Current Issue : Article / Volume 3, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-5038/014
Collection, Generation and Identification of Bone Marrow Mesenchymal Stem Cells (Bm-Mscs), Taken from Mice
1Physiology, Department of Basic Medical Sciences, Medicine Faculty, Selcuk University, Konya, Türkiye.
Kemal Yuce, *
Kemal Yuce, (2024). Collection, Generation and Identification of Bone Marrow Mesenchymal Stem Cells (Bm-Mscs), Taken from Mice. International Journal of Stem cells and Medicine. 3(1). DOI: 10.58489/2836-5038/014
© 2024 Kemal Yuce, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 20-08-2024
- Accepted Date: 23-08-2024
- Published Date: 23-08-2024
Abstract
Mesenchymal stem cells are multipotent cells capable of secreting various factors, including macrophage colony-stimulating factor, transforming growth factor-beta, vascular endothelial growth factor, insulin-like growth factor-1, and exosomes. These cells can differentiate into various cell types and migrate to damaged areas, making them valuable in both preclinical and clinical research. BM-MSCs have potential therapeutic applications for conditions such as heart and immune system disorders, multiple sclerosis, Alzheimerâs disease, spinal cord injuries, bone fractures, and cartilage damage. This study focused on isolating BM-MSCs from mice, producing, and identifying them. For identification, flow cytometry was used. Cells were removed from petri dishes, treated with 1 ml of staining buffer, and incubated with an antibody or isotype control. The analysis was conducted using a BD Facs Aria III instrument. The results showed that the BM-MSCs had the following surface marker expression levels: CD45- at 99.8%, CD105+ at 97.6%, CD29+ at 88.3%, and SCA-1+ at 93%. Overall, MSC research is a prominent and evolving field both nationally and globally, offering significant opportunities for preclinical and clinical applications. Thus, the processes of harvesting, producing, and identifying stem cells are crucial.
Introduction
Mesenchymal stem cells are multipotent and capable of secreting various hormones, growth factors, and exosomes. They can also differentiate into multiple cell types, making them valuable for both preclinical and clinical applications [1]. MSCs can migrate to and engraft in lesioned areas, demonstrating their potential for therapeutic use. Research has shown that transplantation of human MSCs into rodent models of neurodegenerative diseases can provide several benefits, including neurotrophic factor-induced protection, enhanced neurogenesis, regulation of inflammation, and clearance of abnormal proteins [2]. In summary, the mechanisms by which MSCs help reduce nervous system damage are as follows: A) Protection of neural tissues from apoptosis and destruction; B) Differentiation of MSCs into functional neurons; C) Enhancement of synaptic integrity, including promotion of synaptogenesis [3]. MSCs are not only useful for studying nervous system diseases but also have applications in connective tissue disorders, such as bone, cartilage, and tendon diseases, as well as immune system disorders and cancer. This study aims to isolate BM-MSCs from mice, produce these cells, and identify them using flow cytometry.
Materials And Methods
Cells were detached from petri dishes using trypsinization and washed with DPBS. After counting, 2 ml of staining buffer was added to the cells. The mixture was then centrifuged at 1200 rpm for 5 minutes, and the staining buffer was discarded. To block Fc receptors, the cells were incubated with 1 ml of staining buffer containing 0.5% BSA for 5 minutes. Following another centrifugation at 1200 rpm for 5 minutes, the supernatant was discarded, and the remaining liquid and pellet were combined. A 100 µl aliquot of this mixture was transferred to a new tube.
Next, 10 µl of antibody or isotype control (R&D Systems) was added to the tube, and the mixture was incubated in the dark for 1 hour. After incubation, 1 ml of staining buffer was added, and the sample was centrifuged at 1200 rpm for 5 minutes. The supernatant was discarded, and 1 ml of staining buffer was added to the pellet. The cells were then pipetted and analyzed using a BD Facs Aria III instrument.
Statistics
Statistical analysis could not be performed for this study, and statistical methods are not applicable. The reliability of the study is determined by evaluating the presence of three positive markers (CD105+, CD29+, and SCA-1+) and one negative marker (CD45-), each serving as a distinct indicator of BM-MSCSs.
Results
Flow cytometry was used to assess the purity of BM-MSCs. The analysis utilized a flow cytometry kit to measure the expression of specific markers: CD45-, CD29+, SCA-1+, and CD105+. The results indicated a high purity of BM-MSCs, with CD45- showing a negativity rate of 99.8%. The expression levels for CD105+, CD29+, and SCA-1+ were 97.6%, 88.3%, and 93.5%, respectively (Figure 1)
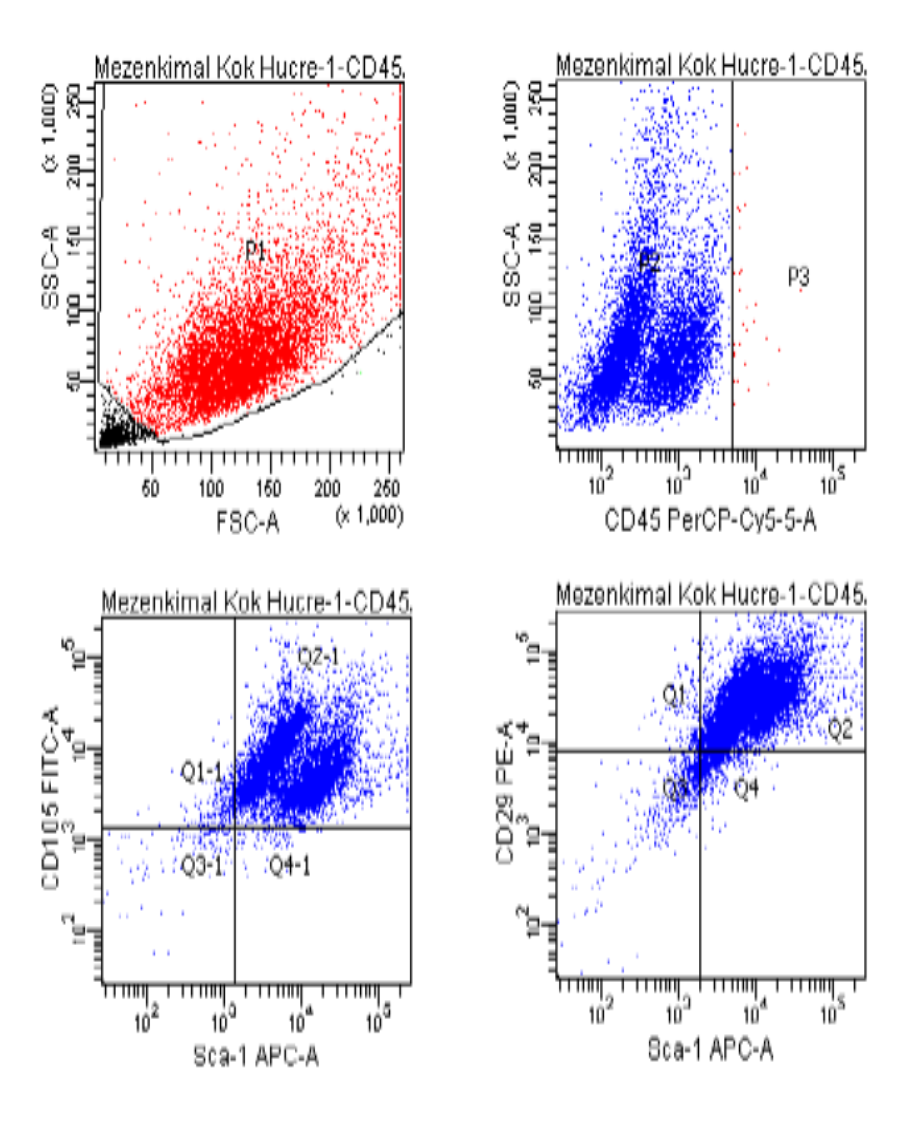
Fig 1. Flow cytometry result.
Fig 1. (Continuation). Flow cytometry result.
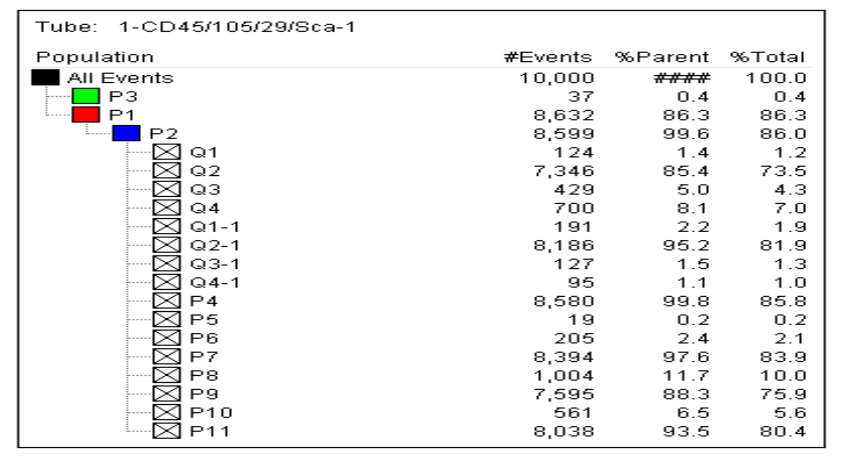
Fig 1. (Continuation). Flow cytometry result.
Discussion
In previous studies, the negativity rate for CD45- in mesenchymal stem cells (MSCs) ranged from 97% to 99%. The positivity rates for CD105+ varied between 86% and 99%, CD29+ ranged from 75% to 99%, and the Sca-1+ positivity rate decreased to as low as 86%. In our study, the CD45- negativity rate was found to be 99.8%. The positivity rates for CD105+, CD29+, and Sca-1+ were 97.6%, 88.3%, and 93%, respectively [4-8] (Figure 1). The results of our study are consistent with findings from previous research. As highlighted, adhering to international standards for producing MSCs allows for their application in a variety of areas, including the treatment of vertebral marrow damage and wound healing in diabetic patients. Therefore, this study is both important and significant in advancing the use of MSCs.
Conclusion
Mesenchymal stem cells are a current issue in the world and have a wide range of experimental and clinical uses. This study provides information to physicians and researchers about how to produce mesenchymal stem cells. Therefore, the current study is important and noteworthy.
Ethics Committee Approval
The current study complies with ethical standards, which is a prerequisite. The Laboratory Animals Ethics Board of Selçuk University Experimental Medicine Research and Application Center gave its approval to the research protocol (Ethics Approval Number: 2016-43).
Conflicts of interest
I declare no conflicts of interest.
Financial Disclosure
This study was supported by the Selcuk University Academic Staff Training Program (ÖYP) Coordination Unit (2013-ÖYP-088)
References
- Wang, J. A. (2009). Mesenchymal Stem Cells for the Heart: From Bench to Bedside.
- Volkman, R., & Offen, D. (2017). Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem cells, 35(8), 1867-1880.
- Gesundheit, B., Ashwood, P., Keating, A., Naor, D., Melamed, M., & Rosenzweig, J. P. (2015). Therapeutic properties of mesenchymal stem cells for autism spectrum disorders. Medical Hypotheses, 84(3), 169-177.
- Hakim, R., Covacu, R., Zachariadis, V., Frostell, A., Sankavaram, S. R., Brundin, L., & Svensson, M. (2019). Mesenchymal stem cells transplanted into spinal cord injury adopt immune cell-like characteristics. Stem Cell Research & Therapy, 10, 1-16.
- Huang, S., Xu, L., Sun, Y., Wu, T., Wang, K., & Li, G. (2015). An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Journal of orthopaedic translation, 3(1), 26-33.
- Miao, Z., Jin, J., Chen, L., Zhu, J., Huang, W., Zhao, J., ... & Zhang, X. (2006). Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell biology international, 30(9), 681-687.
- Shi, L., Li, B., Zhang, B., Zhen, C., Zhou, J., & Tang, S. (2019). Mouse embryonic palatal mesenchymal cells maintain stemness through the PTEN-Akt-mTOR autophagic pathway. Stem Cell Research & Therapy, 10, 1-14.
- Zhang, Y., Ma, L., Su, Y., Su, L., Lan, X., Wu, D., ... & Ji, X. (2019). Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Research, 1725, 146432.


