Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-5038/003
Violations of the Ultrastructure of Neurons in The Cerebral Cortex of Rats with Total Cerebral Ischemia
Grodno State Medical University, Grodno, Republic of Belarus.
Correspondng Author: Bon E. I
Citation: E.I. Bon, Maksimovich N.E., Zimatkin S.M., Ostrovskaya O.B., Smirnov V.Yu., Nosovich M.A., Khrapovitskaya K.A., (2022). Violations of the Ultrastructure of Neurons in The Cerebral Cortex of Rats with Total Cerebral Ischemia. International Journal of Stem cells and Medicine. 1(1). DOI: 10.58489/2836-5038/003.
Copyright: © 2022 E.I. Bon, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-11-04, Received Date: 2022-11-04, Published Date: 2022-12-26
Abstract Keywords: neurons, parietal cortex, hippocampus, ischemia.
Abstract
The ultrastructural characteristics of neuronal organelles are important indicators of the degree of brain damage during ischemic exposure, reflecting the severity of compensation, which necessitates the study of changes in the ultrastructure of brain neurons. With total cerebral ischemia, organelles are disorganized both in the neurons of the parietal cortex and in the neurons of the hippocampus, causing profound disturbances in energy production and metabolism.
Introduction
With cerebral ischemia, a chain of pathogenetic disorders develops in its structures, among which one of the leading ones is energy deficiency, which leads to the development of cellular pathology due to violations of homeostasis, enzyme activity, membrane integrity and energy pumps. Under conditions of cerebral ischemia, synaptic transmission mechanisms are selectively disrupted, which contributes to disruption of autoregulation of local blood flow, the development of vasospasm, increased platelet aggregation and the development of intravascular stasis, aggravating hypoxia and increasing energy deficiency [1, 2, 4, 5].
The ultrastructural characteristics of neuronal organelles are important indicators of the degree of brain damage during ischemic exposure, reflecting the severity of compensation, which necessitates the study of changes in the ultrastructure of brain neurons.
Currently, there are no data on the severity of these disorders depending on the type of ischemic injury and its severity. These studies are relevant, as they allow us to study the nature of disorders of brain neurons at the ultrastructural level, depending on the severity of ischemia.
Materials and methods of research
The experiments were carried out on 12 male outbred white rats weighing 260±20 g in compliance with the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010 on the protection of animals used for scientific purposes.
Сerebral ischemia modeling was carried out under conditions of intravenous thiopental anesthesia (40-50 mg/kg).
The studies used models of total cerebral ischemia. The control group included 4 males; the ischemia group included 8 males.
Total cerebral ischemia was modeled by decapitation of animals. The brain sampling was carried out 1 hour and 24 hours after decapitation. The control group consisted of sham-operated rats of similar sex and weight. Electron microscopic studies were performed in the parietal cortex and hippocampus of the brain of rats.
Immediately after decapitation and quick extraction of the brain, sections were cut out with a blade and placed in 1% osmium fixative in Millonig's buffer (pH=7.4) for 2 hours at a temperature of 4°C.
Next, the sections were washed in a mixture of Millonig's buffer (20 ml) and sucrose (900 mg), dehydrated in alcohols of increasing concentration, a mixture of alcohol and acetone, pure acetone; passed through a mixture of resins (araldite M + araldite H + dibutyl phthalate + DMR-30) and acetone and embedded in a mixture of resins.
Semi-thin sections (about 350 nm thick) were made on an MT-7000 ultramicrotome (RMC, USA), stained with methylene blue, and the sections necessary for study were cut out with a blade.
Ultrathin sections (about 35 nm thick) were made on the same ultramicrotome, assembled on support grids, and counterstained with uranium acetate and lead citrate. To do this, the meshes with sections were dipped into a drop of uranyl acetate and kept for 20 minutes in the dark at room temperature, then washed in 3 portions of bidistilled water for 5 seconds and contrasted with lead citrate for 8 minutes, washed in 3 portions of bidistilled water for 5 seconds.
The resulting preparations were studied under a JEM-1011 electron microscope (JEOL, Japan) and photographed with an Olympus MegaView III digital camera (Olympus Soft Imaging Solutions, Germany).
Morphometry of ultrastructures was performed using the Image Warp program (Bit Flow, USA). Density, size, shape of organelles, density and length of mitochondrial cristae, sizes of lysosomes and their density, the number of endoplasmic reticulum-bound and free ribosomes were measured, and their ratio was calculated.
To prevent a systematic measurement error, brain samples from the compared control and experimental groups of animals were studied under the same conditions.
As a result of the research, quantitative continuous data were obtained. Since the experiment used small samples that had a non-normal distribution, the analysis was performed by nonparametric statistics using the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). The data are presented as Me (LQ; UQ), where Me is the median, LQ is the value of the lower quartile; UQ is the value of the upper quartile. Differences between groups were considered significant at p<0>
Research results
The characteristics of changes in the ultrastructure of neurons in the cerebral cortex of rats with total cerebral ischemia are shown in (Table 1).
When studying the ultrastructure of neurons, there was no change in the number of mitochondria in the cytoplasm of rat neurons during 1-hour cerebral ischemia (p>0.05), but there was a change in their size and shape.
Table 1. Parameters of ultramicroscopic morphometry of neuron organelles in the parietal cortex and hippocampus of rats with total cerebral ischemia (TCI), Me (LQ;UQ).
Index | Parietal cortex | Hippocampus | |||||
Control | TCI 1 hour | TCI 1 day | Control | TCI 1 hour | TCI 1 day | ||
Mitochondria | density | 1,8(1,7;2,2) | 1,6(1,5;1,7) | 1,2(1,0;1,3) *+ | 2,1(1,7;2,2) | 1,7(1,6;1,7) | 1,3(1,1;1,4) *+ |
area, µm2 | 0,26 (0,17;0,37) | 0,11(0,06;0,12) * | 0,10(0,08;0,12) * | 0,21(0,17;0,26) | 0,10(0,08;0,12) * | 0,09(0,08;0,11) * | |
Form factor, unit | 0,63(0,61;0,72) | 0,87(0,78;0,96)* | 0,93(0,83;0,96) | 0,71(0,60;0,75)* | 0,86(0,84;0,95)* | 0,89(0,85;0,93)* | |
Elongation factor, units | 3,8(3,5;4,1) | 1,4(1,3;1,5) | 1,4(1,3;1,5) | 2,1(1,9;2,5) | 1,5(1,3;1,7)* | 1,4(1,2;1,5)* | |
mitochondrial crist density | 76(71;82) | 45(41;51)* | 40(37;45)* | 62(59;72) | 45(41;52)* | 40(37;45)* | |
length of mitochondrial cristae / µm2 | 12(10;15) | 5(4;6)* | 4(3;6)* | 13(12;18) | 6(4;7)* | 5(4;7)* | |
Ribosomes | amount /µm2 | 20,9(19,3;22,7) | 17,6(16,5;18,7) * | 17,4(16,8;19,2) | 20(18,1;22,8) | 16,2(14,6;19,4) * | 16,3(15,0;18,7)* |
free / µm2 | 4,7(4,1;5,8) | 16,0(15,3;16,8)* | 16,5(16,0;17,8)* | 6,0(4,8;7,3) | 14,5(13,3;17,3)* | 15,5(14,3;17,5)* | |
bound /µm2 | 16,2(15,2;16,9) | 1,6(1,2;1,9)* | 0,9(0,8;1,4)* | 14,0(13,3;15,5) | 1,7(1,3;2,1)* | 0,8(0,7;1,2)* | |
ratio of bound and free ribosomes | 3,4 | 0,1* | 0,05* | 2,3 | 0,1* | 0,05* | |
Lysosomes
| density | 0,4(0,3;0,5) | 0(0;0,1) | 0(0;0) | 0,5(0,4;0,6) | 0(0;0,1) | 0(0;0) |
area, µm2 | 0,02(0,01;0,03) | - | - | 0,03(0,02;0,04) | - | - | |
area, µm2 | 0,02(0,01;0,03) | - | - | 0,03(0,02;0,04) | - | - |
Note. * - p<0>TCI 1 hour" group.
With 1-hour TCI, compared with the indicators in the control group, in the parietal cortex, the mitochondrial area decreased by 58% (p<0>
The density of mitochondrial cristae and their length decreased in the parietal cortex by 40% (p<0>
With TCI with an ischemic period of 1 day, there was a decrease in mitochondrial density: by 33% in the parietal cortex (p<0>
At the same time, in the parietal cortex, the area of mitochondria decreased by 59% (p<0>
The density of mitochondrial cristae and their length were less in the parietal cortex by 47% (p<0>0.05), figures 1.2.

Electrongram. Magnification 50000
Figure 1 - Mitochondria of neurons in the parietal cortex of the rats of the control group.
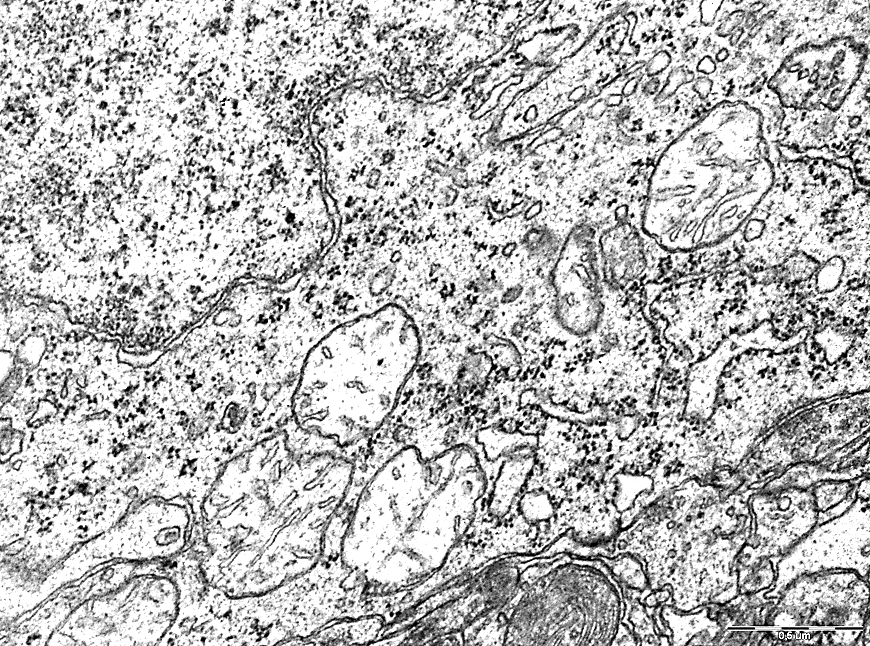
Electrongram. Magnification 50000
Figure 2 - Mitochondria of neurons in the parietal cortex of rats with 1-hour total cerebral ischemia.
Compared with the indicators in the "control" group, the number of free ribosomes during 1-hour TCI increased by 71% in the parietal cortex (p<0>
With 1-day TCI, the number of free ribosomes in the cytoplasm of neurons of the parietal cortex and hippocampus increased by 72% (p<0>0.05). The ratio of the number of bound and free ribosomes decreased from 3.4 in the control group to 0.05 in the parietal cortex (p<0>
There was a significant decrease in the density of lysosomes. With 1-hour TCI, only single lysosomes were observed in the cytoplasm of neurons (p<0>
Along with this, compared with the control group, there was a change in the expansion and vacuolization of the cisterns of the granular endoplasmic reticulum in TCI with a predominance of free ribosomes in the cytoplasm, Figures 3,4. Electrongram. Magnification 50000
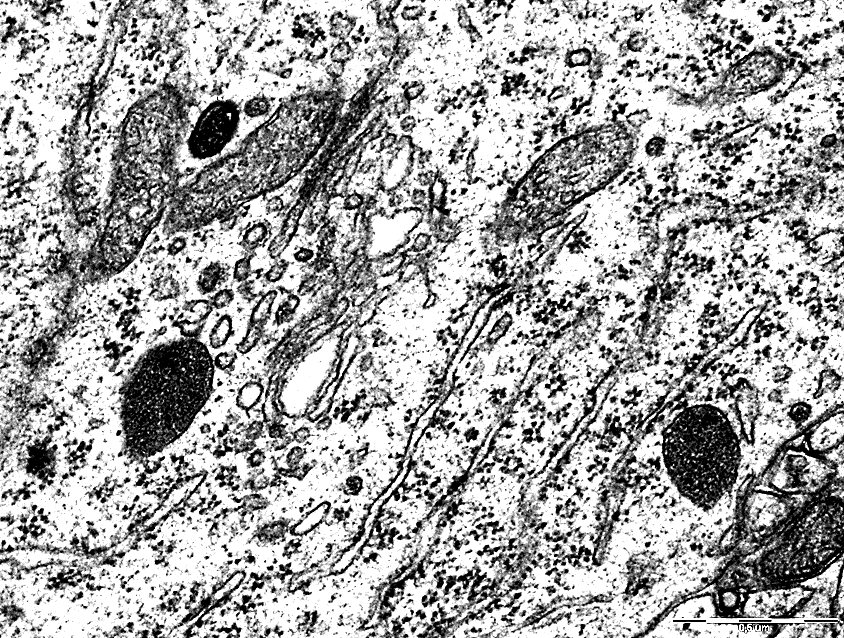
Figure 3 - Endoplasmic reticulum of neurons in the parietal cortex of the control group rats.
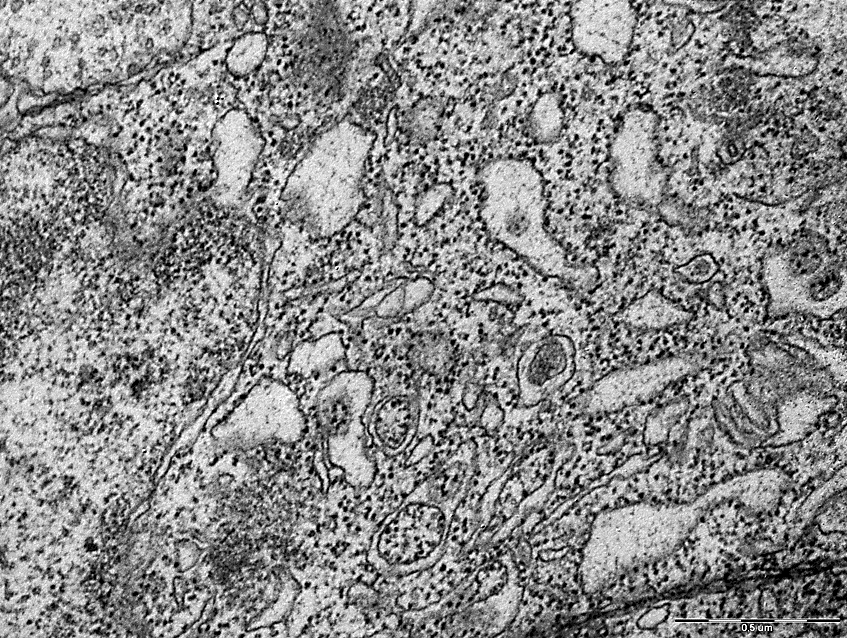
Electrongram. Magnification 50000
Figure 4 - Endoplasmic network of neurons in the parietal cortex of rats with 1-hour total cerebral ischemia
Deformation and vacuolization of the Golgi complex was also noted in comparison with the control group, figures 5,6.
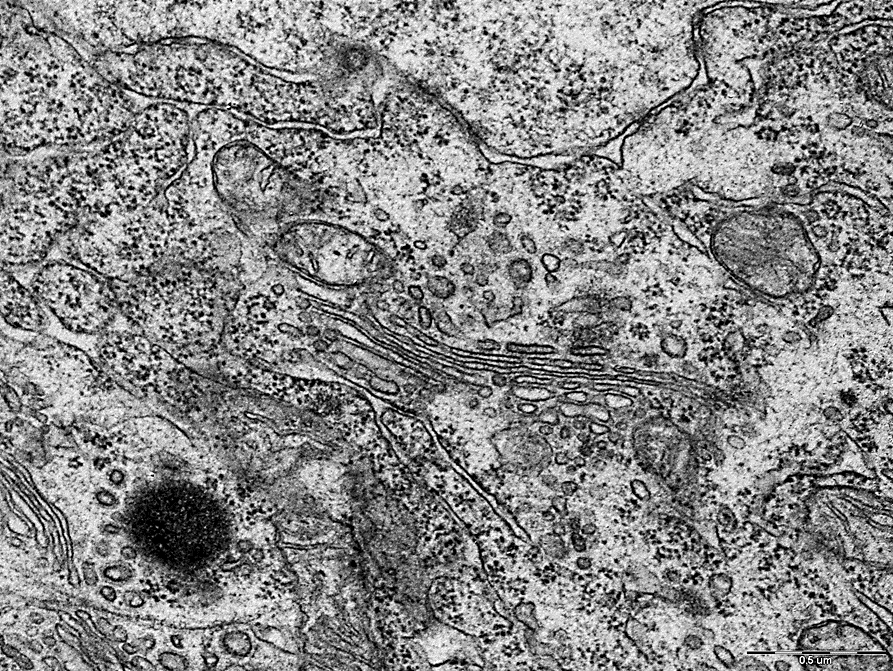
Electrongram. Magnification 50000
Figure 5– Golgi complex of neurons in the pyramidal layer of field CA1 of the hippocampus of rats in the control group.
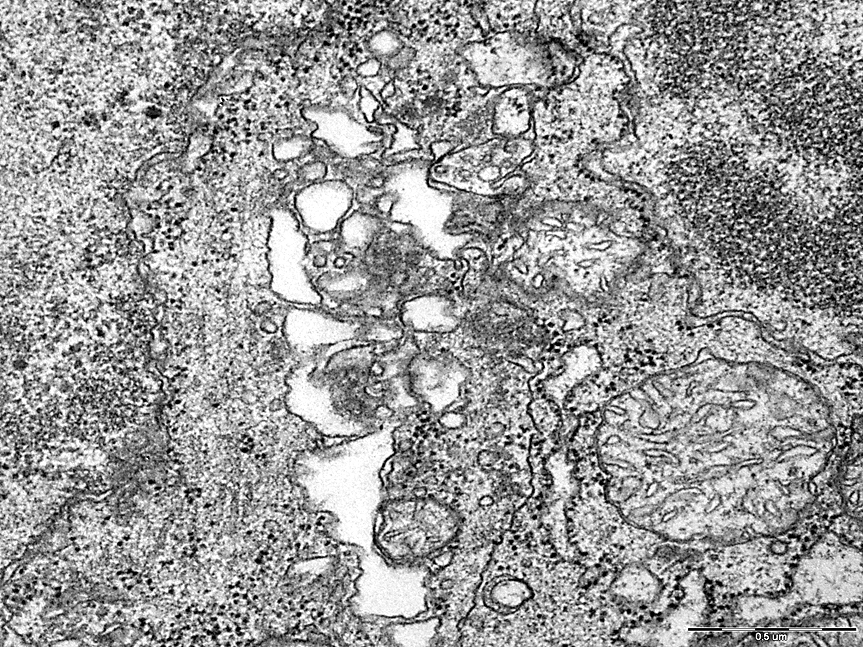
Electrongram. Magnification 50000
Figure 6 - Golgi complex of neurons in the pyramidal layer of the field CA1 of the hippocampus of rats with 1-day total cerebral ischemia.
Ultramicroscopic morphometry of organelles revealed no differences between the groups "TCI 1 hour" and "TCI 1 day" (p>0.05), except for a decrease in the density of mitochondria with 1-day TCI, which indicates a progressive disorder of these organelles, leading to an increase in energy shortages.
Discussion of the obtained data. According to the literature data, changes in the mitochondria of neurons are noted during cerebral hypoxia: swelling, destruction of their cristae, uneven distribution in the cytoplasm, which indicates a violation of the energy supply of neurons [1,2,3,4,6]. The destruction of the outer and inner membranes leads to a decrease in the number of mitochondria due to a violation of the permeability for cations, which leads to edema and rupture. Active swelling of mitochondria is associated with the operation of the electron transport chain. There is an expansion of the tubules of the granular and smooth endoplasmic reticulum, a change in their structure, disintegration into small granules, the appearance of large vacuoles and loops [5,6,7,8]. Free ribosomes predominate, forming extensive clusters. This is one of the manifestations of the energy deficiency that is formed in the cell, since the fixation of ribosomes to the membranes of the rough endoplasmic reticulum with the participation of the ribophorin protein is an energy-dependent process. Under conditions of ischemic exposure, the neuron reduces protein export and seeks to direct it for internal needs. Disorganization of the granular endoplasmic reticulum leads to the accumulation of newly formed proteins in the cytoplasm. As cellular hypoxia and acidosis increase, protein denaturation increases [9]. Due to the accumulation of water, the expansion of the cisterns of the Golgi complex and their partial fragmentation are noted [7, 10]. The average number of lysosomes increases, their sizes increase. There is an exit into the cytoplasm and activation of hydrolytic enzymes of lysosomes - cathepsin, ribonuclease, acid phosphatase, deoxyribonuclease, hyaluronidase and other enzymes, which leads to autolysis [4,5,8,9,11].
The data obtained correspond to the literature data, supplementing them with the features of the development of organelle disorganization during ischemia of varying severity.
Conclusions
Thus, with total cerebral ischemia, organelles are disorganized both in the neurons of the parietal cortex and in the neurons of the hippocampus, causing profound disturbances in energy production and metabolism.
Funding source
This study was not supported by any external sources of funding.
Competing interests
The authors declare that they have no competing interests.
Author contribution
All authors confirm the compliance of their authorship, according to international ICMJE criteria (all authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published).
References
- Hong A, Bing Zh, Xunming J. Mitochondrial quality control in acute ischemic stroke // J Cereb Blood Flow Metab. 2021. Vol. 41, N. 12. P. 3157-3170. DOI: 10.1177/0271678X211046992.
- Nicholls D.G., Budd S.L. Mitochondria and neuronal survival // Physiol Rev. 2000. Vol. 80, N. 315, P. 60. DOI: 10.1152/physrev.2000.80.1.315.
- Bon' E.I., Zimatkin S.M. Dark brain neurons // Morphology. - 2017. - No. 6. - P.81–86. DOI: 10.1007/s11055-018-0648-7.
- Nina J. Solenski, Charles G. diPierro, Patricia A. Trimmer, Aij-Li Kwan and Gregory A. Helms. Ultrastructural Changes of Neuronal Mitochondria After Transient and Permanent Cerebral Ischemia. Stroke. Vol. 33, No. 3. Р. 816–824. DOI: 10.1161/hs0302.104541.
- Mattson M.P., Clumsee C., Yu Z.F. Apoptotic and antiapoptotic mechanisms in stroke // Cell Tissue Re. 2000. Vol. 301. P. 173–87. DOI: 10.1007/s004419900154.
- Zhi H, Niya N, Qiongxiu Zh, Seyed Esmaeil Kh. Mitochondria as a therapeutic target for ischemic stroke // Free Radic Biol Med. 2020. Vol.146. P. 45-58. DOI: 10.1016/j.freeradbiomed.2019.11.005.
- Garcia J.H., Lossinsky A.S., Kauffman F.C., Conger K.A. Neuronal ischemic injury: light microscopy, ultrastructure and biochemistry // Acta Neuropathologica. 1978. Vol. 43. P. 15–24. DOI: 10.1007/BF00685002.
- Snider B.J., Gottron F.J., Choi D.W. Apoptosis and necrosis in cerebrovascular disease // Ann N Y Acad Sci. – 2004. – V. 893. P. 243–253. DOI: 10.1111/j.1749-6632.1999.tb07829.x
- Colbourne F., Sutherland G.R., Auer R.N. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia // J. Neurosci. – 1999. – V. 19. – P. 4200–4210. DOI: 10.1523/JNEUROSCI.19-11-04200.1999
- Belenichev I.F. Neuroprotection and neuroplasticity: monograph. K .: Polygraph Plus LLC, 2014. - 512 p. DOI: 10.1038/s42003-019-0521-4
- Zimatkin, S.M. Maternal alcohol intake induces dramatic ultrastructural changes in offspring brain cortex neurons / S.M. Zimatkin, E.I. Bon // Brain and Nerves. - 2017. Vol. 1. P. 1-4. DOI: 10.15761/JBN.1000105


