on its multiply), being reduced to a stretched and portion spawning (Garlov, 2001).
Thus, the consecutive reactions of PNS during breeding, as one of the topic integrating chains (together with hypothalamo-hypophysial -gonadal, -adrenal, -thyroid, and -somatomedin axis), probably, reflect its participation both in the gradual decrease of a viable degree, or of the resistance of an organism (effects of NpNh influence on reproductive behaviour and on reproductive system as a whole at the moment of spawning), and in the maintenance of metabolic equilibrium (the viscerotropic effect of NpNh). We assume, that the regulation of such cyclic dynamics in changes of a viable degree of an organism in a specimem ontogenesis occurs on a background of an exhaustion of an organism as a result of migrations and spawning and is carried out by a principle of a self-regulation, basically.
Conclusion
It is necessary to take into account, that the level of physiological stability of an organism of breeders (which bright parameter is just a state of PNS), which is reduced as much as possible till the breeding season, shows also especially rigid requirements to development and application of industrial fish-farm bioengineering. So, in a basis of bioengineering of administration of fish breeding process, it should be incorporated, first of all, a principle of physiologically adequate influences (in limit of a "physiological norm" for the present species and stage of a reproductive cycle of the specimen), taking into account the main ecologo-physiological features of an initial state of a certain organism. Thus, it is necessary to combine the complex ecological approach (ecological treatments, approach empirical by origin) with "physiological" one (using the most modern hormonal preparations and other bioactive matters). And, at last, it is necessary to use the basic principle of search of the most effective influences: first of all, upon the centers of integration of a controlled function (it is natural historically, that such treatments educed on a line of a "reversal" communication, e.g.: gonads - hypophysis - hypothalamus) and at the latter - during the natural periods of the functional lability of an organism (between the seasons of a long stabilization of functions – “latent” periods), e.g. at autumn or at spring, when the resistance (or defence ability) of an organism to a various natural influences ("unspecifically") weakens, e.g. at final period of gonadal maturity (just before breeding). And, at last, a certain principle of biotechnical treatment, as a whole, its establishment, choice and usage are determined by its purpose (or the “aim”) corresponding to achievable effect, the major range of which application is necessary to present first of all (e.g., effect: physiological, fish-industrial, economic etc.).
Some of the above specified principles we also tried to take into account in our fish-farm biotechnique elaborations, of which theoretical basis was the representation about dual (alternative) functional value of PNS in neuroendocrine regulation of fish breeding (Garlov, 1971, 1990, 2001; Garlov, Polenov, 1988; Fig. 6).
References
- Anokhin P.K. 1948: Systemogenesis as the general law of evolutionary process. Bull. Exp. Biol. and Medicine, 8: 81-99 (in Russian).
- Arvy L., Fontaine M., Gabe M. 1959: La voie neurosecretrice hypothalamo-hypophysaire des Teleosteins. J. Physiol., 51: 1031-1085.
- Barannikova I.A.1975: Functional bases of migrations of fishes (in Russian). Leningrad.,
- Billard R., Bry C., Gillet C. 1981: Stress, environment and reproduction in Teleost fish. In: Stress and Fish (ed.: A. D. Pickering). Acad. Press, L., N-Y.: 185-208.
- Demski L.S., Sloan H.E. 1985: A direct magnocellular-preopticospinal pathway in goldfish: implications for control of sex behaviour. Neurosci. Lett., 55: 283-288.
- Donaldson E.M. 1981: The pituitary - interrenal axis is an indicator of stress in fish. In: Stress and Fish. L., N-Y.: 11-48.
- Donaldson E.M. 1990: Reproductive induces as measures of the effects of environmental stressors in fish. Amer. Fish. Soc. Symp., 8: 109-122.
- Eddy F.B. 1981: Effects of stress on osmotic and ionic regulation in fish. In: Stress and Fish. Acad. Press, L., N-Y.: 77-102.
- Fontain M. 1972: Endocrine glands and various forms of fish behaviour. In: Acipenserids and Problems of sturgeon fishery farm industry. (Ed.: J.J. Marti, I.A. Barannikova). Moscow: Food-processing industry: 158-166 (in Russian).
- Garlov P.E. 1971: Ecological-hystophysiological research of a neurohypophysis in Russian sturgeon. In book: Scientific Reports of Institute of Marine Biology. Vladivostok, 1: 52-56 (in Russian).
- Garlov P.E. 1976: Morpho-functional analysis of some mechanisms of neurosecretory regulation of reproduction in some fish. Proc. of the VII Internat. Sympos. on Neurosecretion
- Garlov P.E. 1990: New methods of administration of a breeding of trade fishes. Fishery Farm, 11: 43-46 (in Russian).
- Garlov P.E. 2001: Stress as a state of a specific physiological norm, arising at lumpsum spawning at some kinds of fish. In: Ecological Problems in ontogenesis of fish (physiologo-biochemical aspects). Moscow: M. University: 226-282 (in Russian).
- Garlov P.E. 2002: Mорфо-functional basis of a plasticity of neurosecretory cells. Cytology. 44: 747-767 (in Russian).
- Garlov P.E., Altufiev U.V., Polenov A.L., Dubovskaya A.V. 1987: The results of usage of a preparation of the isolated anterior pituitary lobe for a stimulation of female maturing of the Russian sturgeon (Acipenser gueldenstaedtii Brandt) and stellate (Acipenser stellatus Pallas). Questions of Ichthyology, 27: 844-851 (in Russian).
- Garlov P.E., Mosyagina M.V. 1998: Structure and function of myoid-secretory (steroid-producing) cells in follicles of an ovary of sturgeon fishes at spawning season. Cytology, 40: 502-513 (in Russian).
- Garlov P.E., Polenov A.L. 1988: The elaboration of new methods in breeding administration of trade fishes. In: Automatization of physiological Researches. Leningrad, Science: 220-236 (in Russian).
- Garlov P.E., Polenov A.L. 1996: The functional cytomorphology of the praeoptic-pituitary neurosecretory system of fishes. Cytology, 38: 275-299 (in Russian).
- Gerbilsky N.L. 1956: Specificity and problems of ecological histophysiology as one of directions in histological researches. Arhiv Anatom., Histol. and Embriology, 13: 14-21 (in Russian).
- Godwin J., Crews D., Warner R.R. 1996: Behavioral sex change in the absence of gonads in a coral reef fish. Proc. Roy. Soc. London - Ser. B: Biol. Sci., 263: 1683-1688.
- Hoar W.S., Randall D.J. 1969: Fish physiology. N.-Y., L.: Acad. Press, 2: 444p.
- Honma J., Tamura E. 1965: Studies on the Japanese chars of the genus Salvelinus. I. Seasonal changes in the endocrine glands of the Nikko-Iwana, Salvelinus leucomaenus pluvius (Hildendorf). Bull. Jap. Soc. Sci. Fisch., 31: 867-887.
- Kaul S., Vollrath L. 1974: The goldfish pituitary. II. Innervation. Cell Tiss. Res., 154: 231-249.
- Knight W.R., Knight J.N. 1996: Telencephalon removal does not disrupt the vasotocin-induced spawning reflex in killifish, fundulus heteroclitus. J. Exper. Zool., 276: 296-300.
- Krayushkina L.S., Moiseenko S.N. 1983: Hypothalamo-hypophysial neurosecretory system of the ecologically various forms of sturgeon fishes during adaptation to hypertonic medium. In: Questions of Neuroendocrinology. Leningrad, Len. University: 93-103 (in Russian).
- Moiseeva E.B. 1975: Histophysiology of the hypothalamo-hypophysial system of sea fishes in connection with spawning. Proceedings of VNIRO, 111: 106-124 (in Russian).
- Moitra K.S., Medya Ch. B. 1980: Morpho-histology of the hypothalamo-neurohypophysial system in relation to gonadal maturation in Cirrinus mrigala (Ham.), a freshwater indian carp. Anat. Anz., 148: 409-421.
- Oganesyan S.A. 1986: Change of morpho-functional state of hypothalamo-pituitary neurosecretory system of capelin in Barents Sea in connection with migration and breeding. In: Ecology and biological productivity of Barents Sea. Iss.: Polar institute of fishery and oceanography. Murmansk: 239-240.
- Ota Y., Ando H., Ban M. 1996: Sexually different expression of neurohypophysial hormone genes in the preoptic nucleus of pre-spawning chum salmon. Zool. Sci., 13: 593-601.
- Oztan N. 1966: The structure of the hypothalamic neurosecretory cells of Zoarces viviparus L. under the conditions of constant dark and light during the reproductive cycle. Z. Zellforsch., 75: 66-82.
- Peter R.E. 1986: Vertebrate neurohormonal systems. In: Vertebrate endocrinology. Fundamentals and biomedical implications. (Ed.: Pickering A.). N.Y.: Acad. Press, 1: 57-104.
- Pickford G.E., Strecker E.L. 1977: The spawning reflex response of the killifish, Fundulus heteroclitus: isotocin is relatively inactive in comparison with arginine vasotocin. Gen. Compar. Endocrinol., 32: 132-137.
- Polenov A.L., Belenky M.A., Garlov P.E., Konstantinova M.S. 1976: The Hypothalamo-Hypophysial System in Acipenseridae VI. The proximal neurosecretory contact region. Cell Tiss. Res., 170: 129-144.
- Polenov A.L., Garlov P.E. 1971: The hypothalamo-hypophysial system in Acipenseridae. I. Ultrastructural organization of large neurosecretory terminals (Herring bodies) and axoventricular contacts. Z. Zellforsch., 116: 349-374.
- Polenov A.L., Garlov P.E. 1973: The hypothalamo-hypophysial system in Acipenseridae. III. The Neurohypophysis of Acipenser güldenstädti Brandt and Acipenser stellatus Pallas. Z. Zellforsch., 136: 461-477.
- Polenov A.L., Garlov P.E. 1974: The hypothalamo-hypophysial system in Acipenseridae. IV. The functional morphology of the neurohypophysis of Acipenser gueldenstaedti Brandt and Acipenser stellatus Pallas after exposure to different salinities. Z. Zellforsch., 148: 259-275.
- Polenov A.L., Garlov P.E., Konstantinova M.S., Belenky M.A. 1972: The hypothalamo-hypophysial system in Acipenseridae. II. Adrenergic structures of the hypophysial neurointermediate complex. Z. Zellforsch., 128: 470-481.
- Polenov A.L., Garlov P.E., Koryakina E.D., Faleeva T.I. 1976: The Hypothalamo-Hypophysial System in Acipenseridae V. Ecological-histophysiological analysis of the neurohypophysis of the female sturgeon Acipenser gueldenstaedtii Brandt during up-stream migration and after spawning. Cell Tiss. Res., 170: 113-128.
- Polenov A.L., Jakovleva I.V., Garlov P.E., Pavlovic M. 1973: Neuro-endocrine mechanisms of realisation of adaptive reactions in Acipenseridae to changes of water salinity. First Europ. Ichthyological Congress, Paper Abstr. Sarajevo, Jugoslavija (21-29, IX, 1973): P. 120.
- Polenov A.L., Kornienko G.G., Belenky M.A. 1986: The hypothalamo-hypophysial system of the wild carp, Cyprinus carpio L. III.Changes in the anterior and posterior neurohypophysis during spawning. Z. mikrosk. -anat. Forsch., 100: 990-1006.
- Polenov A.L., Pavlovich M., Garlov P.E. 1972: Preoptic nucleus and neurohypophysis in sturgeons (Acipenser güldenstädti Brandt) at different stages of their life cycle and in experiments. Gen. Compar. Endocrinol. (Abstracts of papers of the VI Congr. of Europ. Compar. Endocrinol.), 18: 617.
- Prakash M.M., Shrivastava S.S., Belsare D.K. 1984: Сyclical changes in the hypothalamo-hypophysial-gonadal system in Notopterus chilata (Ham.). Z. mikrosk. -anat. Forsch., 98: 225-240.
- Prasada Rao P.D. 1970: The hypophysis of two freshwater teleosts, Labeo calbasu (Ham.) and Puntius sarana (Ham.). Zool. Anz., 184: 335-348.
- Ramadan A.A., Ezrat A.A., Meguid N.A, Khadre S.E.M., Abdel-Aziz S.N. 1988: Cyclic histological changes in the pituitary gland of Sparus aurata in correlation to the gonadal cycle. Folia morphol., 36: 113-124.
- Robertson O.H., Krupp M.A., Favour C.B., Hane S., Thomas S.F. 1961: Physiological changes occuring in the blood of the Pacific salmon (Oncorhynchus tschawytscha) accompaning sexual maturation and spawning. Endocrinology, 67: 746-773.
- Schiebler T.H., Hartmann J. 1963: Histologische und histochemische Untersuchungen am neurosekretorische Zwischenhirn-Hypophysensys- tem von Teleostiern unter normalen und experimentellen Bedingungen. Z. Zellforsch., 60: 89-146.
- Sirotkin A.V., Polenov A.L., Garlov P.E. 1987: Participation of nonapeptide neurohormones in a regulation of a reproductive function in animals // Results of Science and Engineering. Мoscow. The Edition of State Committee of Science and Engineering (GKNT), All-Union research institute of the scientific and technical information (VINITI), Academy of Sciences of USSR. Neuroendocrine mechanisms of the control of reproduction of wild and agricultural animals, 15: 21-30.
- Sokol H.N. 1961: Cytological changes in the teleost pituitary associated with the reproductive cycle. J. Morphol., 109: 219-236.
- Terlou M., Ekengren B., Hiemstra K. 1978: Localization of monoamine in the forebrain of two salmonid species, with special reference to the hypothalamo-hypophysial system. Cell Tiss. Res., 190: 417-434.
- Thripathi I.M., Pandey K. 1986: Hypothalamo-hypophysial neurosecretory system of the mud eel Amphipnous cuchia (Ham.). Arch. Biol., 97: 267-277.
- Wendelaar Bonga S.E. 1997: The stress response in fish [Review]. Physiol. Rev., 77: 591-625.
- Wong C.J.H. 1997: Afferent and efferent connections of the diencephalic prepacemaker nucleus in the weakly electric fish, Eigenmannia virescens - interactions between the electromotor system and the neuroendocrine axis. J. Compar. Neurol. 383: 18-41.


