Current Issue : Article / Volume 3, Issue 2
- RESEARCH ARTICLE | DOI:
- https://doi.org/10.58489/2836-2330/020
Echocardiogram Diagnosis of Complex Congenital Cardiac Malformations
1MBBS, DCH (CMC&H, Vellore, S. India) (DNB Ped), Consultant Paediatrician, Grace Specialist Clinic, #4 Church Street, 4th Main, Horumavu Main Road, Bansawadi, Bangalore 560043, India.
Grace Lalana Christopher*
Grace Lalana Christopher (2024). Echocardiogram Diagnosis of Complex Congenital Cardiac Malformations. Journal of Clinical and Medical Reviews. 3(2); DOI: 10.58489/2836-2330/020
© 2024 Grace Lalana Christopher, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 17-05-2024
- Accepted Date: 01-06-2024
- Published Date: 06-06-2024
Echocardiogram, Complex Congenital Cardiac Malformations, Age, Sex-wise
Abstract
Background: Complex congenital cardiac malformations rarely reported in literature
Objective: Study was undertaken to define complex congenital cardiac malformations by Echocardiogram
Materials and Methods: Prospective study included 101 children aged 2 to14 years, both male and female during March 2004 to February 2005 and July 2006 who attended in-patients & outpatient departments at Vyedhi Institute of Medical Sciences and Research Centre (VIMS & RC) and Sri Jayadeva Institute of Cardio-vascular Sciences and Research of Cardiology (SJICR), Bangalore, India. Clinical diagnosis was confirmed by relevant investigations and Echocardiography.
Results: Complex congenital cardiac malformations presented in 16 (15.99%) of 101 children raising the total number of cardiac defects to 121. Majority 77 (76.2%) acyanotic cardiac malformations comprised of 29 (28.72%) Atrial Septal Defects (ASD) had 11 complex cardiac malformations. Three cases ASD + Partial Anomalous Pulmonary Venous Drainage (PAPVC), 2 cases ASD + Patent Ductus Arteriosus (PDA) + Mitral Valve prolapse (MVP), 2 cases ASD + Pulmonary Stenosis (PS,) 1 case ASD + PDA + PS, 1 case ASD + Ventricular Septal Defect (VSD), 1 case ASD + Coarctation of Aorta (CoA) and 1 case ASD + MVP. Next 23 (22.77%) VSD had 4 complex cardiac malformation cases, 3 cases of VSD + Aortic Stenosis (AS) of which one case had dextrocardia and 1 case VSD + PS. Followed by AS 8 (7.9%) with 1 case of AS + CoA, another case had VSD + AS. Among 24 (23.76%) cyanotic defects, TOF18 (17.8%) was the commonest, other rare variants included Double Outlet Right Ventricle (DORV) 4(3.96%) one case with dextrocardia. Pentalogy of Fallot 1 (0.99%) and Triology of Fallot 1 (0.99%). The age distribution revealed most 30(29.8%) were young children aged 2-4 years with overall male predominance Male:Female::1.3:1, observed contrasted to female predominance noted in ASD and TOF, M:F::1:2 and M:F::1:1.3 respectively
Conclusion:
Complex congenital cardiac malformations presented in 16(15.99%) of 101 children raising the total number of cardiac defects to 121
Introduction
The incidence of congenital cardiac malformations ranges from 4 to 50‰ live birth but account for 30% of congenital malformations and is the leading cause of neonatal congenital malformations deaths (1-4). While an accurate diagnosis by echocardiogram screening of live births increased incidence to 53.2‰ classified as 26.6‰ severe, 3.5‰ moderate, 5.4‰ mild and 17.7‰ clinically non-significant cardiac malformations. The commonest cardiac malformation was VSD 17.3 ‰, ASD 6.2‰, PDA 1.3‰, Tetralogy of Fallot (TOF) 0.4‰, Single Ventricle (SV) O.4‰, Atrio-ventricular septal defect (AVSD) 0.2‰, and DORV 0.2‰ [5]. By fourth month incidence decreased to 19.5‰ due to spontaneous closure of muscular VSD, one-third by one month, three-fourths by three months and virtually all by one year. Most PDA closes by one month, usually within the first week of life upto one year [5-7]. Clinically symptoms manifests after birth and 40-50% are diagnosed in first week of life, remaining 50-60% by one month or may sometime not manifest at all [3,4].
Incidence of critical congenital cardiac malformations ranges from 1-2‰ up to 12.1‰ live births; require surgery or other procedures within first year of life for survival [8.9]. Screening by pulse oximetry within 48 hours of birth with SpO2 <90>
Advances in palliative and corrective surgery have markedly improved outlook with more patients surviving to adulthood as fatal cardiac defects are now treated successfully. Genetic factors play a role in the cause of these defects but the pattern of inheritance is generally unclear, in fact in all but about 3% of cases the underlying cause of the abnormality cannot be identified [3,4,12].
Cardiac embryonic development starts in the fourth week of pregnancy, the tube gradually increases in length over the next four weeks and loops to form right and left side separated by septum that develops into upper and lower chambers with four valves to keep blood flowing forward and out to all parts of the body. As fetal circulation is independent of lungs for respiration, there’s remarkable degree of cardiac malformations without causing difficulty that assumes importance in the newborn as the two sides of the circulation – lungs and circulatory system are separate and function on their own. Ligation of the umbilical cord at birth initiates the first breath and lung expansion increases pressure in the left side of the heart resulting in closure of foramen ovale in the atrial septum, ductus arteriousus and ductous venosis with left to right shunt and smooth transition to extrauterine life [3,4,13].
Materials and Methods
Prospective study included 101 children attending out-patient and inpatient departments at VIMS & RC and SJICR during 13 months period from March 2004 to February 2005 including July 2006. Inclusion criteria children aged 2-14 years, both male and female with congenital cardiac malformations detected clinically and confirmed by investigations having had no prior palliative or definitive surgical treatment.
Exclusion criteria included other major congenital malformation or associated chromosomal anomalies or syndromes. Chronic debilitating diseases such as tuberculosis or chronic neurological disorders such as cerebral palsy, mental retardation, paralytic poliomyelitis, Endocrinal diseases such as hypothyroidism, diabetes mellitus, chronic renal diseases and obvious metabolic disorders e.g. mucopolysaccaridoses etc.
Diagnosis was based on clinical examination, laboratory investigations such as Haematocrit- Hb, Peripheral blood smear etc, Chest radiography, Electrocardiogram, Echocardiography, Real-Time Doppler echocardiography and cardiac catherization.
Results
Majority 77(76.2%) of 101 children had acyanotic cardiac malformations, mainly ASD 29(287%) followed by VSD 23(22.7%), AS 8(7.9%), PS 8(7.9%), PDA 7(6.9%), CoA 1(0.99%) and CTGA 1(0.99%). Cyanotic malformations 24(23.76%) comprised of TOF 18(17.8%), DORV 4(3.96%), Pentalogy of Fallot 1(0.99%) and Triology of Fallot 1(0.99%). Distribution of 101 children with congenital cardiac malformations by number and percentage seen in Table 1 and Fig. 1 respectively
Table 1: Distribution of Congenital Cardiac Malformations in 101 children
| Congenital Cardiac malformations | Number |
| Atrial septal defect (ASD) | 29 |
| Ventricular septal defect (VSD) | 23 |
| Tetralogy of Fallot (TOF) | 18 |
| Aortic stenosis (AS) | 8 |
| Pulmonic stenosis (PS) | 8 |
| Patent ductus arterious (PDA) | 7 |
| Double outlet Right Ventricle (DORV) | 4 |
| Coarctation of aorta (CoA) | 1 |
| Corrected Transposition of Great Arteries (CTGA) | 1 |
| Pentalogy of Fallot | 1 |
| Triology of Fallot | 1 |
| Total | 101 |
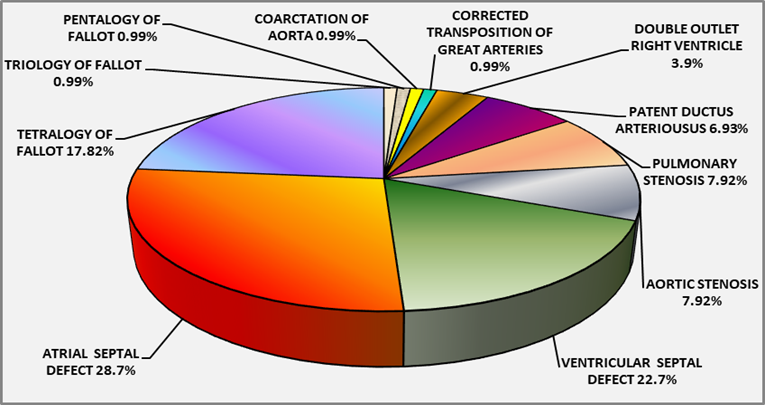
Fig 1: Percentage distribution of 101 children with Congenital Cardiac Malformations
Complex or multiple congenital cardiac malformations occurred in 16 (15.99%) of 101 children, raising the total number to 121 confirmed by echocardiography. Among 29 (28.7%) ASD cases, there were 11 complex cardiac malformations, included 3 cases of PAPVC + ASD, 2 cases PS + ASD, 2 cases MVP + PDA + ASD, 1 case of PDA + PS + ASD, 1 case of ASD + subaortic VSD, 1 case ASD + CoA and 1 case ASD + MVP, while 18/29 (62%) were isolated ASD cases.
Majority 25/29 (86.2%) ASD were ostium secundum defects, remaining 3/29 (10.3%) were sinus venosus and 1/29 (3.4%) large endocardial cushion defect. There were two cases of dual ASDs with septal aneurysm. The sizes of atrial septal defect ranged from small 3 mm to large 2.5 cm endocardial cushion defect. Next 23 (22.7%) VSD presented with 4 complex cardiac malformations, 3 cases VSD + AS of which one had dextrocardia and 1 case VSD + PS. Majority 19/23 (82.6%) VSD cases were isolated.
The sizes of the VSD ranged from small 4 mm up to 2 cm almost like a single ventricle. Most 16/23 (69.5%) VSD were sub aortic, 4/23 (17.3%) peri-membraneous and 3/23 (13%) subpulmonic defects, of which 7/23 (30.4%) had septal aneurysm.
AS 8/101 (7.92%) had one complex cardiac malformations case of AS + CoA and another case had VSD, remaining 7/101 (6.9%) were isolated AS cases, isolated PS 8(7.9%), isolated PDA 7(6.9%) and one each of CoA and Corrected Transposition of great arteries(CTGA) 1(0.99%) respectively. Echocardiogram diagnosis of complex congenital cardiac malformations seen in Table 2
Table 2: Echocardiogram Diagnosis of Complex Congenital Cardiac Malformations
ECHOCARDIOGRAM DIAGNOSIS | Number (n=101) | Percentage % |
| ACYANOTIC CARDIAC MALFORMATIONS | ||
| Atrial septal defect (ASD) Isolated | 18 | 17.8 |
| ASD+PAPVCS | 3 | 2.97 |
| ASD+PDA +MVP | 2 | 1.98 |
| ASD+PS | 2 | 1.98 |
| ASD+PDA+PS | 1 | 0.99 |
| ASD+VSD | 1 | 0.99 |
| ASD+CoA | 1 | 0.99 |
| ASD+MVP | 1 | 0.99 |
| TOTAL ASD | 29 | 28.72 |
| Ventricular septal defect (VSD) Isolated | 19 | 18.81 |
| VSD+AS | 2 | 1.98 |
| VSD+AS+Dextrocardia | 1 | 0.99 |
| VSD+PS | 1 | 0.99 |
| TOTAL VSD | 23 | 22.77 |
| Aortic stenosis (AS) Isolated | 7 | 6.93 |
| AS+CoA | 1 | 0.99 |
| TOTAL AS | 8 | 7.92 |
| Pulmonary stenosis (PS) Isolated | 8 | 7.92 |
| Patent ductus arteriosus (PDA) Isolated | 7 | 6.93 |
| Coarctation of Aorta (CoA) Isolated | 1 | 0.99 |
| Corrected transposition of great arteries (CTGA) | 1 | 0.99 |
| TOTAL ACYANOTIC DEFECTS | 77 | 76.21 |
| CYANOTIC CARDIAC MALFORMATIONS | ||
| Tetralogy of Fallot (TOF) | 18 | 17.82 |
| Double outlet right ventricle + VSD | 4 | 3.96 |
| Pentalogy of Fallot +Dextrocadia | 1 | 0.99 |
| Triology of Fallot | 1 | 0.99 |
| TOTAL CYANOTIC DEFECTS | 24 | 23.76 |
Age distribution revealed most 30 (29.8%) children were aged 2-4 years, incidence of cardiac malformations decreased with increasing age to minimal 9 (8.9%) at 8-10 years with slight increase 15 (14.8%) 10-12 years to 11(10.8%) 12-14 years. Percentage distribution of children with congenital cardiac malformations in age groups 2-14 years seen in Fig 2.
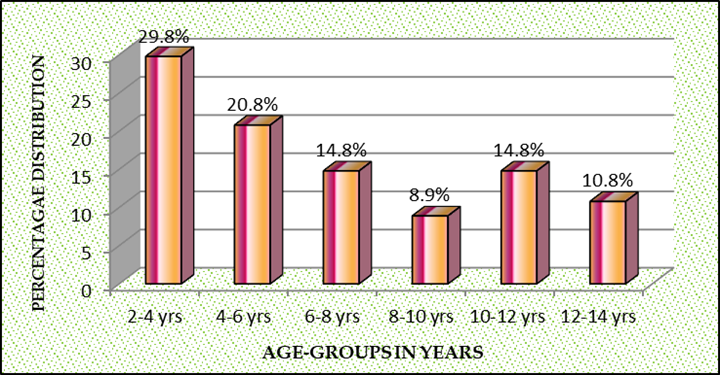
Fig 2: Percentage Age-wise distribution of 101 children
Among total 70 children with ASD, VSD and TOF. ASD 29 /70 (41%) was the commonest followed by VSD 23/70 (32.8%) and TOF 18/70 (25.7%). However majority 25/70 (35.7%) children were in the young age group 2-4 years, next 14/70 (20%) aged 4-6 years and 10/70 (14.2%) were both in age groups 6-8 and 8-10 years, decreasing thereafter to 6/70 (8.5%) in 10-12 years age group to 5/70 (7.1%) in 12-14 years adolescents. Percentage distribution in children 2-4 years was ASD 40%, TOF 32% and VSD 28%. While 4-6 olds majority had VSD 43% which declined to 10% in 6–8-year-olds due to spontaneous closure of muscular VSD but peaked to 60% in adolescents 12-14 years probably due to large defects. However ASD peaked to 50% and 60% in 6-8 and 8-10 years respectively complicated by pulmonary hypertension with large defects, children aged 10-12 years had 50% distribution of ASD and VSD, latter peaked 60% in12-14 years. Cyanotic TOF varied from 32% in 2–4-year-olds to 40% in 6–8-year-olds and thereafter declined above 8 years. The number and percentage age wise distribution in 70 children with ASD, VSD and TOF seen in Table 3 and Fig. 3.
Table 3: Age-wise distribution in children with ASD, VSD & TOF
| Age-wise Distribution of Children with ASD, VSD & TOF | |||||||
| Cardiac Malformations | 2-4 years No. | 4-6 years No. | 6-8 years No. | 8-10 years No. | 10-12 years No. | 12-14 years No. | Total No. |
| ASD | 10 | 4 | 5 | 6 | 3 | 1 | 29 |
| VSD | 7 | 6 | 1 | 3 | 3 | 3 | 23 |
| TOF | 8 | 4 | 4 | 1 | 0 | 1 | 18 |
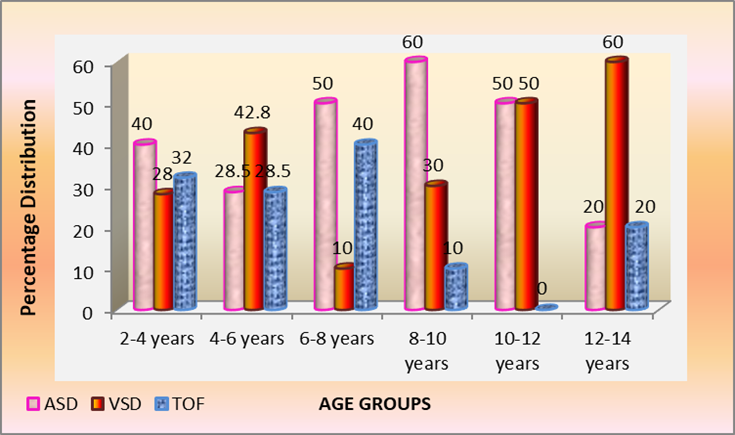
Fig 3: Age-wise percentage distribution of children with ASD, VSD & TOF
Sex distribution revealed overall male predominance M:F::1.3:1, specific congenital cardiac malformations revealed, AS M: F:: 6:1, VSD M:F::3:1, PS ratio M:F::1.7:1, PDA ratio M:F::1.2:1, CTGA and CoA M:F::1:0 with an equal sex distribution in DORV M:F::1:1 contrasted to female predominance in children with ASD M:F::1:2 and TOF ratio of M:F::1:1.3. Sex-wise distribution among 101 children by number and percentage seen in Table 4 and Fig.4
Table 4: Sex-wise distribution of children with Congenial Cardiac Malformations
| CARDIAC DEFECTS | MALE | FEMALE | TOTAL |
| ASD | 11 | 18 | 29 |
| VSD | 17 | 6 | 23 |
| AS | 7 | 1 | 8 |
| PS | 5 | 3 | 8 |
| PDA | 4 | 3 | 7 |
| TOF | 8 | 10 | 18 |
| DORV | 2 | 2 | 4 |
| CoA | 1 | 0 | 1 |
| CTGA | 1 | 0 | 1 |
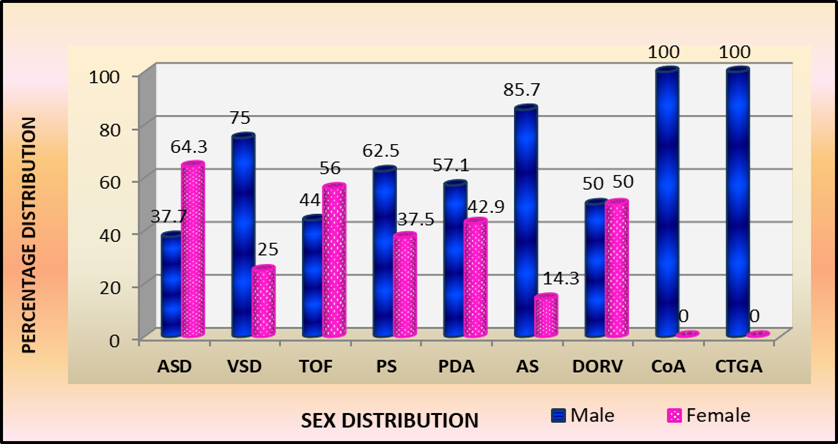
Fig 4: Sex wise percentage distribution of children with specific Congenital Cardiac Malformations
Discussion
The reported incidence of VSD 1:280, ASD 1:1062, Atrioventricular canal (AVC) 1:1372, TOF 1:2375, TGA 1:3175, HypoplastIc Left heart syndrome (HLHS) 1:3759, DORV 1:6369, PA 1:7576, Ebstein’s anomaly 1:8772, Truncus Ateriosus (TA) 1:9346 and Tricuspid atresia is 1:12658 [1,2]. In the present study of congenital cardiac malformations in 101 children, majority 76.2% were acyanotic defects comprised of ASD 28.7%, VSD 22.7%, AS 7.9%, PS 7.9%, PDA 6.9% CoA 0.99% and CTGA 0.99%. Cyanotic cardiac malformations 23.8% included TOF 17.8%, DORV 3.9%, Pentalogy of Fallot 0.99% and Triology of Fallot 0.99%.
Two commonest acyanotic defects ASD and VSD together comprised half 51.5% of all congenital cardiac malformations. Mexico study reported acyanotic cardiac malformations 59.8% compared to the present study with 76%. However majority were PDA as the study included infants above 1 month who were admitted for closure of the ductus, next VSD and thirdly ASD [14].
Three other studies from India reported majority VSD malformations [15-17]. In contrast a study from Turkey reported cyanotic cardiac malformations 65.1% to acyanotic cardiac defects 34.8% [18]. Another hospital based study from India at birth reported acyanotic malformations 88.5%, majority were VSD 31.2%, followed by PDA 24.3% and low ASD 11.5%. Among cyanotic cardiac malformations, TOF 48% was the commonest [19].
Complex congenital cardiac malformations presented in 16 (15.9%) children raised the total number of cardiac defects to 121 confirmed by echocardiogram among 101 children. Among 29 (28.72%) ASD defects, there were 11 complex cardiac malformations included 3 cases of ASD + PAPVC, 2 cases ASD + PDA + MVP, 2 cases ASD + PS, 1 case each of ASD + PDA + PS, ASD + subaortic VSD, ASD + CoA and ASD + MVP. Majority 18/101 (17.8%) were isolated ASD.
Types of defects in ASDs, a majority 24/29 (82.7%) were ostium secundum defect while remaining 3/29 (10.3%) were sinus venosus and 1/29 (3.4%) large endocardial cushion defect. Two cases also presented with dual ASDs and one had septal aneurysm. Sizes ranged from small 3mm to large 2.5cm endocardial cushion defect.
The incidence of pulmonary hypertension in children less than 20 years of age is about 5% in most studies as in spite of large pulmonary flow, pulmonary arterial pressure is usually normal because of the absence of a high pressure communication between pulmonary and systemic circulation but increases to 20% in those aged 20-40 years to 50% in patients older than 40 years. However severe elevation of resistance (Eisengmengers’ reaction) is unusual [3,4]. In the present study 9/29 (31%) ASD cases had complications of pulmonary congestion and hypertension [20].
Increased pulmonary blood flow over many years causes changes in pulmonary vascular bed consisting of intimal fibrosis and endothelial proliferation with less medial muscular hypertrophy and increased pulmonary vascular resistance with right ventricular hypertrophy may increase with decrease compliance resulting in cyanosis with reversal of shunt [3,4,13].
Next VSD 23/101 (22.77%) most were isolated VSD in 19/23 (82.6%), while 4/23 (17.39%) children presented with complex congenital cardiac malformation occurred, of which 3/23 (13%) cases had VSD + AS including one with dextrocardia and 1/23 (0.04%) case VSD + PS. Two-dimensional echocardiogram revealed sizes of VSD varying from small 4 mm up to 2 cm endocardial cushion defect almost like a single ventricle. The position varied from subaortic, 16/23 (69.5%), peri-membraneous 4/23 (17.3%) and subpulmonic 3/23 (13%). Seven cases had septal aneurysm.
Defect in thin membranous septum with ventricular septal aneurysm partially covered by tricuspid valve tissue limits volume of left to right shunt. Echocardiography is useful for estimating shunt size by examining the degree of volume overload of the left ventricle as an increased dimension reflects the size of the left to right shunt; in addition echocardiogram also determines presence of aortic valve insufficiency or leaflet prolapse in the case of supracristal VSDs.
Pulsed Doppler examination reveals pressure restrictive VSD by calculating the pressure gradient across the defect. Spontaneous closure occurs in 40% peri-membraneous while muscular VSD closes within one year, 60-70% by 3 years and 80-90% by 8 years, however inlet and Swiss cheese VSD do not close spontaneously [3,4,6].
AS 8/101 (7.9%) had 1 complex congenital cardiac malformation case of AS + CoA, while 7/101(6.95) AS were isolated. There are two types’ valvular bicuspid and subvalvular aortic stenosis, majority are valvular with thickened semilunar bicuspid valves leaflets, the commissures fused to varying degrees with increased left ventricular systolic pressure and hypertrophy of the ventricular wall due to obstruction to outflow with decreased compliance and increase end diastolic pressure.
Subvalvular (subaortic) stenosis has discrete fibro muscular shelf below the aortic valve, obstruction to left ventricular outflow tract frequently associated with other forms of congenital cardiac disease may progress rapidly in severity but rarely diagnosed in infancy, become apparent after successful surgery for other cardiac defects such as Coartaction of aorta, PDA, VSD etc.
Shone complex is characterized by muscular or membranous subvalvular aortic stenosis, coarctation of aorta, supravalvular mitral membrane and parachute mitral valve, or discrete juxtaductal coarctation, the ascending aortic blood flows through the narrowed segment to reach the descending aorta causes left ventricular hypertrophy [15].
Dominant isolated PS 8(7.9%) while two other cases had small VSD classified as PS + VSD rather than TOF and another PS + ASD. The presence of large left to right or right to left shunt is determined by the degree of pulmonary valve stenosis on which clinical features depends, if stenosis is mild, there is a large left to right shunt with symptoms similar to an isolated ASD or VSD. Arterial oxygen saturation will be normal even in cases of severe pulmonic stenosis unless an intracardiac communication such as VSD or ASD allows blood to shunt from right to left. Severe pulmonary stenosis in a neonate markedly decreases right ventricular compliance, causing right to left shunting through a patent foramen ovale, termed as critical PS (3-5).
There were 7(6.9%) isolated PDA, other complex cardiac malformations with PDA, included 2 cases of PDA + ASD + MVP and 1 case of PDA + ASD + PS. PDA is usually due to maternal rubella infection in early pregnancy and common in preterm infants. Functional closure of the ductus usually occurs soon after birth or within two weeks upto one year (3,4,7).
Isolated CoA 1/101(0.99%), two other cases of complex cardiac malformation included CoA + ASD and CoA + AS. Isolated CTGA 1/101 (0.9%) had double inversion of the atrioventricular and ventriculoarterial relationships resulting in desaturated right atrial blood reaching the lungs and oxygenated pulmonary venous blood flowing appropriately to the aorta.
The commonest among cyanotic congenital cardiac malformation 24/101 (23.76%) was TOF 18/101 (17.8%). TOF consist of four basic components, VSD, PS, aorta unusually positioned above the VSD, thickening of the right ventricular muscle wherein upper portion of the ventricular septum aligns incorrectly, resulting in the presence of a large subaortic VSD such that it appears that the aorta arises from both the left and right ventricles.
Rare variants included DORV + VSD + PS 4/101 (3.9%) of which one case had dextrocardia has both aorta and pulmonary artery arising from the right ventricle with PS and outlet from the left ventricle through VSD into the right ventricle, aortic and mitral valves separated by smooth muscular conus similar to that seen under the normal pulmonary valve and aorta may override VSD by at least 50% of right ventricle, variant may be viewed as part of a continuum of Tetralogy of Fallot depending on degree of aortic override (3,4).
Other rare cyanotic congenital cardiac variants included Pentalogy of Fallot 1/101 (0.9%) consists of unrestricted large anterior subaortic perimembranous misalignment that leads to equalization of right and left ventricular pressures, right ventricular hypertrophy secondary to outflow tract narrowing or complete obstruction with infundibular pulmonary stenosis and overriding of aorta due to mal-alignment type of VSD with part of aorta existing from right ventricle [22], and Trilogy of Fallot 1/101(0.9%) occurs in 1% of live births had dual atrial septal defect, pulmonary stenosis, right ventricular hypertrophy and overriding of aorta wit right to left shunt, while literature reported only single ASD [23,24].
Overall age-wise distribution majority 30% were 2-4 year olds followed by 20.8% 4-6 years, 14.8% 6-8 years and 10-12 years age groups, incidence decreased to 8.9% in 8-10 years with subsequent increase to 14.8% to 10.8% in 10-12 and 12-14 years age groups respectively. Both present and Mexico study (14) reported low 10.8% and 7.1percentage adolescents respectively probably due to either spontaneous closures of cardiac defects or prior surgical intervention.
In the present study, three commonest congenital cardiac malformations ASD 29/101 (28.7%), VSD 23/101 (22.7%) and TOF 18/101 (17.8%) comprised over two-thirds 70 (69.3%) of cardiac defects in children aged 2-14 years. ASD was the commonest defect peaking to 60% in children aged 8-10 years while VSD 30% and TOF was 10%.
However among children aged 6-8 years revealed ASD 50% while TOF was 40% and VSD 10%, while VSD increased to 42.8% to 28.5% ASD and 28.5% TOF in younger children 4-6 years
TOF decreased to zero cases in 10-12 year olds with 50% ASD and VSD among children with hospital attendance. VSD peaked 60% in adolescent 12-14 years with equal 20% distribution of ASD and TOF. However among 2-4 year olds revealed ASD 40%, TOF 32% and VSD 28%.
Sex distribution noted overall male predominance M:F::1.3:1, however specific cardiac malformations revealed AS M:F::6:1, VSD M:F::3:1, PS M:F::1.7:1, PDA M:F::1.7:1, both CTGA and CoA M:F::1:0, contrasted to DORV M:F::1:1, while female predominance observed in ASD M:F::1:2 especially in ostium secundum type, described as tall, thin and gracile and TOF M:F::1:1.3. Mexico study reported an overall female preponderance M:F::1:1.1 [14] while Mumbai reported more males, M:F::1.88:1 [15].
Conclusion
Complex cardiac malformations presented in 16 (15.99%) of 101 children confirmed by echocardiogram raised total number of cardiac defects to 121.
Most 76.2percentage acyanotic malformations, majority being ASD 29/101 (28.7%) with 11 complex cardiac malformations, 3 cases of ASD + PAPVC, 2 cases ASD + MVP + PDA, 2 cases ASD + PS, 1 case of ASD + PDA + PS, 1 case ASD + VSD, 1 case ASD + CoA and 1 case ASD + MVP. However majority 18/29 (62.5%) were isolated ASD.
Next VSD 23/101 (22.7%) had four complex cardiac malformations, 3 cases VSD + AS including one with dextrocardia and 1 case of VSD + PS, most 19/23 (82.6%) were isolated VSD. Among AS 8/101 (7.9%) majority 6/8 (75%) were isolated, complex cases included 1 case of AS + CoA and another of VSD +AS.
Isolated PS comprised 8/101 (7.9%), however there were four other complex malformations, 2 cases of ASD + PS, 1 case ASD + PDA + PS and 1 case of VSD + PS. Isolated PDA 7/101 (6.9%) with other three complex malformations, 2 cases of ASD + MVP + PDA and 1 case ASD + PS + PDA. Isolated CoA 1/101 (0.99%) however had two other cases of complex cardiac malformation included 1 case of ASD + CoA and 1 case AS + CoA and an isolated CTGA 1/101 (0.99%).
Cyanotic malformations comprise 24/101 (23.6%) among 101 children in the study. Commonest cardiac malformation was TOF 18/101 (17.8%) other rare variants included DORV 4/101 (3.9%) of which one case had dextrocardia, Pentalogy of Fallot 1/101 (0.99%) and Trilogy of Fallot 1/101 (0.99%).
Age-wise distribution revealed majority 30% were 2-4 year olds. While specific ASD peaked 40%, 50%, and 60% in 2-4, 6-8 and 8-10 years age group respectively. VSD peaked 42.8% to 60% in 4-6 years and 12-14 years respectively. There were zero TOF cases with 50percentage each of ASD and VSD in 10-12 year olds.
Sex distribution noted an overall male predominance M:F::1.3:1, while specific cardiac defects revealed AS M:F::6:1, VSD M:F::3:1, PS M:F::1.7:1, PDA M:F::1.2:1, CTGA and CoA M:F::1:0, contrasted to female predominance in ASD M:F::1:2 and TOF M:F::1:1.3.
This study of Echocardiogram diagnosis of complex congenital cardiac malformations in Indian children is reported for the first time in literature.
Declarations
Funding: No external Funding sources:
No conflict of interest
References
- Van Der Linde, D., Konings, E. E., Slager, M. A., Witsenburg, M., Helbing, W. A., Takkenberg, J. J., & Roos-Hesselink, J. W. (2011). Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology, 58(21), 2241-2247.
- Hoffman, J. I., & Kaplan, S. (2002). The incidence of congenital heart disease. Journal of the American college of cardiology, 39(12), 1890-1900.
- Daniel Bernstein. Congenital heart disease. Kliegman, Behrman, Bonita F.S, Joseph W, Nina.F.S (eds) Nelson Textbook of Paediatrics, 21st edition vol II, Elsevier; 2019: pp 449-461.
- Wernovsky G, Gruber PJ: Common congenital heart disease: Presentation, management, and outcomes. In: Ballard RA, ed. Avery’s Diseases of the Newborn. 9th Ed. Philadelphia, Pa: WB Saunders; 2012: pp699-788.
- Zhao, Q. M., Ma, X. J., Jia, B., & Huang, G. Y. (2013). Prevalence of congenital heart disease at live birth: an accurate assessment by echocardiographic screening. Acta Paediatrica, 102(4), 397-402.
- Du, Z. D., Roguin, N., & Wu, X. J. (1998). Spontaneous closure of muscular ventricular septal defect identified by echocardiography in neonates. Cardiology in the young, 8(4), 500-505.
- Mitchell, S. C. (1957). The ductus arteriosus in the neonatal period. The Journal of Pediatrics, 51(1), 12-17.
- Oster, M. E., Lee, K. A., Honein, M. A., Riehle-Colarusso, T., Shin, M., & Correa, A. (2013). Temporal trends in survival among infants with critical congenital heart defects. Pediatrics, 131(5), e1502-e1508.
- Chang, R. K. R., Gurvitz, M., & Rodriguez, S. (2008). Missed diagnosis of critical congenital heart disease. Archives of pediatrics & adolescent medicine, 162(10), 969-974.
- Mahle, W. T., Newburger, J. W., Matherne, G. P., Smith, F. C., Hoke, T. R., Koppel, R., ... & Grosse, S. D. (2009). Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation, 120(5), 447-458.
- Plana, M. N., Zamora, J., Suresh, G., Fernandez‐Pineda, L., Thangaratinam, S., & Ewer, A. K. (2018). Pulse oximetry screening for critical congenital heart defects. Cochrane Database of Systematic Reviews, (3).
- Pierpont MEM, Moller JH: Genetics if Cardiovascular Disease. Boston, Martinus Nijhoff, 1987.
- Park MK, TroxierRG: Pathophysiology of left to right shunt. In: Pediatric Cardiology for Practitioners, 4th ed. 2002; ch. 9: p100-102.
- Villasís-Keever MA e. [Frequency and risk factors associated with malnutrition in children with congenital cardiopathy]. - PubMed - NCBI [Internet]. Ncbi.nlm.nih.gov. 2016 [cited 8 August 2016]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11547592
- Kasturi L, Kulkarni AV, Amin A, Mahashankar VA. Congenital heart disease: clinical spectrum. Indian Pediatr 1999; 36: 953.
- Jain, K. K., Sagar, A., & Beri, S. (1971). Heart disease in children. The Indian Journal of Pediatrics, 38, 441-448.
- Bidwai, P. S., Mahajan, C. M., Walia, B. N., & Berry, J. N. (1971). Congenital heart disease in childhood--a clinical study. Indian Pediatr.
- Varan, B., Tokel, K., & Yilmaz, G. (1999). Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Archives of disease in childhood, 81(1), 49-52.
- Wanni KA, Shahzad N, Ashraf M, Ahmed K, Jan M, Pasool S . Prevalence and spectrum of congenital heart disease in children. Heart India 2014; 2:76-79.
- Christopher GL. Growth Patterns in Asian Children with Congenital Heart diseases. Grace Lalana Publications. 2023, ch 1-6. https//: www.newgenparenting.com
- Aslam, S., Khairy, P., Shohoudi, A., Mercier, L. A., Dore, A., Marcotte, F., ... & Mongeon, F. P. (2017). Shone complex: an under-recognized congenital heart disease with substantial morbidity in adulthood. Canadian Journal of Cardiology, 33(2), 253-259.
- Topol, E. J., & Califf, R. M. (Eds.). (2007). Textbook of cardiovascular medicine. Lippincott Williams & Wilkins.
- “Fallot triology (Concept Id: C0041022)-MedGen – NCBI”. www.ncbi.nim.nih.gov. retrieved 2021-08-07
- Swan H, Marchioro T,Kinard S, Blount SG. Triology of Fallot. Experience with twenty two surgical cases. Archives of Surgery 1960; 81:291-298. Doi:10.1001/archsurg.1960.01300020119018. ISSN 0004-0010. PMID 13836013.


