Article In Press : Article / Volume 3, Issue 2
- IMAGE ARTICLE | DOI:
- https://doi.org/10.58489/2836-2330/024
Challenges for Pacemaker Packaging and Clinical Trial in Microgravity (Mars and Moon)
Packaging and Polymer Science Technologist (PG), Principal Consultants, Bioxytran Inc.MA, Boston, USA.
Anupam Chanda*
Anupam Chanda, (2024). Challenges for Pacemaker Packaging and Clinical Trial in Microgravity (Mars and Moon). Journal of Clinical and Medical Reviews. 3(2); DOI: 10.58489/2836-2330/024
© 2024 Anupam Chanda, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 31-07-2024
- Accepted Date: 10-08-2024
- Published Date: 31-08-2024
Abstract
Introduction
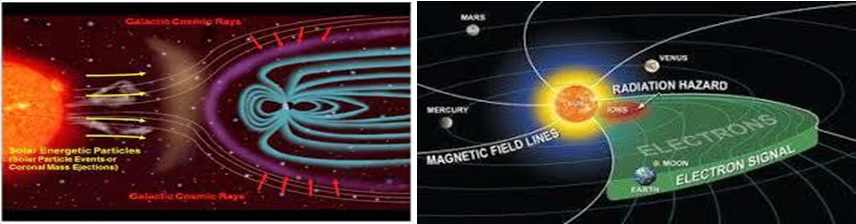
pace travel involves significant gravitational and radiation stress on both cellular and systemic physiology which directly effect in cardiovascular changes. Packaging Technologists have significant roles to pack safely Pacemakers that during transit damage will not occur. As we all know microgravity environment is too extreme to survive without any special pacemaker. Pacemakers already designed and several Clinical trials have been carried out in different type of patients in microgravity environments. Our Aim is to prepare pacemakers in microgravity environment and all Raw materials will be available over there. We know petrochemicals not yet discovered in microgravity, so there is no possibilities to get “wide range of Polymers” those are using in pacemakers. So, we have to depend on glass and Aluminium.
Please see below shown how “Patient will sit” inside the space craft while Clinical trial activities will carry out.

Pacemaker Packaging Design
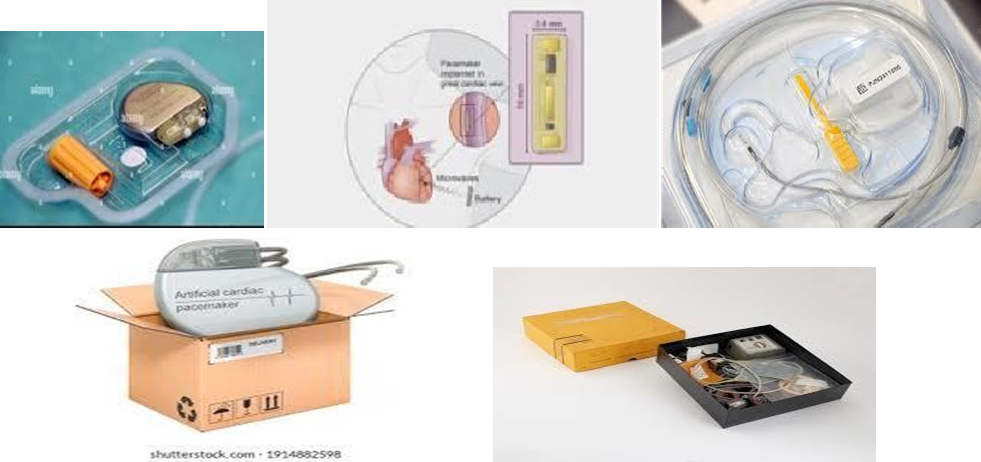
Following Packaging materials are using wildly for packaging of Pacemakers
- HIPS Tray with multiple pockets.
- Folding cartons
- 3 ply or Micro-fluted corrugated Boxes.
- PS Tray
- Aluminium Tray (For microgravity Environments)
Following precautions need to take designing the Pack
- Pacemaker should tightly fit to the cavity (Primary pack), slightest shaking is not accepted.
- Vacuum pack is preferred to avoid moisture.
- Secondary and Tertiary packs should be durable enough to protect the Primary pack.
Pacemaker Packaging USFDA Regulations
This device is falling under Class 3 category.- This is falling in 21CFR, Section # 820.130.
- Under 21 CFR 806, Medical Device Correction and Removals, manufacturers and importers are required to make a report to FDA of any correction or removal of a medical device(s) if the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Act caused by the manufacturers.
- Recall of Pacemakers or any other Medical Devices
- Manufacturers voluntarily recall the medical devices under 21 CFR 7.
- FDA may issue a recall order to the manufacturer under 21 CFR 810, when manufacturers are failed to recall the Medical devices.
Sterilization of Pacemakers Essential Guidance to follow
- AAMI TIR104:2022: Sterile processing of medical devicesand their environment.
- AAMI TIR17:2017/(R)2020 : FDA guidancedocument on managing materials compatibility. Compatibility of materials subjectedto
- sterilization, addresses materials compatibility for polymers, ceramics, metals, and other materials used in healthcare products subject to sterilization modalities such as ethylene oxide, steam, and radiation.
- ANSI/AAMI PC76:2021: Guidance documentfor patients safety with pacemakers and ICDs exposedto magnetic resonance imaging.
- ANSI/AAMI/ISO TIR10974: 2012: Guidance document for standard applies to “transvenous pacemaker, ICD [implantable cardioverter-defibrillator], and CRT [cardiac resynchronization therapy] systems intended to be used in patients who undergo a magnetic resonance scan,” and provides testing guidelines that can be used to demonstrate that a device conformsto its MR Conditional [MagneticResonance Conditional] labelling.
Sterilization Dose Sterilization dose assurance level of10-6 or less for radiation sterilization of health care products.
Radiation Sterilization for Pacemaker Pack - Radiation sterilization utilises ionising radiation to sterilise medical devices, electron beam sterilisation has also use of radiation for sterilization, Gamma rays from a cobalt-60 (60Co) isotope source or machine-generated accelerated electrons are used. Gamma irradiation is the most popular form of radiation sterilization and is used when materials are sensitive to the high temperature of autoclaving but compatible with ionising radiation. Exposure is achieved when the packages are transported around an exposed 60Co source for a defined period of time.
- The most commonly validated dose used to sterilize medical devices is 25 kGy.7
- The bactericidal effect of gamma irradiation is dependent on oxidation of biological tissue. It is a simple, rapid and efficacious method of sterilisation. However, high capital costs are a major disadvantage. Most metal-based medical devices can be sterilised using radiation. However, sterilisation of biomedical polymers using gamma irradiation is known to result in physical changes, including embrittlement, discolouration,15–17 odour generation, stiffening,18,19 softening, an increase or decrease in melt temperature20 and decrease in molecular weight.11,13,18
- The two mechanisms involved in these changes are chain scission and cross-linking and mechanical properties including tensile strength, elastic modulus, impact strength, shear strength and elongation may be affected. Decrease in fatigue strength have also been reported in some biomedical polymers following gamma irradiation.19 Embrittlement may occur and crystallinity may also change.21 Gamma irradiation has also been reported to magnify surface defects in some biomedical polymers6 10,21 and Fourier transform infrared (FTIR) studies12,19,22 have indicated significant oxidation of the surface of some biomedical polymers.
Gamma irradiation also has undesirable consequences due to the potential production of toxic degradation products such as 4,4’-methylenedianiline (MDA) that can be produced when a high-molecular-weight polyurethane material decomposes as a consequence of irradiation.6,14,23 Cytotoxic effects have also been reported after contact with gamma-irradiated polyurethane samples believed to result from the effect of a low-molecular-weight by- product.24
Pacemaker Packaging Validation Flow chart
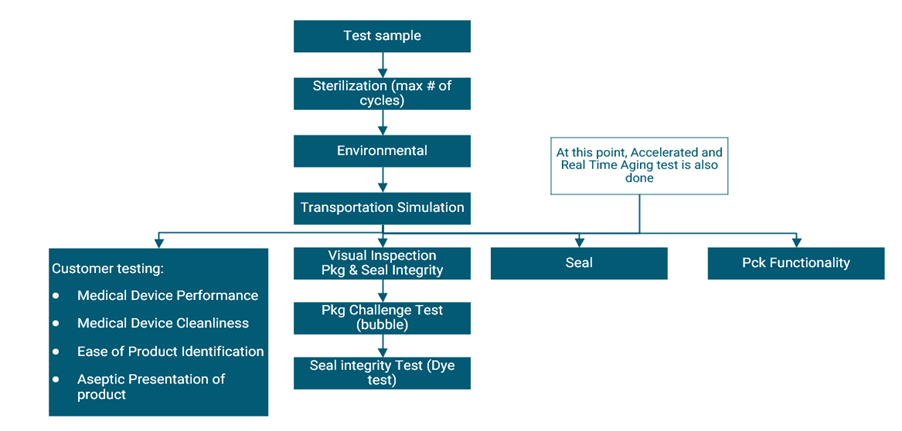
Essential Guide lines from FDA
- ISO 16628:2022, Anaesthetic and respiratory equipment - Tracheobronchial tubes
- ISO 10993-2:2022, Biological Evaluation of medical devices - Part 2: Animal welfare requirements
- ISO 14708-2:2019, Implants for surgery - Active implantable medical devices - Part 2: Cardiac pacemakers
- ISO 14708-6:2019, Implants for surgery - Active implantable medical devices - Part 6: Particular requirements for active implantable medical devices intended to treat tachyarrhythmia (including implantable defibrillators)
- ISO 81060-3, Non-invasive sphygmomanometers - Part 3: Clinical investigation of continuous automated measurement type
- ANSI/AAMI PC76:2021, Active implantable medical devices - Requirements and test protocols for safety of patients with pacemakers and ICDs exposed to magnetic resonance imaging
- ANSI/AAMI PB70:2022, Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities
- ISO/TS 16775:2021, Packaging for terminally sterilized medical devices - Guidance on the application of ISO 11607-1 and ISO 11607-2
- ISO 22441:2022, Sterilization of health care products - Low temperature vaporized hydrogen peroxide - Requirements for the development, validation and routine control of a sterilization process for medical devices
- AAMI TIR104:2022, Guidance on transferring health care products between radiation sterilization sources
- AAMI TIR17:2017/(R)2020, Compatibility of materials subjected to sterilization
- IEC 60601-2-52:2015, Medical electrical equipment - Part 2-52: Particular requirements for the basic safety and essential performance of medical beds
- IEC 60601-2-39:2018, Medical electrical equipment Part 2-39: Particular requirements for basic safety and essential performance of peritoneal dialysis equipment
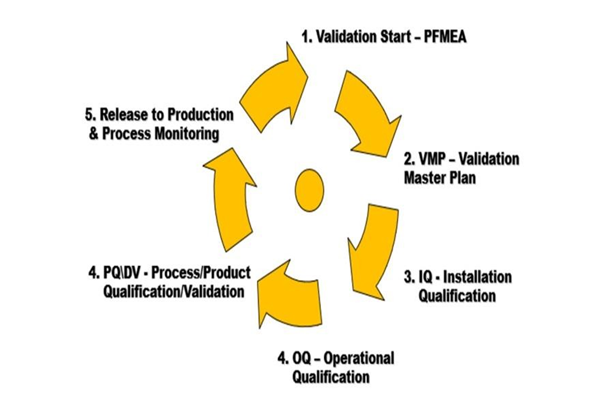
Validation Protocol
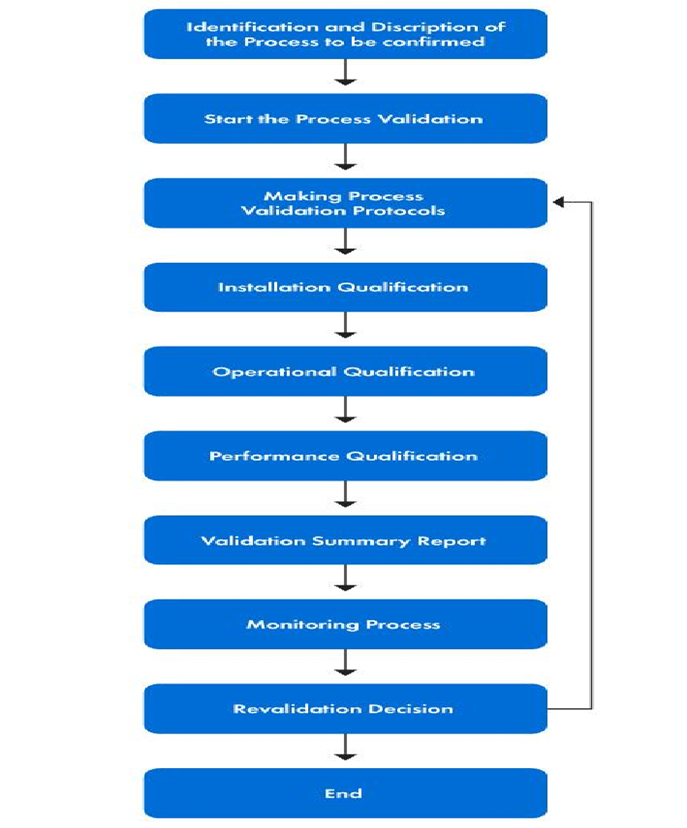
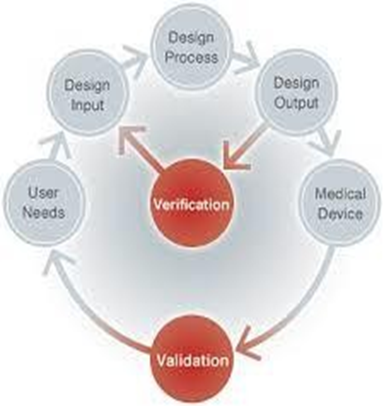
Design Verification
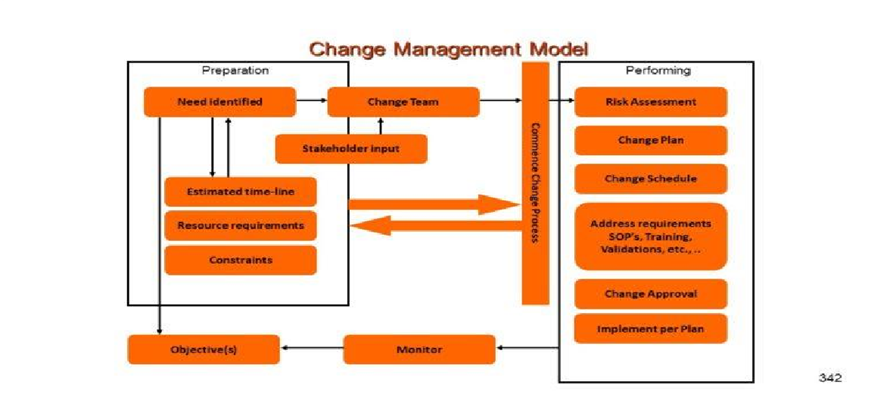
Pacemaker Packaging Process Validation Flow chart
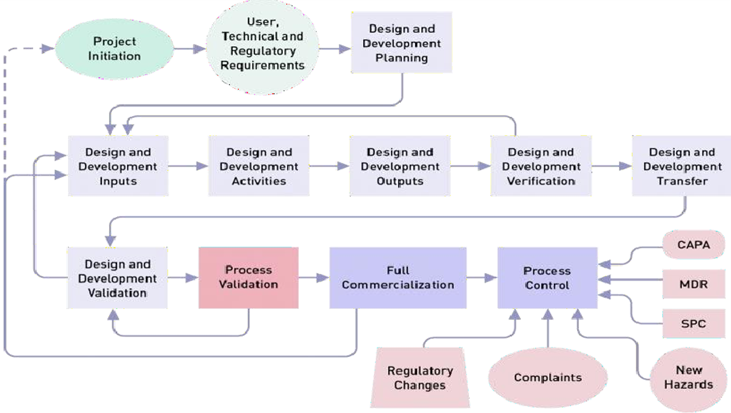
Pacemaker Packaging Validation & Verification
Heart Beat
There are differences between normal pacemakers using in Earth and going to use in MARS and MOON.
Please see below most important points:
- Earth Pacemakers have slow heartbeat whereas due to Low gravity in Microgravity environments heartbeat as high.
- Chances of Cardiac failures are more in Microgravity due to low pressure.
- Infuture all Pacemakers are going to make in Microgravity without “Polymers” since those are not available.
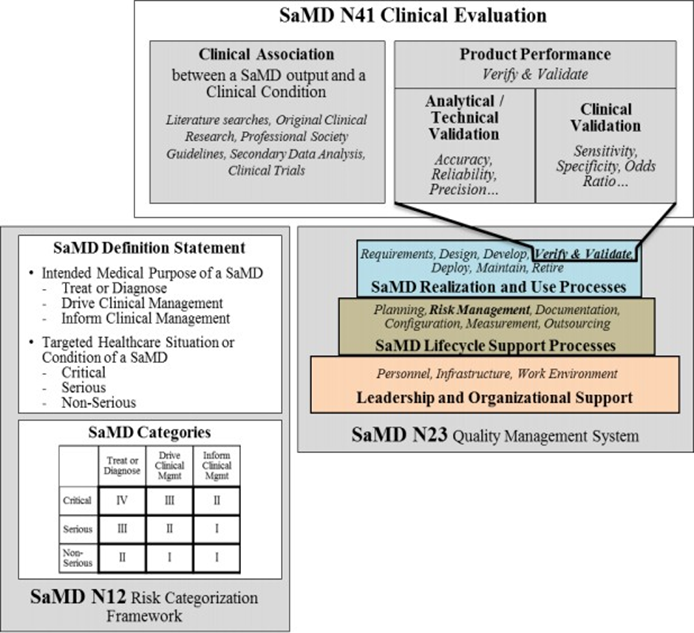
Clinical trials are possible to carry out on the Microgravity environments.
Medical Device (Pacemaker) Clinical Trial Flow
References
- https://spinoff.nasa.gov/Spinoff2019/hm_1.html
- https://array.aami.org/content/news/fda-recognizes-aami-guidance-pacemakers-radiation-sterilization-and-more
- https://prorelixresearch.com/software-as-medical-device-clinical-trials-as-per-us-fda/


