Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-2276/003
Lesion Characterizations, Associated Risk Factors and Financial Implication of Zoonotic Hydatid Cyst of Dromedary Camels Slaughtered at Addis Ababa, Akaki Kality Municipal Abattoir, Ethiopia
1 Affiliation; Jigjiga University, College of Veterinary Medicine, Department of Veterinary Pathology and Parasitology, Somali, Ethiopia.
2 Affiliation; Affiliation; Department of Clinical Studies, College of Veterinary Medicin and Agriculture Addis Ababa University, Bishoftu, Ethiopia.
3 Department of pathology and Parasitology, Addis Ababa university, College of Veterinary medicine and Agriculture, Bishoftu, Ethiopia.
4 Affiliation; Department of pathology and parasitology, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia.
Correspondng Author: Elias Gezaw Anbu
Citation: Elias Gezaw Anbu, Yacob Hailu Tolossa, Abdi Feyisa, Jirata Shiferaw Abosse, (2022). Lesion Characterizations, Associated Risk Factors and Financial Implication of Zoonotic Hydatid Cyst of Dromedary Camels Slaughtered at Addis Ababa, Akaki Kality Municipal Abattoir, Ethiopia. Journal of Food and Nutrition. 1(1). DOI: 10.58489/2836-2276/003
Copyright: © 2022 Elias Gezaw Anbu, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-09-07, Received Date: 2022-09-07, Published Date: 2022-10-26
Abstract Keywords: Abattoir, Camels, Dromedary, Financial loss, Histopathology, Hydatid cyst, Lesion, Liver, Lung.
Abstract
Hydatid cyst, is zoonotic helminthic parasites of Taeniid families having significant economic crisis in the world. A cross-sectional study was conducted from October 2021 to May 2022 to characterize hydatid cyst lesions, analysis of associated risk factors and estimate annual direct financial loss due to hydatid cyst infected organs condemnation during postmortem examination in dromedary camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia. A total of 370 camels were purposively sampled and systemic meat inspection was employed to detect the presence of hydatid cyst. Out of 370 hydatid cysts like cases examined in different organs, 96 (25.9%) camels were found harboring hydatid cyst. The percentage of hydatid cyst in adult camels were 29.67%; OR= 1.54 while in young it was 18.55%. Likewise, the prevalence of hydatid cyst in poor, medium and good body condition score was 61.4%; OR= 10.79, 30.89%; OR= 3.30 and 12.11% respectively. Results indicated that age and body condition score had statically significant effect (P<0.05) on prevalence of hydatid cyst. The overall prevalence of hydatid cyst in male dromedary camel was (14.59%; OR=0.83;) found higher than female (11.35%). The overall prevalence of hydatid cyst in camels originated from Borana, East Hararge, Jigjiga, Karrayyu, Matahara, Minjar-shenkora and Wollo were 9.18%, 0.54%; OR= 2.12, 4.86%; OR= 2.06, 3.51%; OR= 1.18, 3.78%; OR= 0.94, 1.08%; OR= 0.78 and 2.97%; OR= 1.31, respectively. Result indicated, origin and sex didnât have significant effect (P>0.05) on the overall prevalence of hydatid cyst. Out of 96 total infected camels, only 9 (9.38%) cases had hydatid cyst on both lung and liver while the remaining 87 (90.62%) cases had hydatid cysts only in single organs. Of 105 total infected organs, percentage of distribution of hydatid cyst in lungs, livers, spleen and heart were 87.61%, 10.47%, 0.95% and 0.95% respectively. Result showed that out of 105 examined organs of camels, 51 organs harboring less than three hydatid cysts number while 54 organs had greater than or equal to three hydatid cysts. Grossly, hydatid cyst of lung had a shape of cotton ball, implanted in lung parenchyma, filled with clear to slightly turbid fluid, soft and malleable to touch and inside white germinal layer while hydatid cyst on the livers was firm, calcified and strong when it was about to be cut off. Microscopically, hydatid cyst structure overlying organs had a fibrous layer on the external (pericyst), an acellular eosinophilic laminated membrane layer on the middle (ectocyst) and a germinal layer internally (endocyst) and protoscolices were also seen in a lung section. Fibrous layer had infiltration of epitheliod macrophages, lymphocytes and eosinophils showed inflammatory reaction response to hydatid cyst layer and cellular infiltration was lessened when they went away from hydatid cysts. Histopathology of lung infected with hydatid cyst displayed massive alveolar damage, some alveoli was emphysematous, congested capillary, hemorrhage and atelectasis in the fibrous layer. In hydatid cyst infected liver histology, there was hemorrhage, hepatocyte degeneration and cytoplasmic swelling with dilation of nucleus in fibrous layer. Microscopically, hydatid cyst infected heart had not a visible protoscolices and it had weak germinal and lamination layer followed by lymphocyte and epitheloid macrophages infiltration. The total annual direct financial loss due to hydatid cyst was estimated at 86,209.63 Ethiopian birr. Hereafter, this study helps to stress the economic consequences and pathological patterns of hydatid cysts at abattoir, and creation of public awareness and control of stray dogs in order to reduce infection by the parasite were suggested recommendation.
Abbreviations
AHI: Animal Health Institute; CSA: Central Statistical Authority: EITB: Enzyme-linked Immunoelectron Transfer Blot; ELISA: Enzyme-linked Immunosorbent Assay; ETB: Ethiopian Birr; FAO: Food and Agriculture Organizations; IGAD: Intergovernmental Authority on Development; MRI: Magnetic Resonance Imaging; NMA: National Meteorological Agency; NMSAE: National Meteorology Service Agency of Ethiopia; OR: Odds ratio; SNNP: South Nations and Nationalities Peoples’ Region; WHO: World Health Organization; OIE: World Organization for Animal Health, formerly the Office International des Epizooties.
1. Introduction
In the world there are about 30 million dromedary camels, but most are in the Africa and Middle East (Food and Agriculture Organizations/FAO, 2016). The dromedary camels are used for various purposes; which able to produce milk, meat, wool and hides in dry-land regions of pastoralist in East Africa (Somalia, Sudan, Ethiopia, Kenya, Djibouti and Eritrea) and the pastoral area of East Africa is home to about 14.5 million dromedary camels (FAO, 2009).
In Ethiopia, around half of a country has arid and semi-arid climatic conditions (Coppock, 1993) and this area is thought to be home to 6-10% of the Ethiopian population (Ayele, 1989). In Ethiopia, there were about 1.23 million camels (Central Statistical Authority/CSA, 2016). Dromedary camels, have exceptional anatomical, behavioral features and physiological natures, are well adapted and known to provide basic necessities to pastoralists who live in arid and semi-arid regions. It is a source of food and power, respect and cash, and means of storing wealth (Zeleke and Bekele, 2000). The unique behavior of dromedary camels other than animals is their resilience withinside the arid environments, giving an essential source of meal and profits for human beings in dry climates (Watson et al., 2016). However, pastorals’ production remains low due to lack of improved genetics, feed shortages, disease, poor animal health care and low technology (Tefera, 2012).
Camel meat is a good and preferred food for pastoralist and Sumale ethnic groups in the Addis Ababa, capital city of Ethiopia (Muskin et al., 2011), especially for those living in the Bole area. However, parasitic diseases of camels cause various economic problems in terms of minimizing the work, reducing the growth and production of meat and milk and condemnation of edible organs (Dakkak, 2010). Also, a few camel’s parasites cause troubles with human fitness (Romazanvoc, 2001; Sazmand and Joachim, 2017).
The most common sources of protein come from animals. Parasites, however, undermine their profit by impeding the health and production. Mortality of animals from parasitic diseases won’t be horrifying at the moment however they lessen milk, meat, wool, skin and hide manufacture, infertility and bring about lack of power of working animals (Derbala and Zayed, 1997).
Hydatid cyst is a zoonotic disease caused by Echinococcus spp. (Echinococcus granulosus and Echinococcus multilocularis) and contracted when a person eats an embryonated egg in green food, parasite-contaminated egg materials and drinks egg contaminated water (Muller, 2002). Other Echinococcus species which can cause hydatidosis are E. oligarthrus and E. vogeli. Hydatidosis in humans is mostly caused by infection with the larval stage of the dog tapeworm, Echinococcus granulosus (WHO, 2015). Camels are infected with metacestodes of Echinococcus species (El-Metenawy, 1999).
Hydatid has an indirect life cycle using dogs, jackals and foxes as definitive hosts and produces eggs which handed into the feces and because of ingestion of the eggs, infection passes to the natural herbal intermediate host, generally herbivores such as camels, sheep, goats, cattle, cervids, swine, and horses whilst grazing. Adult Echinococcus granulosus tapeworm survives in the small intestines of definitive hosts, namely canids specifically in dogs (Ahmadi and Meshkehkar, 2011; The Center for Food Security and Public Health, 2012). Besides, people are infected when eggs or larvae from definitive hosts are launched into the surrounding with feces, and people by accident devour infected green foods or water (Jenkinsa et al., 2005; Santivanez and Garcia, 2010). When the egg is eaten up by way of means of an intermediate host, it releases embryos (oncospheres); embryos tour through the small intestinal wall until it reaches the small vessels of the intermediate host and migrate to the organs which include liver, lung, kidney, heart and spleen through blood circulation and or lymphatics and expand in to a hydatid cyst. Most of them are caught in the hepatic sinusoids. Embryos with much less than 0.03 mm may pass through the hepatic sinusoids and relax in the lungs (Sarkar et al., 2016). The life cycle of the E. granulosus is finished whilst the definitive host ingested an inflamed lung, liver, spleen, heart and kidney of intermediate hosts.
Hydatid cyst due to E. granulosus frequently predominant in sheep-raising parts of the Australia, Mediterranean, South America, New Zealand, South Africa and the Middle East including Saudi Arabia (Toulah et al., 2012). It is reported in Africa mostly where cattle raised in a free range related closely with dogs (Abebeand Yilma, 2013). Hydatid cyst is major cestodes which are described from camels in India (Parsini et al., 2008).
Knowing where the infection found plays an important role in preventing the spread of the disease. Hydatid cyst is widely distributed worldwide on different hosts with varying prevalence (World Health Organization/ World Organization for Animal Health (WHO/OIE), 2001). Highest prevalence of hydatid cyst in humans and animals is found in rural and temperate areas, specifically in growing international locations in which there’s near contact among canine and intermediate hosts (Eckert and Deplazes, 2004). In many African countries, the lack of proper meat inspection, especially in rural areas and presence of a huge populace of stray canine has caused an excessive prevalence of disorder (Magambo et al., 2006).
Risk factors such as proper climatic and ecological conditions, slaughter of animals at home and the presence of many stray dogs make hydatid cysts survive (Seimenis et al., 2006). In areas with a large number of livestock breeders which don't have proper veterinary service assistance and the offal of slaughtered animals has easy access to a dog, the transmission and prevalence of hydatid cyst is high enough (Eckert et al., 2001).
In livestock hydatid cyst doesn’t show main clinical signs and it is noticed only at a time of postmortem examination and causes economic losses by the way of condemnation of infected organs (liver, lung, heart, spleen and kidney) and lowered meat and milk production (Torgerson, 2003). Hydatid cyst frequently grows silently over many years and clinical signs are immediately associated with the predilection site, size and burden of cysts exist (Zhang et al., 2014). If large cyst is located in an area of the body, with rigid bounders, such as the lungs or brain, the significances can be very serious (Daryani et al., 2007). Little information indicates it has an effect on production of the animal especially decrease in carcass weight, milk production, and fleece value, losses of offal and fertility losses (Yildiz and Gurcan, 2003). Generalized pruritis and fever are systemic symptoms frequently related with hydati-dosis. Rupture of cysts, principally into serosal cavities, may cause acute and sometimes fatal anaphylactic reaction (Parry et al., 2004). The adult Echinococcus is considered to be harmless to the definitive host, apart from when it occurs in large numbers, which may cause severe enteritis (Dunn, 1987).
There are no sufficient tests to diagnose hydatid cysts in animals as in human beings (Njoroge et al., 2002). However, hydatid cysts are diagnosed in laboratory by CT-scan, MRI, X-ray, many serological methods: Enzyme-linked immunosorbent Assay (ELISA) and Fast-ELISA with sensitivity (Rokni et al., 2006), Dot ELISA (Dalimi et al., 2000), Enzyme linked Immuno electron Transfer Blot (EITB) (Rokni and Aminian, 2006), ELISA (Haniloo et al., 2005). At present, ELISA method using AgB is the most efficient test and is accessible in some la-boratories (Sadjjadi et al., 2007). The analysis of intrathoracic hydatid cyst is primarily based totally at the imaging characteristics and serologic tests. Infrequently, infection and degeneration of the cyst may hamper the accurate diagnosis and, in those cases, histology confirms the proper diagnosis and prognosis (Alloubi, 2013). However, there is largely unsuccessful serological diagnosis in intermediate hosts when compared with the examination in humans, due to the coexistence of multiple infections with taeniid species, cross reactivity of antigens and low-level antibody response to infections. Now, postmortem examination is the test of high-quality for the diagnosis of hydatid cyst in the intermediate host. Nevertheless, out of postmortem positive intermediate hosts might actually be false positive due to condition like unclear granulomas, pseudotuberculosis, fatty degeneration, abscesses, caseous enlargement of lymph node usually due to infection and larval stage of Taenia hydatigena can confuse the positive results. Additionally false negative diagnosis could also be made due to small intra-parenchyma cysts (Zhang et al., 2012).
Hydatid cyst have round cotton ball shaped, fluid-filled, unilocular cyst that consists of an inner germinal layer of cells maintained by a characteristic acidophilic-staining, acellular, laminated membrane of variable thickness containing numerous tiny protoscoleces. Hydatid cyst most often mature in the liver and lungs and also develop in the spleen, kidney, nervous tissue, bone and other organs (Seimenis, 2003).
The histopathological structure of not unusual hydatid cyst in the organs, comprising a germinal layer, cuticular membrane or laminated cyst wall, fibrous tissue capsule and cellular infiltration. The fibrous capsule consists of a thick connective tissue layer which has inflammatory infiltrates holding masses of lymphocytes, eosinophils, histiocytes and plasma cells in surrounding tissue (Bektas et al., 2016).
Camel lung affected with hydatid cyst wall designed of thick fibrous connective tissue, cyst lumen filled with clear to turbid liquid and the adjacent tissues showed atelectasis, hemorrhage and emphysema. Hydatid cyst overlying the liver is filled with caseated materials which is mixed with dystrophic calcification and surrounded by fibrous capsule and mononuclear inflammatory cells. And camels’ liver shows hyperplasia, thickening of bile duct and atrophy of liver cells due to fibrous proliferation (Dyab et al., 2020). Microscopic result of liver show multilocular cysts of finely and amphophilic laminated membrane (ectocyst) and geminal layers (endocyst) surrounded by fibrinous walls (pericyst) (Wei-Hsin et al., 2005).
Microscopic tissue examination confirms the cyst diagnosis after 10% buffered formalin fixed tissue is processed with the aid of different staining methods. Microscopically, growth and the development of hydatid cyst structure belong to host tissue and hydatid cysts. Hydatid cyst has an outside cuticular membrane and an internal proligerous membrane produced by budding proligerous vesicles, the cyst is full of a clean to turbid liquid. The adventitious is fibrous area corresponding to host reaction to the cyst (Carmen et al., 2010).
Chemotherapy with benzimidazole compounds and, more of late, treatment with cyst puncture, aspiration, injection of chemicals and re-aspiration have been introduced and, gradually, have replaced surgery as the favored treatment (Pawlowski et al., 2001). The combination of praziquantel and albendazole has been used successfully in the treatment of hydatid disease. Praziquantel used at 50 mg/kg in different regimens (once daily, once weekly, or once every two weeks) with albendazole formed very effective and quick outcomes associated with albendazole therapy alone. Albendazole (10-15 mg/kg/day) and mebendazole (40-50 mg/kg/day) have established usefulness; though, the outcomes for albendazole have been superior, possibly because of its pharmacokinetic profile, which favors intestinal absorption and penetration into the cyst (Cobo et al., 1998). Another treatment choice of hydatid cysts consists of: (1) percutaneous puncture using sonographic guidance, (2) aspiration of substantial amounts of the liquid contents, (3) injection of a protoscolicidal agent (e.g., 95% ethanol or hypertonic saline) for at least 15 minutes, and (4) re-aspiration (PAIR, puncture, aspiration, injection, and re-aspiration) (Smego et al., 2003).
The preventive and control programs need disturbing the life cycle of the Echinococcus granulosus acting on the epidemiological chain (Guisantes, 2014). Systematic screening and treatment of diseased dogs was successfully eradicated the disease in endemic area of New Zealand. Inhibiting the feeding of infected raw offal’s by proper disposal of hydatid cysts possessing condemned offal’s at local slaughterhouses, abattoirs, back yards and on farms. Introduction of appropriate meat inspection, creation of local slaughterhouses, educating people, effective application of legislative measures, burning or burial of condemned offal’s and sterilization of offal’s when it is going to be used as dog feed is additional control method of hydatid diseases. (Craig et al., 2007). Health education is another method for the control and prevention of echinococcosis which consists of three activities which rely on each other: 1) Information which comprises the transfer of professional knowledge to the group targeted and highlight certain ideas to get the community to actively participate in preventive activities. 2) Health education sensu strictu that designed to target groups who are not professionally concerned about the problem, such as schoolchildren or the public at large. 3) Occupational training which targets those who must implement health standards in their professional doings (such as farmers and butchers) (Parodi et al., 2001).
Health education, hygiene perfection, meat examination and abattoirs facilities, and rising safe water supplies (horizontal approach) and testing dogs to check for parasites, systematic dosing of dogs with praziquantel and immense lessening of dog numbers, including baseline surveys and continuing surveillance of livestock and human infections (vertical approach) were two main control programs employed (Gemmell, 2001).
In human being, the prevention is mostly depending on personal hygiene which includes avoiding contact with unknown dogs that may be infected with parasite, washing hands before eating a food, and washing raw vegetables before eating them. Treat the dogs regularly with praziquantel and avoid feeding them with raw offal potentially infected with cysts. Examination of dog feces over the study of coproantigens is another measure of care at the household level. All person managing dogs in endemic areas must be aware of the health risk of acquiring hydatidosis and get safety measures (Guisantes, 2014).
Hydatid diseases have major economic significance in both animals and humans (Torgerson and Budke, 2003). Hydatid cyst causes considerable economic importance due to reduced productivity and monetary losses because of carcass and organ condemnations at abattoir in different countries of the world (Scala et al., 2005). The levels of hydatid cysts impose a huge medical, economic and social burden in the affected areas. In view of to its importance, treatment modalities for patients with the disease embrace chemotherapy via albendazole and mebendazole or praziquantel, puncture aspiration injection reaspiration (PAIR) and surgery (Ahmadnia et al., 2013). Large economic loss occurs through prohibitions of importation and exportation of animals and their products especially from endemic areas (Sariozkan and Yalcin, 2009). In humans, the economic damage is related with the direct financial loss due to diagnosis, hospitalization, and surgical or percutaneous treatments. Medication, post-treatment assistance, travel by the patient and family to treat him and various expenses incurred in the hospital can be cited as economic problem. In addition, if the sick person is not tested, the disable will cause him to stop working. This means, that a country’s productivity decreases and also 1%-2% of hydatidosis cases can be fatal (Torgerson et al., 2003).
Abundant research carried out in our country, Ethiopia on cattle, sheep and goat hydatid cysts (Abriham, 2021). Irrespective of the wonderful environmental and financial fee of the dromedary camels to the livelihood of the pastoral society who do now no longer have any opportunity mode of production system, there are uncommon research facts on hydatid cyst while as compared with that of different home animals in Ethiopia; the ones uncommon research have been said with the aid of using Abdiselam et al. (2014); Aboma et al. (2015); Bayleyegn et al. (2013); Boru et al. (2013); Dawit et al. (2019); Etana et al. (2015); Mekuanent et al. (2015); Abebe et al. (2021), and Zeleke and Berkeley (2000). Yet, camels are intermediate hosts for hydatid cysts like different livestock (Omer et al., 2004). In this regard, abnormalities of carcasses suitable for eating organs put the community at risk of acquiring zoonotic food borne illnesses. Also, organ condemnation marks critical monetary loss in the country, Ethiopia. Besides, histopathology of hydatid cyst in camels has now no longer been carried out in our country, Ethiopia. To avoid these problems, abattoir-based research is needed to study associated risk factors on occurrence of hydatid cysts, lesions characterization in organs of camels and this will expect its monetary and public health significance.
Therefore, the objectives of current study are:
- To estimate the prevalence of hydatid cyst in dromedary camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia.
- To assess the association risk factors with the occurrence of hydatid cyst.
- To calculate direct annual financial loss because of organ condemnation in dromedary camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia.
- To characterize hydatid cyst lesions in dromedary camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia.
2. Materials and Methods
2.1. Study Area Description
The study was conducted at Addis Ababa, Akaki Kality Municipal abattoir from October 2021-May 2022, which is located in Addis Ababa; (Capital city of Ethiopia) with mean annual minimum and maximum temperature of about 21-27oC, respectively. Addis Ababa is positioned at 9°1’48’ North 38° 44°-24’ East/ 9.030000N 38.740000E, at a mean altitude of 2500 m above sea stage. The annual rainfall is about 800 to 1100 mm (National Meteorology Service Agency of Ethiopia (NMSAE), 2012). Even if the camel meat is not commonly known in Addis Ababa city, Somali community, some people come from abroad and a smaller number of Muslim communities who live in the city are the main consumers of camel meat from Akaki Kality slaughterhouse. As a result, the Akaki Kality abattoir usually slaughters an average of seven camels per day. But there is also another slaughtered animal daily (cattle, sheep and goats) in Akaki Kality Municipal abattoir and then gives service to the hotels and restaurants of the Akaki town district. Camels slaughtered at Akaki Kality Municipal abattoir were come from Borana, East Hararge, Jigjiga Karrayyu, Minjar-Shenkora district and Wollo.
Borana zone is found in Oromia regional state, southern part of Ethiopia and has 962,489 total populations of peoples (CSA, 2007). It is bordered by Kenya in the South, West Guji zone in the North, Somali region and Guji zone in the East and South Nations and Nationalities Peoples’ Region (SNNPR) in the West. Astronomically, this area stretches from 3°30’ N to 5°25’ N latitude and 36°40’ E to 39°45’ E longitude. Yabelo is the capital town of Borana zone and located at about 570 km South of Addis Ababa. It is almost 48,360 km2 out of which more than 75% is lowland. It has a mean monthly precipitation of 39.19mm and a monthly mean maximum temperature and minimum temperature of 29.660Cand 16.310C respectively (Worku et al., 2022). The comfortably high temperature length withinside the 12 month is from March to May, even as the bottom yearly minimum temperatures get up among November and January (National Meteorological Agency (NMA) of Ethiopia, 2007). The average annual rainfall ranges from 350 mm to about 900 mm which is distributed through the two rainy time of year from March to May and September to October (Debela et al. 2019).
East Hararge area is sited withinside the eastern Ethiopia and bounded through Bale quarter to the south and southwest, Dire Dawa administration council to the north; Somali regional state to the north, east and south east and west Hararghe sector to the west. Totally, Harari area is surrounded with the aid of using east Hararge neighborhood. To the east, Harar is 510 km from Addis Ababa, Ethiopia. The altitude ranges from 500-3,400 meters above sea levels. The area takes three agro-ecological zones; baddaa (upland raises above 2,300m, rainfall 1,200mm-2,000mm), bad-daree (midlands-elevation among 1,500m and 2,300m, rainwater 600mm3-2,000mm3 and gammoojjii (lowland- under 1,500m). Total area of the east Hararge is 24,247.7km2 (Degefa and Tesfaye, 2008). East Hararghe quarter hold 216,943 overall populations of humans (Central Statistical Authority/CSA, 2007).
Jigjiga (capital city of Ethiopian Somali Regional State) located at 9o 20' north latitude and 42o 47' east longitude and has a distance of 628km to the east of Addis Ababa. The elevation of the region levels from 900-1600 meters above sea level and gets a yearly rainfall of 300-500mm with the mean minimal and most annual temperatures of 20o C and 28°C, respectively. The peoples of this region are pastoralist and agro-pastoralist and, whose livelihood is based on high milk they get from camels, cows and goats (Somali Regional State, Ethiopia, 2004) and also bringing goods from Togo Wuchale to Jigjiga town and marketing it.
The Karrayyuu is indigenous peoples of Fantalle district and they are the clans of Oromo (Gebre, 2009). Karrayyuu have fundamental clans particularly Baso (stay in Eastern of a district) and Dullacha (live in western part). Karrayyu located two hundred kilometers East from Addis Ababa, Ethiopia (Debela, 2014). Estimated total populations of Fantalle district (Karrayyu) is 70,049 (CSA, 2006). Fantalle has 18 agropastoral affiliation and two urban centers namely Matahara and Haroo Adii which are approximately 5 km apart. Yearly rainfall is set about 500mm and anticipated to get 100C and 420C minimal and most temperature, respectively (Beyene and Gudina, 2009).
Minjar-Shenkora district in the northern shoa zone is an Amhara regional state which is located at 129km east of Addis Ababa. Approximately the district has an entire area of 229,463 hectares and a complete populace of 152,430. It raises varieties from 1,040 to 2,380 meters directly above sea level. Flat landscape (84%) with a small number of ragged mountains is the principal topography of a district. The average temperature varies from 14 ºC to 27 ºC whereas the annual rainfall changes between 780mm and 900 mm. The important farming scheme in this area is blended crop and small and large ruminant production (Setotaw et al., 2014).
South Wollo is located in the northern components of Ethiopia (CSA, 2008). The area is known by different mountain, hilly, plateaus and sloppy areas, streams, rivers and lakes. The altitude tiers from 1500-2600 meters above sea level. The middling rate of rainfall ranges from 39.63mm to 1000 millimeters. This area has minimum and maximum temperature of 11.6 0C and 23.9 0C, respectively. The relative humidity of the place varies from 23% to 79% (Hailu et al., 2013). North Wollo management sector is positioned north of Addis Ababa and eastern of Amhara regional state within 8095’- 1208’N longitude and 3805’-40020’E latitude. The altitude varies from 700-4100 meters above sea levels. The annual rainfall varies from 650 to 1200mm, and the minimum and most temperature is 16 0C and 25 0C, respectively (Belay, 2010). This administrative zone has 1,731,849 human populations with an expected area of 16,400.98 km2 (CSA, 2003).
2.2. Study Animal
All camels (Camelus dromedarius) which came for slaughter at Addis Ababa, Akaki Kality Municipal abattoir included in the study population. Both gender of camels (male and female) was subjected during the study period and age of the camels turned into grouped primarily based totally on teeth (deciduous dental formulation in camel; 2(I 1/3, C 1/1, P 3/3 and M 0/0=24) and permanent dental formulation; 2(I 1 / 3, C 1 / l, P 3 / 2 and M 3 / 3) =34 (Kertesz, 1993). And the age became classified as young when they were in deciduous dental formula (much less than five years) and adult once they were grouped in permanent dental formula (≥ five years of age) in accordance with standard specified by Bello et al. (2013). The body condition grading classified as poor, medium and good based on the rule of thumb given through Faye et al. (2001) for dromedary camels. This turned into via way of means of observing on the back (ischial tuberosity, sacrotuberous ligament, anogenital region, spinous apophysis) and flank (coxal tuberosity, hole of the flank, transverse apophyses, ribs and their humps).
2.3. Study Design
A cross-sectional study category executed from October 2021 to May 2022 in Addis Ababa, Akaki Kality Municipal abattoir. Data of lesion related to hydatid cyst on camels were gathered to signify the zoonotic Hydatid Cyst of dromedary camels, associated risk factors and direct yearly monetary loss because of condemned carcass and body parts like liver, lung, kidney, heart, spleen and brain.
2.4. Sampling Method and Sample Size Determination
The purposive sampling method was used for sampling until the end of the required sample size reached. On the day of investigation, all camels presented for slaughter (per day average of seven camels which was too small) were sampled and a total of 370 camels were examined based on the formula given by Thrusfield and Christley, (2018) and with expected prevalence of 38.22% (Abebe et al., 2021). Through guideline of thumb there had been 38.22% expected prevalence of hydatid cysts. Using 5
2.5. Study Methodology
Both ante-mortem and post-mortem meat inspection strategies were performed at Akaki Kality Municipal abattoir (n= 370).
2.5.1. Ante-Mortem Examination
Ante-mortem examination was conducted on individual and cluster of dromedary camels once they entered into the lairage. Right and left aspects of the camels inspected at rest and in motion. Additionally, the overall conduct of the camels, cleanness, and signal of illnesses and abnormality became documented in line with the usual ante-mortem inspection methods (FAO, 1994). Origins, sex, body conditions score and age of the camels have been recorded earlier than slaughter of camels. The body condition rating of the camels has been assessed in step with Faye et al. (2001) after which it is grouped as poor (score zero and one), medium (score two and three) and good (score four and five). The age of the camels anticipated the use of rostral dentition Bello et al. (2013) and after which they were classified as young (much less than five years) and adult (≥ five years of age).
2.5.2. Post-Mortem Examination and Gross Pathology
Postmortem examination was carried out through visible inspection, palpation and systemic incision of every visceral organ particularly the lung, liver, heart, kidney and spleen according to producer of Getaw et al. (2010). One or more incisions of every organ were made with the knife after the blood has been cleansed from it with water. Whenever cysts had been present, they have been removed, the gross pathology lesions were described, characterized and the illustrative of the alternative tissue become taken and placed into prefilled universal bottles with 10% buffered formalin, labeled and then taken to the laboratory for further studies.
2.5.3. Amount of Hydatid Cyst on Organs
All organs which had hydatid cysts on their seen surface and of their parenchyma have been gathered and the entire number of hydatid cysts in every affected organ turned in to count to compute the load of burden on the organ. After counting the cyst quantity on every organ, it was then labeled as less than three cysts and more than or equal to three cysts. This class becomes primarily based totally at the frequency of cyst quantity on examined organs at some point of the look at duration of study.
2.5.4. Sample Collection and Transportation
After the dromedary camels had been slaughtered in the Addis Ababa, Akaki Kality Municipal abattoir, systematic postmortem exams were taken to check the presence of zoonotic hydatid cyst. Organs like liver, lungs, kidney, spleen and heart have been the most considered and, the carcass and gastrointestinal tract became additionally taken into consideration for exam. During study period only lung, liver, heart and spleen were positive, and 20 representative hydatid cyst infected organs had been incised and put into a universal tissue collector bottle filled with 10% buffered formalin and transported to Animal Health Institute, Sabata Veterinary parasitology and pathology departments. All cystic contents (germinative membrane, sandy rock water) dispatched for histopathological examination. All precautions took no longer to spill off the cystic contents once it was taking and transporting the samples to Animal Health Institute, Sabata.
2.5.5. Histopathological Examination
Representative 20 tissue samples associated with hydatid cyst in different organs (lung, liver, heart and spleen) have been accrued and preserved in 10% neutral buffered formalin. The fixed tissue samples of about 1cm3 in size became trimmed to 5mm and processed in tissue processor (dehydrated via a chain of ascending grades of ethanol alcohol; 75%, 95%, three changes in 100% ethanol alcohol (100% I, 100% II, 100% III) cleared in three modification of xylene (xylene I, II and III) and impregnated two times with paraffin wax (paraffin wax II and paraffin wax II)) and finally, embedded in melted paraffin at 600c. The tissues have been then sectioned through Microtome at 5µm and stained routinely with hematoxylin and eosin, for microscopic examination (Belina et al., 2015).
2.5.6. Financial Implication
The financial loss because of zoonotic hydatid cyst, only direct loss was assessed and the calculation was based on condemned organs. By contemplating the average number of camels which were slaughtered and the degree of organ condemnation per annum become direct monetary loss attributed to metacestodes and average organ price and price index was used to calculate the loss (Getaw et al., 2010). Accordingly, the once a year direct monetary losses because of the hydatid cyst were expected through multiplying the average selling market price of the organs, by the average annual number of camels slaughtered, percent of hydatid cyst consistent with organ and prevalence of hydatid cyst in that study. Thus, the loss resulted from organs condemnation on the abattoir becomes assessed using the following formula;
ADFLC = (ACS* Ph*PLi* ACLi) + (ACS*Ph*PLu* ACLu) + (ACS*Ph*PHr* ACHr) + (ACS*Ph*PSpl* ACSpl). Where, ADFLC= Annual direct financial loss due to organ condemnation, ACS= Average number of camels slaughtered per year at Addis Ababa, Akaki Kality Municipal abattoir, Ph= Prevalence of hydatid cyst, ACLi= Average cost of liver, PLi= Percentage of hydatid cyst in liver, ACLu= Average cost of lung, PLu= Percentage of hydatid cyst in lung, ACHr= Average cost of heart, PHi= Percentage of hydatid cyst in heart, ACSpl= Average cost of spleen, PSpl= Percentage of hydatid cyst in spleen (Dawit et al., 2019).
2.6. Data Management and Analysis
The data which was collected from Akaki Kality Municipal abattoir each at antemortem and postmortem inspection have been saved into Excel spreadsheet (Microsoft excel 2007). Before being subjected to statistical analysis, the data was properly coded and carefully screened for errors. Descriptive statistical analysis including frequency distributions, percent and proportion were used to summarize in table and present the collected data. Chi-square (χ2) test was used to decide the association in zoonotic hydatid cyst and risk factors (ages, sex, body conditions score and origins). The overall prevalence was calculated by dividing the number of zoonotic hydatid cyst positive dromedary camels by the entire number of dromedary camels which were examined. For (χ2) test, p- value < 0> 0.05 was considered non-significant. Logistic regression was employed to analyze association of Hydatid cyst occurrence with the potential risk factors using R studio statistical software. The degree of risk factors associated with the disease occurrence was further analyzed using odds ratio.
2.7. Ethical Considerations
The study protocol was reviewed and approved by the research ethics review committee of Addis Ababa University, college of Veterinary Medicine and Agriculture (VM/ERC/13/02/14/2022). The objective and procedure of the study were explained to Addis Ababa abattoir enterprise and Akaki Kaliti Municipal abattoir for their voluntary use of their dromedary camels and they provided permission regarding camels related studies.
3. Results
3.1. Prevalence and Risk Factors of Hydatid Cyst in Dromedary Camels
The number and the infection rate of the infected dromedary camels are shows in the table 1. It was found that of 370 examined camels, 96 dromedary camels were infected with hydatid cyst (the infection rate of 25.9).
Table 1- Overall prevalence of Hydatid Cyst in dromedary camels at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia (n= 370).
Examined dromedary camels | Frequency | Prevalence |
Diseased | 96 | 25.9 |
Not diseased | 274 | 74.1 |
Total | 370 | 100 |
The distribution of hydatid cysts in different age groups, body condition score, sex and origin are illustrated in table 2. The prevalence and likelihood of occurrence of hydatid cyst was significantly (P value=0.03) higher (29.67%; 73/246, OR= 1.54) in adult age group than young dromedary camels (18.55%; 23/124). It’s true that the overall prevalence of hydatid cyst in adult dromedary camels was significantly (P= 0.03) higher (19.73%;73/370) than overall prevalence of young dromedary camels (6.23%; 23/370).
There was no statically significance difference between sex group (P value= 0.53) and origin of the camels (P value= 0.437). Out of 220 males examined, 54 camels were infected with hydatid cysts (24.5%, OR= 0.83) and from 150 female examined, 42 camels were infected (28%). The overall prevalence of hydatid cyst of males was 14.59%; 54/370 and of females was 11.35%; 28/370. Out of 146 examined camels from those came from Borana, 34 camels were positives (23.28%; 34/146) and the overall prevalence of hydatid cyst of camels from those came from Borana was 9.19%; 34/370.
Table 2- Prevalence analysis of hydatid cyst by age, body condition score, sex and origin (n= 370).
Risk Factors | Category level | Examined Number | No. of positive | Prevalence within group | Overall prevalence | P-value | Odds Ratio | ||
Age | Young (< 5> | 124 | 23 | 18.55 | 6.22 |
0.03 | Ref*
1.54 | ||
Adult (>= 5 years) | 246 | 73 | 29.67 | 19.73 | |||||
Body condition score | Poor | 57 | 35 | 61.4 | 9.46 |
0.00
0.53
0.43 | 10.79 | ||
Medium | 123 | 38 | 30.89 | 10.27 | 3.30 | ||||
Good | 190 | 23 | 12.11 | 6.22 | Ref* | ||||
Sex |
| Male | 220 | 54 | 24.54 | 14.59 | 0.83 | ||
| Female | 150 | 42 | 28 | 11.35 | Ref* | |||
Origin | Borana | 146 | 34 | 23.28 | 9.19 | Ref* | |||
East Hararghe | 5 | 2 | 40.00 | 0.54 | 2.12 | ||||
Jigjiga | 47 | 18 | 38.29 | 4.86 | 2.06 | ||||
Karrayyu | 49 | 13 | 26.53 | 3.51 | 1.18 | ||||
Matahara | 63 | 14 | 22.22 | 3.78 | 0.94 | ||||
Minjar-shonkora | 21 | 4 | 19.05 | 1.08 | 0.78 | ||||
Wollo | 39 | 11 | 28.205 | 2.97 | 1.31 | ||||
| Total | 370 | 96 | - | 25.94 | - | |||
In this study, the prevalence of hydatid cyst associated with body condition score was statically significant (P < 0 xss=removed xss=removed>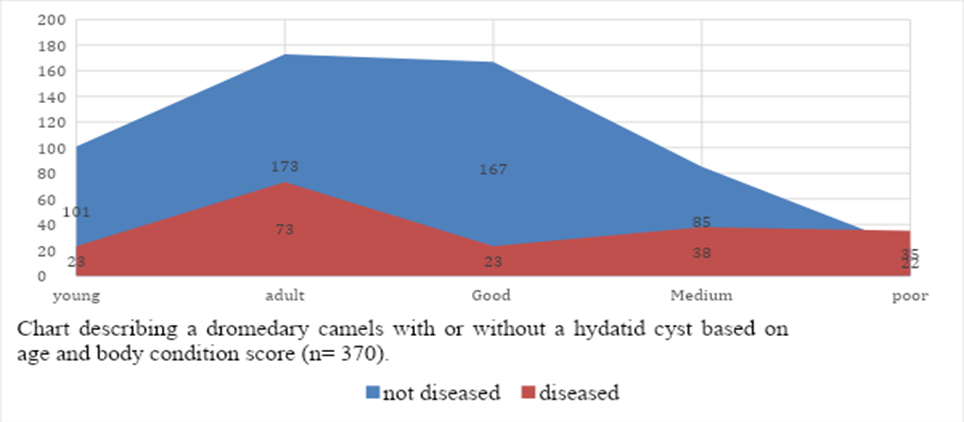
Out of 96 infected camels, 1 (1.04%) camel’s heart, 2 (2.08%) camel’s liver, 83 (86.46%) camel’s lung, 9 (9.38%) camel’s lung and liver, and 1 (1.04%) camel’s spleen had hydatid cyst in them. Among camels infected with a cyst, 90.62% had cysts only in a single organ while the remaining 9.38 had hydatid cyst on lung and liver. The overall frequency of hydatid cyst in different organs from the total examined camel is shown in table 3 below.
Table 3- Distribution of hydatid cyst in different organs
Organs | Numbers infected | Percent from infected dromedary camels | Prevalence from total examined dromedary camels |
Heart only | 1 | 1.04 | 0.27 |
Liver only | 2 | 2.08 | 0.54 |
Lung only | 83 | 86.46 | 22.43 |
Lung and liver | 9 | 9.38 | 2.43 |
Spleen only | 1 | 1.04 | 0.27 |
Total | 96 | 100 | 25.9 |
Out of total infected organs (105), infected lung was 92 (87.61%), liver was 11(10.47%) and the others spleen and heart were only one in number (0.95
3.2. Antemortem Examined Dromedary Camels
The dromedary camels were examined in the lairage both in motion and at rest for any abnormality (fracture, emaciation and presence of external parasite) in order to exclude those positive for slaughter and different parameters (body condition score, age, sex and origin) were recorded before their slaughter. Accordingly, there were no visible clinical signs recorded to protect them from slaughtering (figure 4).

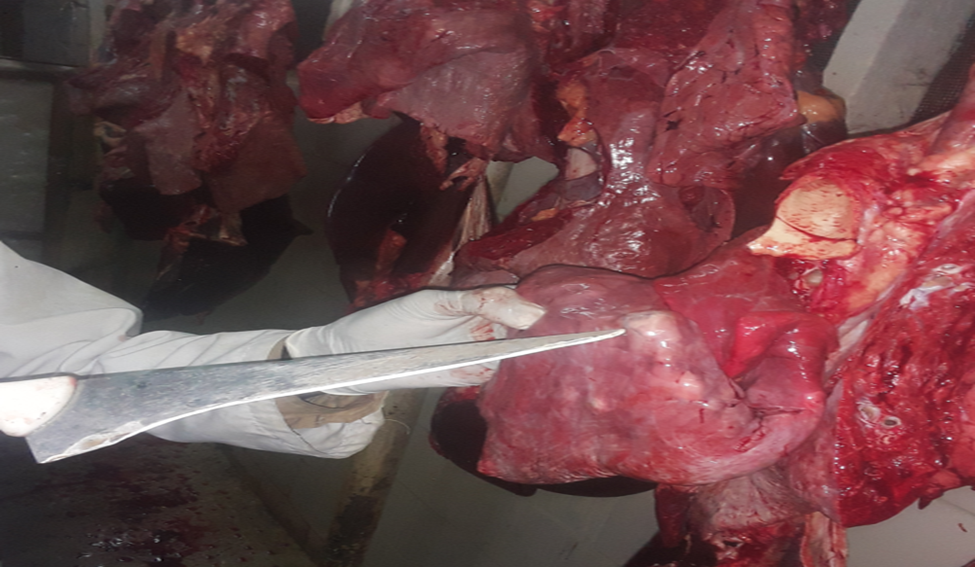
3.3. Gross Pathology
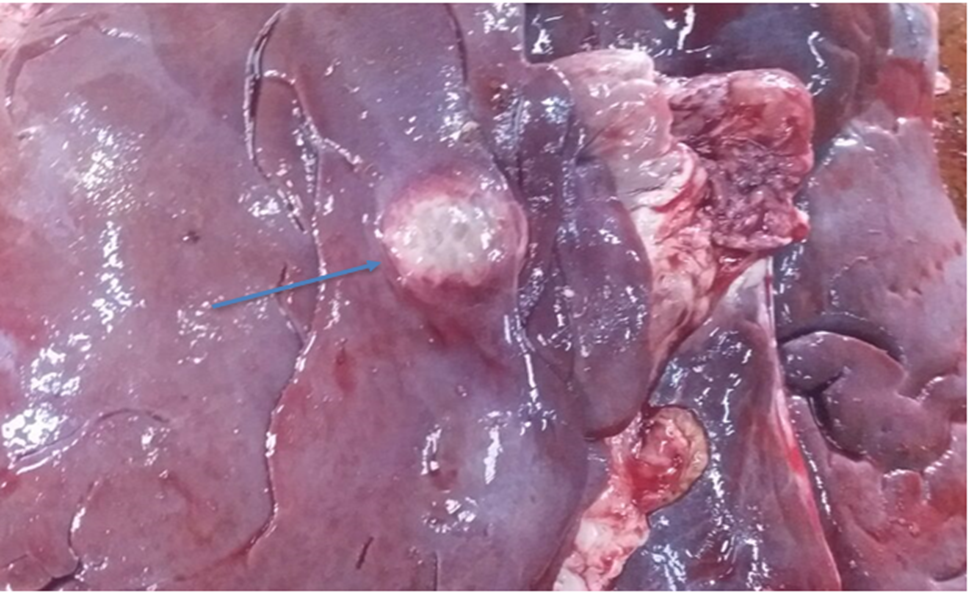
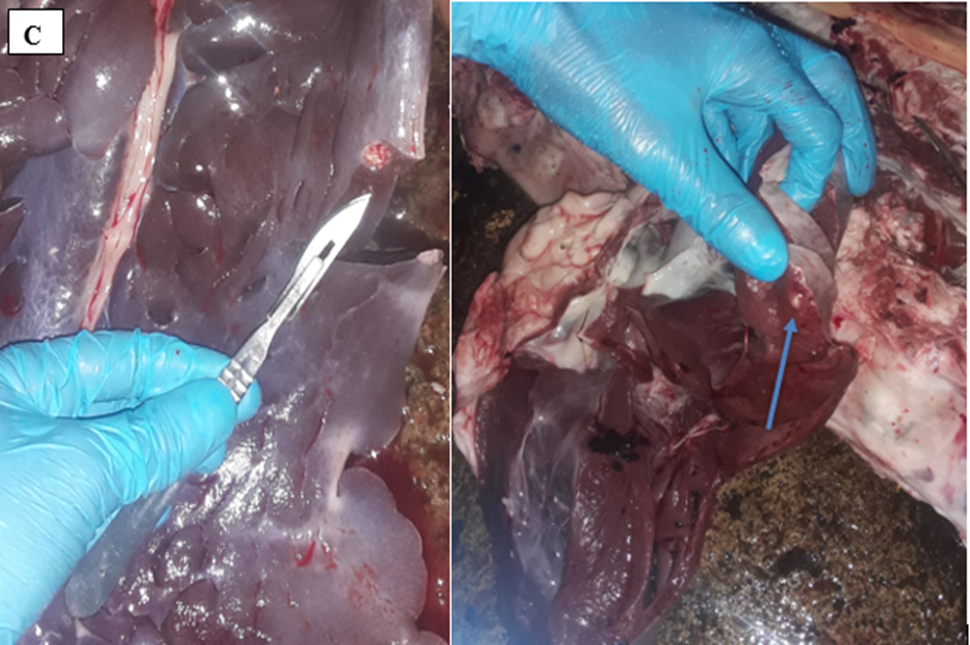
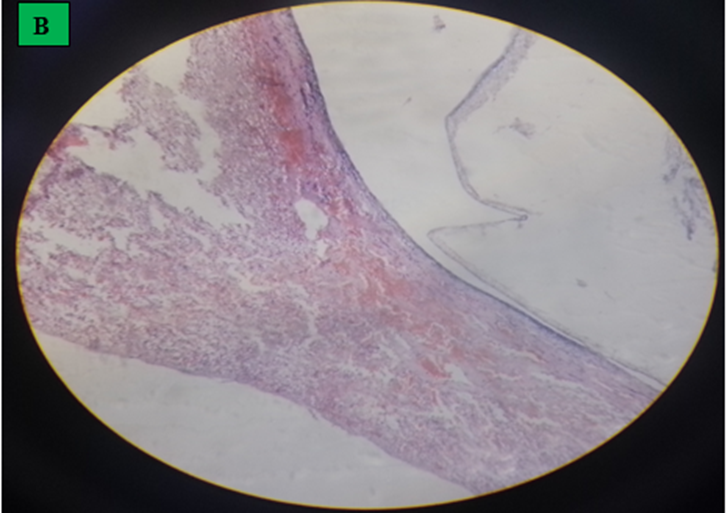
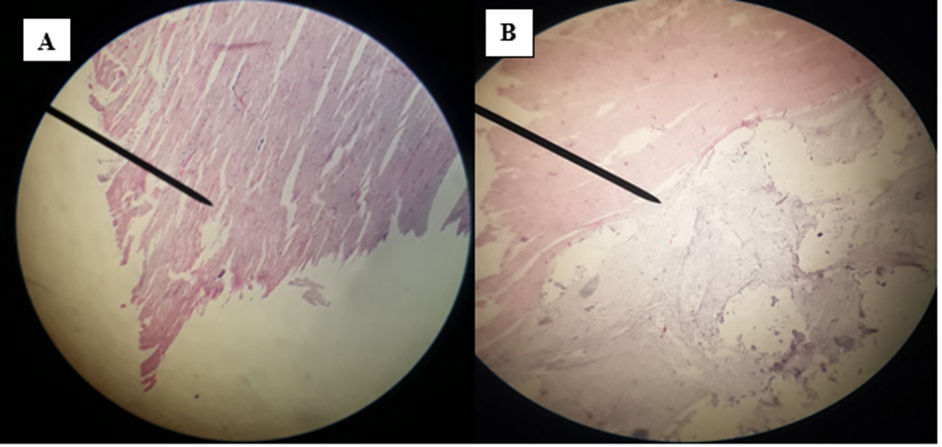
The affected organs were examined for any gross changes related to the cyst. The lung is exposed single to multiple hydatid cysts with water under pressure in the pulmonary parenchyma with different sizes. They have a shape of cotton ball and the cysts were implanted in the lung parenchyma or have been in part embedded after they have been seen from the lung surface. Dorsal and ventral portions of the lung had been affected. The cysts were filled with clean to incredibly turbid fluid, soft consistency and malleable to the touch in the lung of affected dromedary camels. Once the fluid was eliminated from the hydatid cyst of the lung tissue, the cysts collapsed and looked like an internal whitish germinal layer. Single to more than one hydatid cysts having small pea size were found from the visceral and or parietal surfaces of the liver. But, maximum cyst on livers was looking firm, calcified and hard to cut. The spleen was infected with the hydatid cysts on its dorsal part and the hydatid cysts on a spleen were doughy to the touch. Heart becomes affected with the hydatid cyst across the apex side and it has pliable consistency (figure 5).
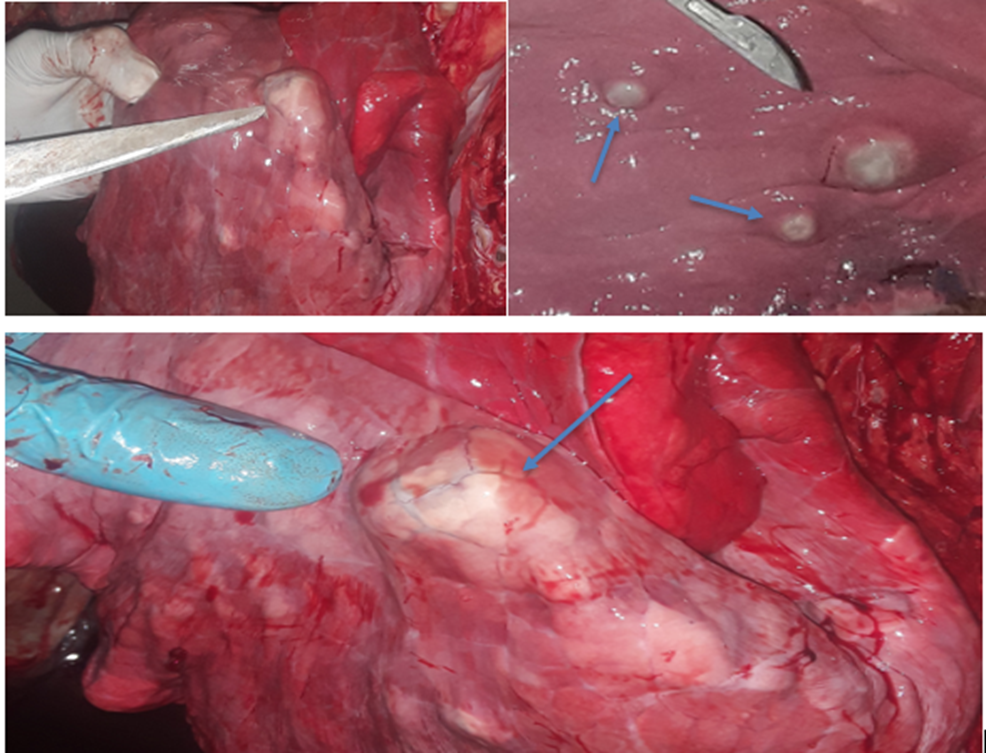
3.4. Microscopic Examination
Hematoxylin and Eosin-stained segment of lung showed the structure of hydatid cyst as outer fibrous layer (pericyst) which constitute host reaction to the parasite; has infiltrated with aggregates of lymphocytes and epithelioid macrophages cells revealing inflammatory response to cyst layers, middle acellular eosinophilic laminated membrane layer (ectocyst) and inner germinal layer (endocyst) and it changed into full of clean hydatid fluid. Segment of lung hydatid cyst fluid had protoscolices (figure 6- A and B).
Typically, Hematoxylin and Eosin-stained sections found out that the histological structure layer and infiltrated cell in hydatid cyst infected liver and heart were similar to that seen in the lung even if it had varied thickness of lamination; the cyst seemed with three layers.
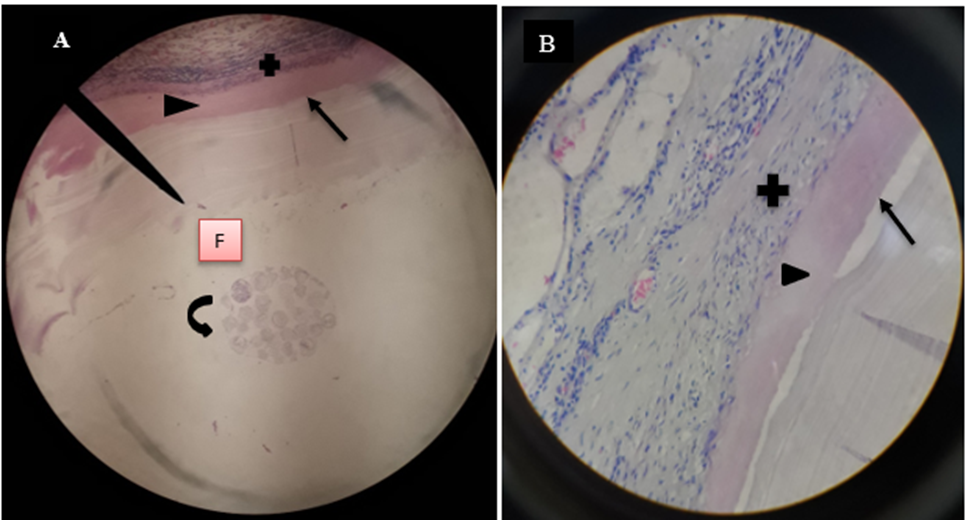
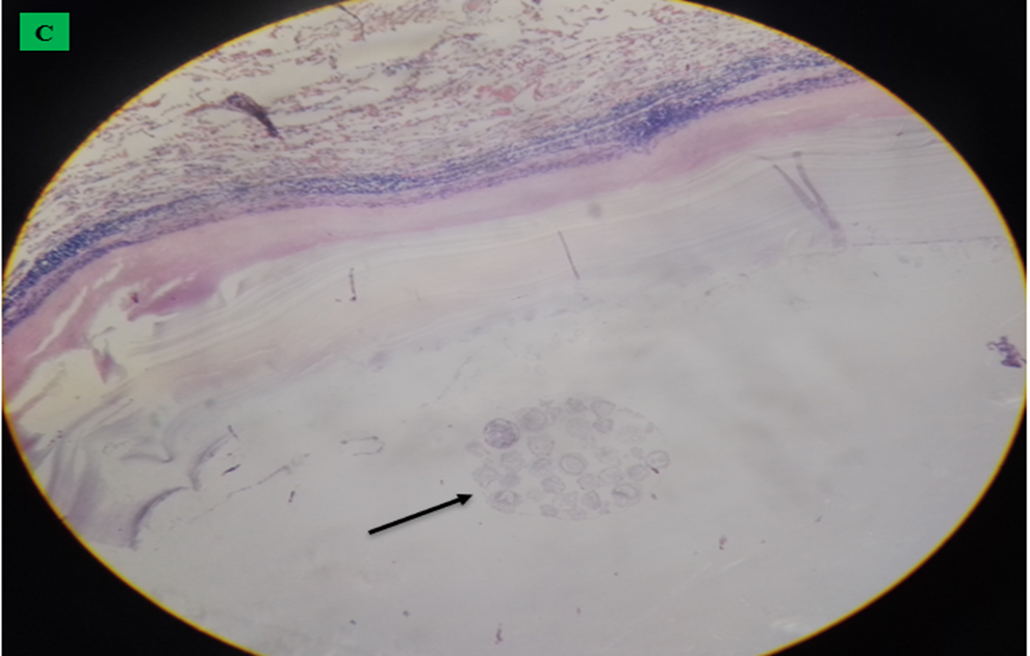
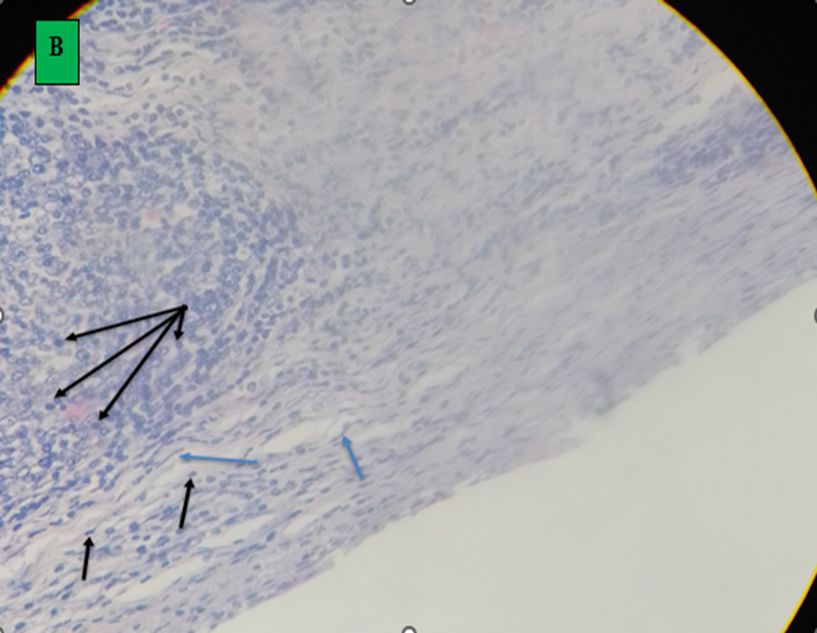
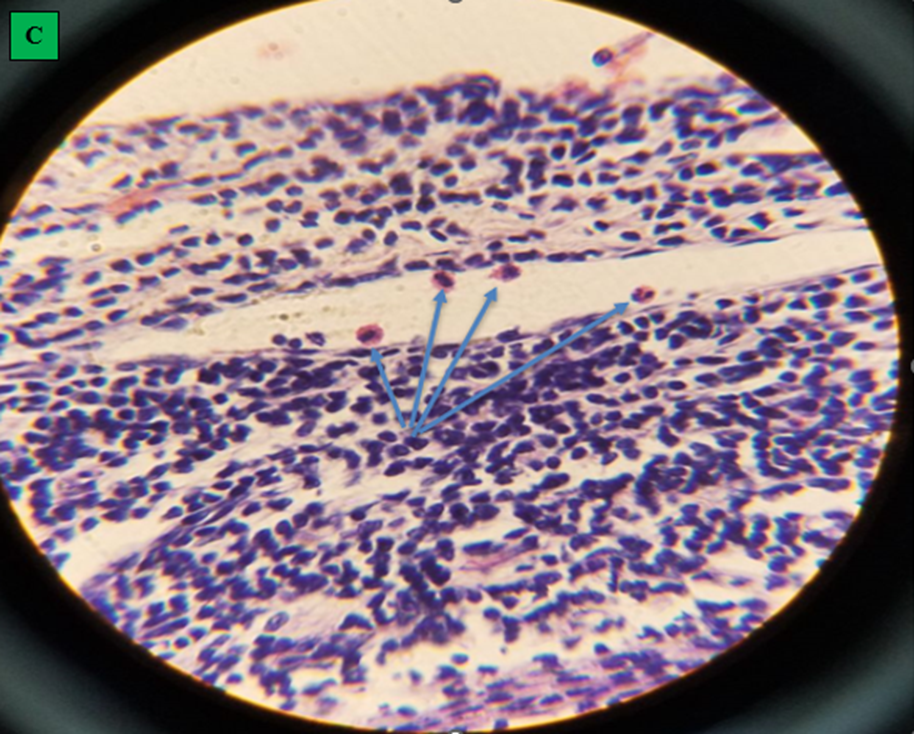
Microscopic finding of camel’s lung suffering from by hydatid cyst which shows pericyst (infiltrated with aggregates of lymphocytes and epithelioid macrophages cells revealing inflammatory response reaction to cyst layers and in to surrounding alveoli (cross shape), ectocyst; acellular laminated membrane (arrow head) and endocyst (germinal layer) (thin arrow). There was also ovoid protoscolices (curved arrow on picture A) within the fluid contained in the lumen (F) (All hematoxylin and eosin, seen under 100x (A) and 400x (B).
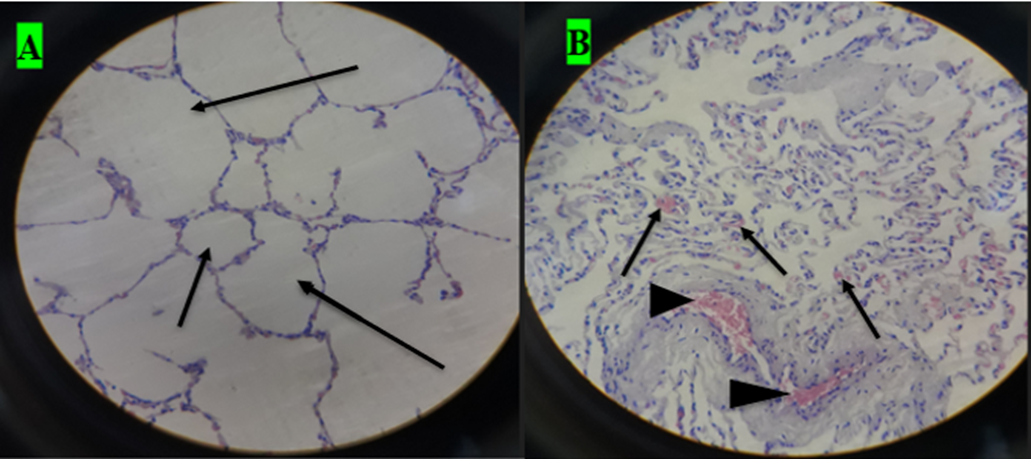
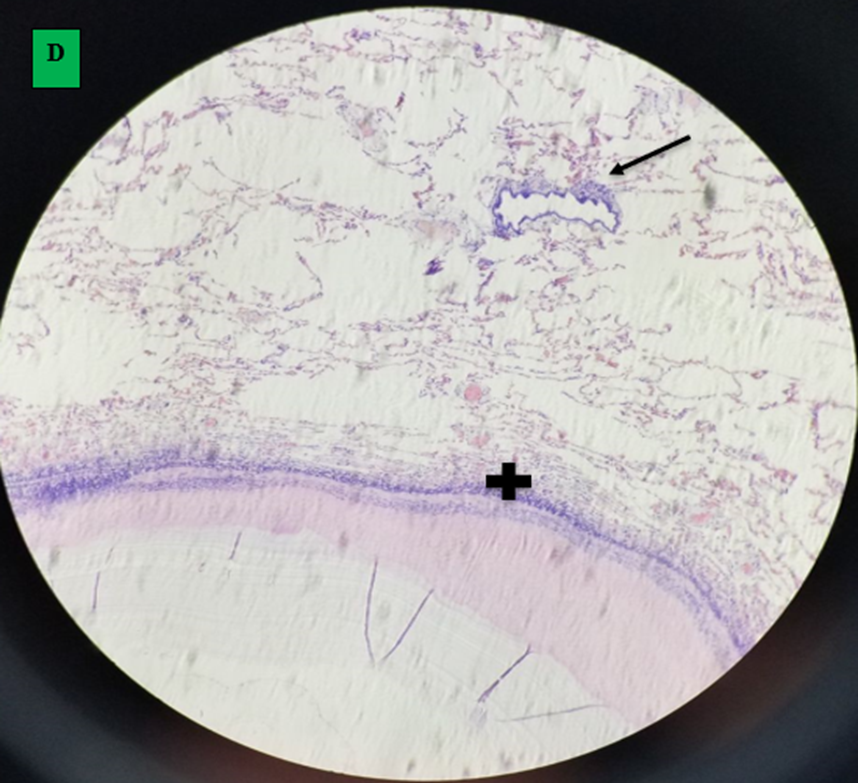
A. small cavity of alveoli structure; normal expansion of air space (small arrow) far away from cyst wall and other alveoli was emphysematous (larger arrow). B. Histopathological result of hydatid cyst in lung: inflammatory infiltrate in fibrous layer, capillary congestion (arrow head), hemorrhage (arrow), massive alveolar damage, atelectasis; collapsed alveoli (throughout slide). C. Hydatid cyst wall in lung, protoscolices (arrow). D. Hydatid cyst infected histopathology of lung with cellular infiltration surrounding bronchioles (arrow), cellular infiltration (cross) and hemorrhage (H and E stain, 400x).
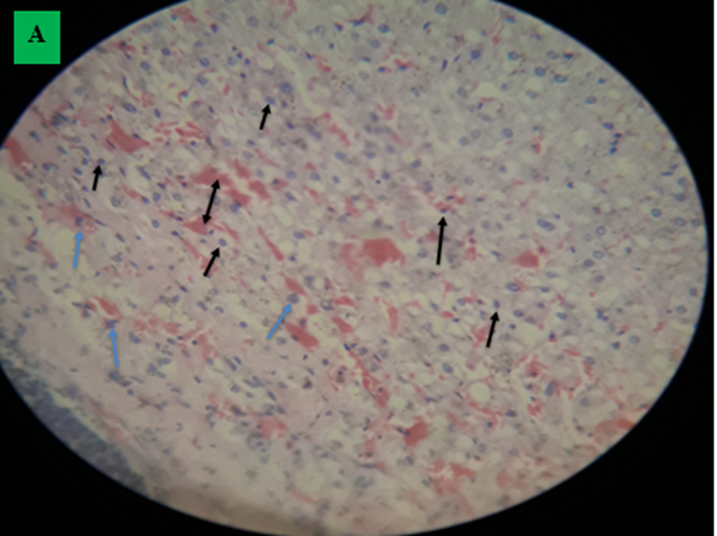
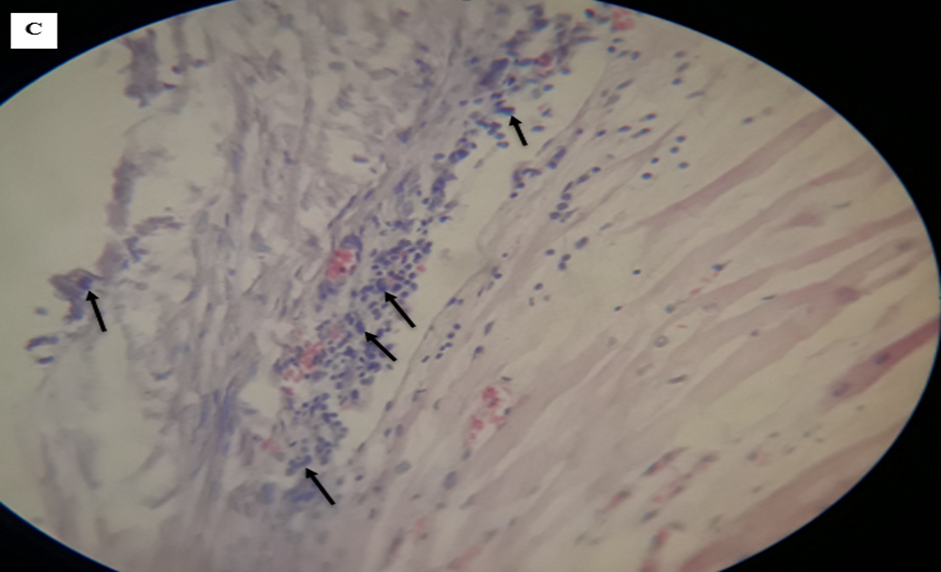
A. Infiltration of cell: Lymphocyte (black arrow), macrophages (green arrow). There was enormous hemorrhage (double arrow) and, hepatocyte degeneration and cytoplasmic swelling with dilation of nucleus. B. Fluid of hydatid cyst which had not a protoscolices, thin lamination free of a cell, hemorrhage and cellular infiltration forms broken fibrosis (H and E stained, 400X).
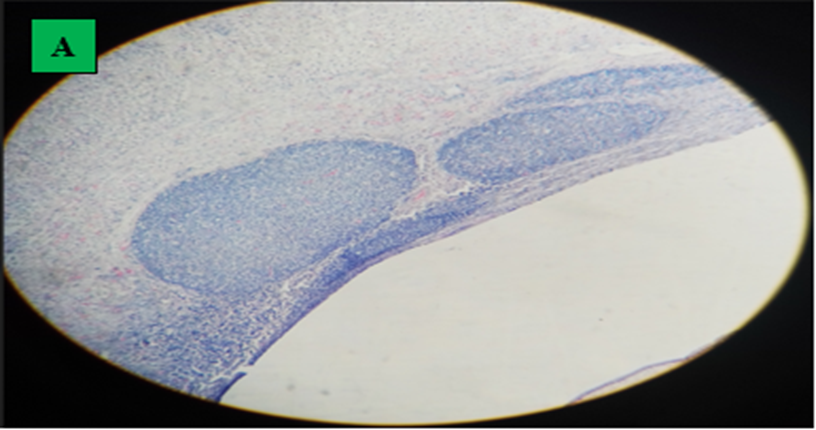
A. Histopathology of camel’s liver which indicated inflammatory nodules. B. Inflammatory nodules with lymphocyte (dominant) (interconnected arrow) and epitheliod macrophages (black arrow), collagen fiber as wave shape (blue arrow) and fibroblast nucleus, thin lamination of hydatid cyst, hydatid cyst fluid, C. Eosinophils migration in vessels (arrow) (H and E stained, 400x).
A. Branch and anastomosis which contain a nucleus in heart muscle and interconnected with a subsequent branch at an intercalated disk. B. The hydatid cyst had not a visible protoscolexs and it had weak germinal and lamination layer followed by cellular infiltration. C. Lymphocyte (dominant) and epitheloid macrophages infiltration (arrow) (H and E stained, 100x and 400x).
3.5. Assessment of Direct Annual Financial Loss
The foremost market fee of camels’ organs at Addis Ababa city was gained from abattoir employees and butchers for the duration of the study period. Average marketplace cost of camel’s lung, liver, spleen and heart at Addis Ababa town had been 15 Ethiopian Birr (ETB), 1000ETB, 5ETB and 120ETB respectively. The abattoir document displays that the mean yearly number of camels slaughtered in the course of the beyond three years become 2,555. Annual direct monetary loss was assessed in view of annual slaughter average of camel, proportion of affected organ and prevalence of hydatid cyst per organ and was expected to be 86,209.63ETB (1,657.8775 US Dollar), (table 6).
Table 5- Estimated annual direct financial losses due to organ condemnation in camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia.
Infected organ | Number infected | Proportion affected | Prevalence of hydatid cyst | AANCS | AMP in Birr (ETB) | ADFL in Birr |
Heart | 1 | 1.04% (1/96) | 25.9% | 2,555 | 120 | 826 |
Liver | 2 | 2.08% (2/96) | 25.9% | 2,555 | 1000 | 13,764.29 |
Lung | 83 | 86.46% (83/96) | 25.9% | 2,555 | 15 | 8,582.17 |
Lung and Liver | 9 | 9.38% (9/96) | 25.9% | 2,555 | 1,015 (1000 + 15) | 63,002.76 |
Spleen | 1 | 1.04% (1/96) | 25.9% | 2,555 | 5 | 34.41 |
Total | 96 | 100% | 25.5% | 2,555 | 2,155 | 86,209.63ETB (1,637.32$) |
AANCS= Average annual number of camels slaughtered; AMP= Average market price; ADFL= Annual direct financial loss; ETB= Ethiopian Birr; USD= United States Dollar. Note: 1 USD= 52.651ETB; 10 August, 2022.
4. Discussion
Hydatid cyst is a zoonotic helminthic parasite caused by Echinococcus granulosus affecting every livestock and human populations (Singh et al., 2010). The present study found that the prevalence of hydatid cyst in slaughtered dromedary camels at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia became 25.9%, which is comparatively less than earlier study reported as 28.7% by Hayer et al. (2014), 38.22% of Abebe et al. (2021), 61.6% by Boru et al. (2013), 65% by Regassa et al. (2015) and 65.47% by Gizachew et al. (2013). However, it is higher than the preceding reports 5.1% by Alembrhan and Haylegebriel (2013), 16.62% Tenaw et al. (2015), 18.79% by Dawit et al. (2019), 18.8% by Woldemeskel et al. (2001), 22.6% by Muskin et al. (2011) and 23% by Debela et al. (2014) from various area of Ethiopia. The higher prevalence in the present study might be due to the presence of higher numbers of dog; a definitive host which are closely associated with camels in the field and barn, along with high population of wild carnivores in majority of study area (Borana) (Balako, 1999) and there was lack of proper condemnation of infected organs in pastoral zones (Bekele, 2008).
Higher prevalence than the current studies were likewise reported from different country such as 32.85% by Mohamed (2010) from Saud Arabia, 35.2% by Ahmadi (2005) from Iran, 59% by Omer et al. (2010) from Sudan, and smaller prevalence of hydatid diseases than the present studies was reported from other countries; 8.25% (Draw city) and 8.5% (Aswan city) by Dyab et al. (2018), 14.64 % by Mohammad et al. (2016) from Tabriz area, Northwest Iran and 19.49% by Ohiolei et al. (2019) from Nigeria. Difference of prevalence in different regions in a country might related with aspects like difference in societal activity, livestock farming system, cultural variation, high number of stray dogs, lack of responsiveness of people, lack of suitable removal of infected carcass and visceral organs, lack of systematic deworming of a dog, and approach of camels to definitive host; a dog (Garippa et al., 2004; Yifat et al., 2011). Specifically, in the pastoralist areas of Ethiopia, typically in Borana, Jigjiga and Karrayyu area, the relationship between stray dogs and camels is very close. This causes the hydatid cyst to be more widespread than elsewhere.
In present study among one humped camel harboring hydatid cyst, lungs have been the most commonly affected visceral organs (87.61%; 92/105) followed by liver (10.47%; 11/105) and the remaining affected spleen (0.95%; 1/105) and heart (0.95%; 1/105) were minimum in proportion. From standard examined whole camels (370), organ distribution of cyst became 22.43% in lungs alone, 2.43% in both lung and liver whereas the remaining infected organs were less percent (0.54%, 0.27% and 0.27% in liver, spleen and heart alone respectively). This study is consistent with Abo El-Ala (2014), Dyab et al. (2018) and Fathi et al. (2012).
Lung infection with hydatid cyst was statistically highly significant in association with other predilection sites (P value < 0>
In the current study, the highest number of hydatid cyst per infected organ was recorded in the lung (less than three cysts 10.54%; 39/370 and greater or equal to three cysts 14.32%; 53/370) than liver (2.7%; 10/370). Spleen and heart had only less than three cyst numbers. Such differences in cyst quantity might be due to the spatial distribution and the infectivity of E. granulosus eggs and the exposure and self-protective capabilities of the host that agree with the study of Macpherson et al. (1985). Likewise, the high number of hydatid cysts in the lung may be due to the relatively softer consistency of lung compared to the liver. The lung tissue is softer in consistency and allows the easier development of the cyst than the liver tissue (Dyab et al., 2018). Small amount of hydatid cyst from the livers and caused it to become a calcified cyst was due to relatively the number of reticuloendothelial cells was high and connective tissue reaction of organs was ample. There was also a small hydatid cyst on the liver at the time of the study and it became supposed that the immunological response of the host avoids it from widening in extent (Larrieu et al., 2001; Torgerson, 2002).
In the present study, the degree of hydatid cyst prevalence within a male (24.55%, 54/220) and females (28%; 42/150) was not statically significant (P value= 0.53, OR= 0.83). But there was smaller prevalence in male than female camels. This is in line with the study of Rokni (2009) which reported that the degree of prevalence between males (34.4%) and females (36.6%) was not statistically significant. Also, Mohammad et al. (2016) reported that there were no significant differences found between male and female rates. However, the present study did not agree with the study of Boru et al. (2013) and Abebe et al. (2021), which reports as the prevalence of hydatid cyst was significantly higher in female camels (61.8%) than the male camels (40%). This might be due to a higher number of females (760) than male camels (only 10) were inspected during the previous study of Boru et al. (2013). This was probably caused the significant effect of hydatid cyst with sex because when a female camel came to the house area for milking, it directly or indirectly comes into contact with the dog which is definitive host of hydatid disease.
There was significant variation (P= 0.03, OR= 1.54) in camels with different age groups where hydatid cyst was higher in adult camels (greater or equal to 3 years old) with the prevalence within age group of 29.67%; 73/246 and 18.55%; 23/124 in young (smaller than 3 years old). This study agrees with the previous study of Azlaf and Dakkak (2006) and Osman and Abdalla (2013). Also, Dyab et al. (2018) found that there was a statistically important difference (P<0>
Analysis of body condition scores of camels had significant association (P<0 OR=10.79).>
The present finding revealed there was no statistical variation (P value= 0.43) in the prevalence rates between the areas where the examined dromedary camels originated (Borana, East Hararge, Jigjiga, Karayyu, Matahara, Minjar Shenkora and Wollo). The reason for the absence of variation in prevalence from those camels that came from different places may be related with the presence of very similar environmental situations. This study is consistent with the study of Hayer et al. (2014) and Regassa et al. (2015) which conveyed that there was no statistical difference (P> 0.05) in prevalence rate of hydatid cyst between study areas. The reason for the absence of an important difference in the prevalence in those different places might be associated with the existence of very similar ecological conditions from the major origin of slaughtered camels; Jigjiga, East Hararge, Borana and Matahara.
In the current study, there was gross expansion of affected organs, appearing as a couple of solid to soft on touch, tumor like cysts developing on the inner and outdoor parenchyma of affected organs and it was in line with the result of Romig et al. (2011). Mostly, affected lung had big sized cyst of soft consistency which had a shape of tennis ball, the cysts have been filled with clear to noticeably turbid fluid and moreover spleen and heart had soft whitish cyst while liver had small pea sized solid cyst were observed from the visceral and parietal surfaces of the liver. Similar findings were stated from other study areas by investigators (Akeel et al., 2017a, Canda et al., 2003, Ibrahim and Gameel, 2014 and Rashed et al., 2004).
In present study, histopathology of hydatid cyst infected organs (lung, liver, heart and spleen) had three parted structures called pericyst (outer adventitial layer), ectocyst (laminated layer eosinophilic in nature) and endocyst (thin inner germinal layer). In the most inspected segment of organs, the parasitic membranes (laminated membrane and germinal layer) were clearly seen in histopathology of lung. Endocyst membrane is the thinnest, small and translucent which produces and surrounds the cyst fluid. In this study, laminated membranes had varied thickness and number of laminations. Proliferation of monocyte-macrophage systems were stimulated by cuticular membranes. This finding is in agreement with the previous study of Canda et al. (2003) reported as the wall of hydatid cyst has three distinct layers; the outer acellular laminated membrane, germinal membrane and protoscolices and hydatid cyst might be walled by either a fibrous capsule or granulation tissue including inflammatory infiltrate.
In current study, the microscopic examination indicated that the tissue section (lung, liver, heart and spleen) had signs of reaction among the host (cellular) and hydatid cyst, characterized by fibrous layer infiltration with lymphocyte (dominant), epithelioid macrophages and eosinophils. Lung of hydatid cyst infected camels displayed massive alveolar damage, some alveoli was emphysematous, capillary congestion, hemorrhage and atelectasis; collapsed alveoli. Hydatid cyst layer was responded to by inflammatory reactions (lymphocytes and epithelioid macrophages) which decreased as they far away from the cyst layer. The inflammatory reaction regularly moved into the surrounding alveoli and small bronchioles. Typically, a hydatid cyst infected liver showed infiltration of inflammatory cells and nodules formation with intermixed lymphocyte (dominant) and epithelioid macrophages, collagen fiber and fibroblast nucleus and thin lamination of hydatid cyst. Enormous hemorrhage, hepatocyte degeneration and cytoplasmic swelling with dilation of nucleus in fibrous layer was another finding. Branch and anastomosis which contain a nucleus in heart muscle and interconnected with a subsequent branch at an intercalated disk and the cyst had not a visible protoscolices and it had weak germinal and lamination layer followed by lymphocyte (dominant) and epithelioid macrophages infiltration. This finding is in line with Akeel et al. (2017a), Akeel et al. (2017b), Dyab et al. (2020) and Khadidja et al. (2014).
The current study indicated that less financial losses; 86,209.63ETB (1,637.32 US Dollar), were recorded due to organ condemnations than the previous study of Dawit et al. (2019), reported as 142, 133.55ETB (5214.95US Dollar) and Gizachew et al. (2013), estimated the financial loss as 1,089,758.8ETB (61,222.4 US Dollar). This less economic loss was due less hydatid cyst prevalence (25.9%) than that of Gizachew et al. (2013) (65.5%), and hydatid cyst had higher proportion in only lungs (86.46%) than only livers (2.08%) when compared to the previous study of Dawit et al. (2019) (proportion of infected only lung and liver was 51.28% and 32.05%, respectively which might be resulted in higher annual direct financial losses than the present study. But all hydatid cyst infected organs are equally important to transmit toward a definitive host (dog and wild carnivorous). So that attention should be made to control and prevent the camel hydatid cyst.
5. Conclusion and Recommendations
In conclusion, the prevalence of hydatid cyst of dromedary camels slaughtered at Addis Ababa, Akaki Kality Municipal abattoir, Ethiopia was identified as 25.9%. Age and body condition score were significant associated risk factors and play a role in the likelihood of yielding hydatid cyst. Hydatid cyst was most commonly found on the single organ of camels. Percentage distribution of hydatid cyst was higher in the lung than the liver. According to systematic meat inspection conducted, many visceral organs had a cyst number of three or more. Grossly, a hydatid cyst of lung shaped like a cotton ball, implanted in the lung parenchyma, filled with clear to slightly turbid fluid, soft to touch and whitish inside. However, in the liver it was firm, calcified and hardened when cut. Histological study of hydatid cyst in dromedary camels allowed us to identify the structural layers of this zoonotic parasite in to three structures on infected visceral organs namely; fibrous layer, an acellular laminated membrane layer and germinal layer from external to internal respectively. Fibrous layer had infiltration of lymphocytes, epithelioid macrophages and eosinophils indicative of inflammatory reaction response for the cyst layer. The number of infiltrated cells becomes smaller as it moves away from the cyst layer. Total annual direct financial loss on the study area plays a role in the economy. According to the above conclusion, the following recommendations are given:
- It would therefore be necessary to keep camel health management.
- Dromedary camels need to be divided into their age and body condition scores and protect them.
- It is obligatory to teach the life cycle and significance caused by hydatid cyst on animals and humans.
- More studies and research on hydatid cyst of dromedary camel should be conducted in different parts of Ethiopia to continue and deepen this study by molecular characterization, know economic loss, clarify the epidemiological role of different animals in the transmission of the cyst in the regions and so as to provide effective prevention.
References
- Abdiselam M.H., Mersha C.K. and Ismail W. (2014). Prevalence, Economic and Public Health Significance of Camel Hydatidosis in Dire Dawa Municipal Abattoir, Eastern Ethiopia. Acta. Parasitologica Globalis, 5 (2): 98-106. DOI: 10.5829/idosi.apg.2014.5.2.84209
- Abebe F. and Yilma Y. (2013). Estimated annual economic loss from organs condemnation, decreased carcass weight and milk yield due to bovine hydatidosis. Ethiop. Vet. J., 16(2): 1-14(22). Schantz PM (1990) Parasite zoonosis.
- Abebe N., Biruhtesfa A. and Berhanu M. (2021). Prevalence of Cystic Echinococcosis in One-Humped Camels Slaughtered at Addis Ababa Municipality Abattoir, Ethiopia. Ethiop. Vet. J., 25 (1): 43-57. https://dx.doi.org/10.4314/evj.v25i1.3.
- Abo El-Ala S.M.E. (2014). Studies on some Metacestodes Infecting Ruminants. MVSC. Thesis of veterinary parasitology, Cairo University.
- Aboma R., Nesibu A., Birhanu H., Yisehak T. and Teshale S. (2015). Internal and external parasites of camels (Camelus dromedarius) slaughtered at Addis Ababa Abattoir, Ethiopia. J. Vet. Med. Anim. Health., 6(7): 30-60. DOI: 10.5897/JVMAH2014.0346.
- Abriham A.T. (2021). Review on Hydatidosis, Its Epidemiology and Economic Importance in Ethiopia. Hadiya Zone, Mirab Badawacho Woreda Livestock and Fishery Resource Development Office, SNNPR, Ethiopia. Int. J. Adv. Res. Biol. Sci., 8(7): 126-137.
- Ahmadi N.A. (2005). Hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Iran. Published online by Cambridge University Press. J. Helminthol., 79(2): 119-125. Doi: 10.1079/joh2005279. PMID: 15946390.
- Ahmadi N.A. and Meshkehkar M. (2011). An abattoir-based study on the prevalence and economic losses due to cystic echinococcosis in slaughtered herbivores in Ahwaz, south-western Iran. J. Helminthol., 85(1): 33-39. Doi:10.1017/S0022149X10000234. Epub 2010 Apr 19. PMID: 20398435.
- Ahmadnia S., Moazeni M., Mohammadi-Samani S. and Oryan A. (2013). In vivo evaluation of the efficacy of albendazole sulfoxide and albendazole sulfoxide loaded solid lipid nanoparticles against hydatid cyst. Exp. Parasitol., 135: 314-319.
- Akeel B.B., Mohmommad M.D., Samina B., Bisma K., Aazima Sh. and Showkat A.Sh. (2017a). Pathological and histochemical studies of the effects of cystic echinococcosis in sheep. Comp. Clin. Path. https://doi.org/10.1007/s00580-017-2606-0.
- Akeel B.B., Mohmommad M.D., Samina B., Bisma K., Aazima Sh. and Showkat A. Sh. (2017b). Gross and histopathological alterations associated with cystic echinococcosis in small ruminants. J. Parasit. Dis. 41(4): 1028–1033. Doi 10.1007/s12639-017-0929-z.
- Alembrhan A. and Haylegebriel T. (2013). Major causes of organ condemnation and economic loss in cattle slaughtered at Adigrat municipal abattoir, northern Ethiopia. Vet. World., 6(10): 734- 738. Doi: 10. 14202/vetworld.2013.734-738.
- Alloubi I. (2013). Thoracic hydatid cyst: Clinical presentation, radiological features and surgical treatment. Principle and Practice of Cardiothoracic surgery. http://dx.doi.org/10.5772/53533.
- Ayele G. (1989). The future of camel rearing for food production in Ethiopia. In: Tegegne T. Edited, Camel pastoralism as food system in Ethiopia. Addis Ababa, Ethiopia, Institute of Development Research (IDR)/Uppsala, Sweden, Scandinavian Institute of African Studies (SIAS), pp. 1-8.
- Azlaf R. and Dakkak A. (2006). Epidemiological study of cystic echinococcosis in Morocco. Department of Parasitology and parasitic diseases, Institut Agronomique et Veterinaire Hassan II, BP 6202, Rabat-Instituts, Agdal, Rabat, Morocco. Vet. Parasitol., 137: 83-93.
- Balako G. (1999). Observation on diseases of one humped camel in Southern Ethiopia, Abattoir Survey. DVM thesis, Addis Ababa University, Faculty of Veterinary Medicine, DebreZeit, pp. 1-43.
- Bayleyegn G., Fikadu K. and Biruhtesfa A. (2013). Camel hydatidosis: Prevalence and economic significance in pastoral regions of Ethiopia. College of Veterinary Medicine, Haramaya University. J. Parasitol. Vector. Biol., 5(6): 90-95. https://doi.org/10.5897/JPVB2013.0115.
- Behnke R., (2010). The contribution of livestock to the economies of IGAD member states: Study findings, application of the methodology in Ethiopia and recommendations for further work. IGAD Livestock Policy Initiative Working. Pp. 02–10.
- Beigh A.B., Darzi, M.M., Bashir S., ShahA. and Shah, S.A. (2018). The pathology of cystic echinococcosis and structural details of hydatid cyst and protoscolex. Indian J. Vet. Pathol., 42(1): 8-14. DOI: 10.5958/0973-970X.2018.00002.0
- Bekele J. and Butako B. (2011). Occurrence and financial loss assessment of cystic Echinococcosis (hydatidosis) in cattle slaughtered at WolaitaSodo municipal abattoir, Southern Ethiopia. Trop. Anim. health. Prod. 43(1): 221-8. Doi: 10.1007/s11250-010-9680-5. Epub 2010 Aug 30. PMID: 20803350.
- Bekele S.T. (2008). Gross and Microscopic lesions of camels from Eastern Ethiopia. Trop. Anim. Health. Prod., 40: 25-28. https://doi.org/10.1007/s11250-007-9046-9.
- Bektas S., Erdogan N.Y., Sahin G., Kir G.and Adas G. (2016). Clinicopathological Findings of Hydatid Cyst Disease: A Retrospective Analysis. Ann. Clin. Pathol., 4(3): 1071.
- Belay, T. (2010): Adapting to the impacts of climate variability and change on agriculture: A model-based exploration across different scales in Ethiopia. PhD proposal.
- Belina D., Demissie T., Ashenafi H. and Tadesse A. (2015). Comparative pathological study of liver fluke infection in ruminants. Haramaya University, College of Veterinary Medicine. Addis Ababa University CVMA. Hawasa University, school of veterinary Medicine, Awasa. Indian J. Vet. Pathol., 39(2): 113-120. DOI: 10.5958/0973-970X.2015.00027.9
- Bello A., Sonfada M.L., Umar A.A., Umaru M.A., Shehu S.A., Hena S.A., Onu J.E. and Fatima O.O. (2013). Age estimation of camels in Nigeria using rostral dentition. Sci. J. Anim. Sci., 2(1): 9-14. ISSN 2322-1704.
- Beyene S. and Gudina D. (2009): Reviving a traditional pasture management system in Fantale, East Central Ethiopia. J. Ecol. Anthropol., 13(1): 57-72. DOI: 10.5038/2162-4593.13.14.
- Boru B.G., Tolossa Y.H., Tilahun G. and Ashenafi H. (2013). Study on prevalence of hydatidosis and cyst characterization in camels (Camelus dromedarius) slaughtered at Akaki abattoir, Ethiopia. J. Vet. Med. Anim. Health., 5: 329-333. DOI 10.5897/JVMAH2013.0239. http://www.academicjournals.org/JVMAH.
- Canda M.S., Guray M., and Canda T. (2003). The Pathology of Echinococcosis and the Current Echinococcosis Problem in Western Turkey (A Report of Pathologic Features in 80 Cases). Turk. J. Med Sci., 33(6): 369-374. DOI: 10.3906/sag-0209-8.
- Carmen S., Gheorghe S., Mariana I., Doru V.H. and Ioan L.M. (2010). Histological aspects of cystic echinococcosis in goats. Sci. Parasitol., 11(4): 191-198.
- Central Statistical Authority (CSA) (2016). Federal Democratic Republic of Ethiopia Agriculture sample survey, Addis Ababa. Staticall Bulletin, 2: 583.
- Cobo F., Yarnoz C., Sesma B., Fraile P., Aizcorbe M. and Trujillo R. (1998). Albendazole plus praziquantel versus albendazole alone as a preoperative treatment in intra-abdominal echinococcosis caused by Echinococcus granulosus. Trop. Med. Int. Health., 3: 462-6.
- Coppock D.L. (1993). International Livestock Centre for Africa, Executive Summary. Systems Study: The Boran Plateau of Southern Ethiopia: Synthesis of pastoral research development and change, 1980-1991. Addis Ababa, Ethiopia, ILRI. PO BOX 5689, Addis Ababa, Ethiopia.
- Coppock D.L., (1994). The Borana plateau of southern Ethiopia: Synthesis of pastoral research, development and change, 1980–91. Addis Ababa: ILCA systems study no. 5, International Livestock Centre for Africa. Pp. 374.
- Craig P.S., McManus D.P., Lightowlers M.W., Chabalgoity J.A., Garcia H.H., Gavidia C.M., Gilman R.H., Gonzalez A.E., Lorca M., Naquira C., Nieto A. and Schantz P.M. (2007). Prevention and control of cystic echinococcosis. Lancet. Infect. Dis., 7: 385–394. http://dx.doi.org/10.1016 /S1473-3099(07)70134-2.
- CSA (2003). Statistical report on livestock and farm implements, part IV, Addis Ababa, Ethiopia.
- CSA (2006). Oromia livelihood zone reports (OLZR) at 2008 total population of Fantalle district.
- CSA (2007). Oromia statistical population and housing census 2007.
- Dakkak A. (2010). Echinococcosis/hydatidosis: A severe threat in Mediterranean countries. Vet. Parasitol., 174(1-2): 2–11. DOI: 10.1016/j.vetpar.2010.08.009. Epub 2010 Aug 20. PMID: 20888694.
- Dalimi A.A., Madani R., Ghorbankhani D. and Salami S. (2000). Comparative evaluation of serodiagnosis techniques in cystic hydatid disease. Arch. Inst. Razi., 51: 85-93.
- Daryani A., Alaei Arab R., Sharif M., Dehghan M.H. and Ziaei H. (2007). The prevalence intensity and viability of hydatid cyst in slaughtered animals in the Arab provinces of North West Iran. J. helminthol., 18: 13-17.
- Dawit K., Mustafe A. and Hailemariam K. (2019). Magnitude of Camel Hydatidosis, Associated Risk Factors and its Economic Significance at Jigjiga Municipal Abattoir, Eastern Ethiopia. EAJVAS; East African Journal of Veterinary and Animal Sciences., 3 (1): 47-54. https://creativecommons.org/licenses/by-nc/4.0/.
- Debela E., Abdulahi B., Megersa B., Kumsa B., Abunna F., Sheferaw D. and Regassa A. (2014). Hydatidosis of camel (Camelus dromedarius) at Jijiga municipal abattoir, Eastern Ethiopia: prevalence, associated risk factors and financial implication. J. Parasit. Dis., 39(4): 730–735. DOI: 10.1007/s12639-014-0403-x.
- Debela G.A. (2014): Traditional camel management as an adaptation strategy to ecological changes: The case of Karrayyu Oromo of Ethiopia. Thesis submitted for the Degree of Master of Philosophy in Indigenous Studies. Faculty of Humanities, Social Sciences and Education UiT the Arctic University of Norway.
- Debela N., McNeil D., Bridle K. and Mohammed C. (2019): Adaptation to climate change in the pastoral and agropastoral systems of Borana, South Ethiopia: options and barriers. Am. J. Clim. Chang., 8(1): 40–60. DOI: 10.4236/ajcc.2019.81003.
- Degefa T. and Tesfaye T. (2008): Linkages between Water Supply and Sanitation and Food Security. A case study in four villages of East Hararge zone, Oromia region. Research-inspired policy and Practice Learning in Ethiopia and the Nile region (RiPPLE). Pp. 1-51.
- Derbala A.A. and Zayed A.A. (1997). Prevalence, fertility and viability of cysticercosis and hydatidosis infections in some domestic animals. J. Union Arab Biol., 7: 109-123.
- Dunn A. (1987). Veterinary Medicine, Addis Ababa University, Ethiopia. Handbook for Ethiopia. Pp. 2.
- Dyab A.K., Ahmed M.A. and Ahmed G. (2020). Histopathological Studies on parasitic affections of lung and liver of Humped Camels in Aswan Slaughter houses, Egypt. By Department of Parasitology, Faculty of Medicine Assiut University, Egypt. https://www.researchgate.net/publication/340621494.
- Dyab A.K., Marghany M.E., Othman R.A., Ahmed M.A. and Abd-Ella O.H. (2018). Hydatidosis of camels and sheep slaughtered in Aswan Governorate, Southern Egypt. Russ. J. Parasitol., 12(3): 33–41. DOI: 10.31016/1998-8435-2018-12-3-33-41.
- Eckert J. and Deplazes P. (2004). Biological, epidemiological and clinical aspects of echinococcosis a zoonosis of increasing concern. Clin. Microbiol. Rev., 17(1):107-135. DOI: 10.1128/CMR.17.1.107-135.2004. PMID: 14726458; PMCID: PMC321468.
- Eckert J. and Deplazes P. (2004). Biological, epidemiological and clinical aspects of echinococcosis a zoonosis of increasing concern. Clin. Microbiol. Rev., 17(1):107-135. DOI: 10.1128/CMR.17.1.107-135.2004. PMID: 14726458; PMCID: PMC321468.
- Eckert J., Schantz P.M., Gasser R. B., Torgerson P.R., Bessonov A.S., Movsessian S.O., Thakur A., Grimm F. and Nikogossian M.A. (2001). Geographic distribution and prevalence. In: WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern, Eckert J., Gemmell M.A., Meslin F.X. and Pawlowski Z.S. (Ed). Paris: World Organization for Animal Health, pp. 101–143. 929044522X.pdf. https://apps.who.int/iris/handle/10665/42427.
- El-Metenawy T.M. (1999). An abattoir survey of metacestodes among slaughtered ruminants of al- Qassim, Saudi Arabia, Ministry of Agriculture and water, Bureidah (Saudi Arabia), Qassim Veterinary Diagnostic Lab. Pakistan Vet. J., 19(2): 84-87.
- Etana D., Buckhary A., Bekele M., Bersissa K., Fufa A., Desie Sh. and Alemayehu R. (2015). Hydatidosis of camel (Camelus dromedarius) at Jijiga municipal abattoir, Eastern Ethiopia: prevalence, associated risk factors and financial implication. J. Parasit. Dis., 39(4): 730–735. DOI: 10.1007/s12639-014-0430-x.
- Fathi S., Dehaghi M.M. and Radfar M.H. (2012). Occurrence of hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Kerman area, southeast of Iran. Comp. Clinic. Pathol. J., 21 (5): 921–927. DOI: 10.1007/s00580-011-1200-0.
- Faye B., (2015). Role, distribution and perspective of camel breeding in the third millennium economies. Emir. J. Food Agric., 27 (4): 318-327. http://www.ejfa.info/.
- Faye B., Bengoumi M., Cleradin A., Tabarani A. and Chilliard Y. (2001). Body condition score in dromedary camel: A tool for management of reproduction. Emir. J. Agric.Sci., 13: 1-6.
- Food and Agricultural Origination (FAO) (1994). Manual on meat inspection for developing countries, FAO, Rome Italy, by Herenda D., Chambers P.G., Ettriqui A., Seneviratna p. and da Silva T.J.P.
- Food and Agriculture Organizations (2009): The State of Food and Agriculture “Livestock in the Balance”. Food and Agriculture organization of the United Nations Rome, Italy. Pp. 1-180. http://www.fao.org/catalog/inter-e.htm.
- Food and Agriculture Organizations (2016): Food and Agriculture Organization corporate statistical database. http://www.fao.org/faostat/en/ #data.
- Garippa G., Varcasia and Scala A. (2004). Cystic echinococcosis in Italy from the 1950s to present. Parassitologia., 46(4): 387-391. PMID: 16044697.
- Gebre A. (2009): When pastoral commons are privatized: Resource deprivation and changes in land tenure systems among the Karrayyu in the upper Awash valley region of Ethiopia. In: Proceedings of the 16th International Conference of Ethiopian Studies, eds by Svein E., Harald A., Birhanu T. and Shiferaw B., Trondheim. Pp. 283-197.
- Gemmell M.A., Roberts T.C., Beard S., Campano Diaz J.R, Lawson J.R and Nonnemaker J.M. (2001). Control of Echinococcus granulosus. In: Eckert J., Gemmell M.A., Meslin F.X, Pawlowski Z.S editors. WHO/OIE Manual on echinococcosis in humans and animals. Paris: World Health Organization for Animal Health; Pp. 195–204.
- Getaw A., Beyene D., Ayana D., Megersa B., Abuna F. (2010). Hydatidosis prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir. Central Oromia, Ethiopia. Acta. Trop., 113(3): 221-5. DOI: 10.1016/j.actatropica.2009.10.019. Epub 2009 OCT 31. PMID: 19883622.
- Gizachew B., Kibru F. and Asrade B. (2013). Camel hydatidosis: Prevalence and economic significance in pastoral regions of Ethiopia. J. Parasitol. Vector Biol., 5(6): 90-95, DOI: 10.5897/JPVB2013.0115. http://www.academicjournals.org/JPVB.
- Guisantes J.A. (2014). Control and Prevention of Hydatidosis: Turgut M.(ed.): Hydatidosis of the Central Nervous System: Diagnosis and Treatment. DOI: 10.1007/978-3-642-54359-3_25. Pp. 305-14.
- Hailu D. and Tegene D. (2013): Hydatidosis of cattle and sheep, its economic importance and Echinococcus granulosus among stray dogs in South Wollo, Ethiopia. Ethiop.Vet.J., 17(2): 101-119. http://dx.doi.org/10.4314/evj.v17i2.8.
- Haniloo A., Massoud J. and Rokni M.B. (2005). Evaluation and comparison of antigen B-ELISA and antigen B-immunoblotting in immunodiagnosis of cystic hydatid disease. Pak. J. Med. Sci., 21(3): 352-356.
- Haridy F.M., Ibrahim B.B., Elshazly A.M., Awad S.E., Sultan D.M., EL-Sherbini G.T. and Morsy T.A. (2006). Hydatidosis granulosus in Egyptian slaughtered animal in the year 2000-2005. J. Egypt Soc.Parasitol., 36(3): 1087-100. PMID: 17153715.
- Hayer A.M., Kebede C.M. and Warsame I. (2014). Prevalence, Economic and Public Health Significance of Camel Hydatidosis in Dire Dawa Municipal Abattoir, Eastern Ethiopia. Acta. Parasitol. Glob., 5 (2): 98-106. DOI: 10.5829/idosi.apg.2014.5.2.84209.
- Ibrahim M.M., Ghamdi M. and Gahmid M.M. (2008). Helminths community of veterinary importance of livestock in relation to some ecological and biological factors. Turkiye. Parasitol. Derg, 32(1): 42–7. PMID: 18351550.
- Ibrahim S.E.A. and Gameel A.A. (2014). Pathological, histochemical and Immunohistochemical studies of lungs and livers of cattle and sheep infected with hydatid disease. In: Conference Proceedings- U. of. K. Graduate College and Scientifi Research. 5th annual conference-Agricultural and veterinary research. Khartoum, Sudan., 2: 328–344.
- Jenkinsa D.J., Romig T. and Thompson R.C.A. (2005). Emergence or re-emergence of Echinococcus spps. A global update. Int. J. parasitol., 35(11-12): 1205- 1219. DOI: 10.1016/J.IJpara.2005.07.014. PMID: 16157340.
- Kebede N., Mekonnen H., Wossene A. and Tilahun G. (2009). Hydatidosis of slaughtered cattle in Wolaita Sodo Abattoir, Southern Ethiopia. Trop. Anim. Health Prod., 41(4): 629-33. DOI: 10.1007/s11250-008-9234-2. Epub 2008 Sep 12. PMID: 18787969.
- Kertesz P. (1993). A Colour Atlas of Veterinary Dentistry and Oral Surgery. Wolfe Publishing, London. 5(3): 174-174. https://doi.org/10.1111/j.2042-3292.1993.tb01033.x.
- Khadidja H., Achour Y., Houcin B. and Vasile C. (2014). Histological Appearance of Echinococcus Granulosus in the Camel Species in Algeria. Bulletin UASVM Veterinary Medicine., 71(1): 79-84.
- Larrieu E., Costa T., Cantoni G., Alvarez R., Cavagion L., Labanchi L., Bigatti R., Araya D., Herrero E., Alvarez E., Mancini S. and Cabrera P. (2001). Ovine Echinococcus granulosus transmission dynamics in the province of Rio Negro, Argentina, 1980–1999. Vet. Parasitol., 98(4): 263-72. DOI: 10.1016/S0304-4017(01)00442-3. PMID: 11423184.
- Macpherson C. N. L. (1985). Epidemiology of hydatid disease in Kenya: A study of the domestic intermediate hosts in Masailand. Trans. R. Soc. Trop. Med. Hyg., 79(2): 209-17. DOI: 10.1016/0035-9203(85)90337-2.
- Magambo J., Njoroge E. and Zeyhle E. (2006). Epidemiology and control of echinococcosis in sub-Saharan Africa. Parasitol. Int., 55: 193-195. DOI: 10.1016/j.parint.2005.11.029.
- Mekuanent T., Teka F., and Bosenu A. (2015). Major Causes of Organ Condemnation in Camels Slaughtered at Akaki Abattoir, Addis Ababa, Ethiopia. College of Veterinary Medicine, College of Dryland Agriculture, Jigjiga University, Ethiopia. J. Anim. Health Prod., 3(1): 14-20. DOI: http://dx.doi.org/10.14737/journal.jahp/2015/3.1.14.20.
- Mohamed M.I. (2010). Study of cystic echinococcosis in slaughtered animals in Al Baha Region, Saudi Arabia: Interaction between some Biotic Factors. Acta. Trop. 113(1): 26-33. DOI: 10.1016/j.actaatropica.2009.08.029.
- Mohammad M., Hadi R., Ahmad N. and Javad A. (2016). Survey of hydatidosis infection in slaughtered camels (Camelus dromedarius) in Tabriz area, Northwest Iran. J. Parasit. Dis., 40(2): 444–447. DOI 10.1007/s12639-014-0523-6
- Muller R. (2002). Worms and human diseases. 2nd ed. The London School of Hygiene and Tropical Medicine, Wallingord: CABI International, Oxon, UK.
- Muskin S., Hailu D. and Moti Y. (2011). Infection Rates, Cyst Fertility and Larval Viability of Hydatid Disease in Camels (Camelus dromedarius) from Borena, Kereyu and Harare Areas of Ethiopia. Jimma university, college of Agriculture and Veterinary Medicine, P.O. Box: 307, Jimma, Ethiopia. Glob. Vet., 7(6): 518-522.
- National Meteorological Agency (NMA) of Ethiopia (2007): Initial National Communication of Ethiopia to the United Nations Framework Convention on Climate Change (UNFCCC). National Meteorological Agency, Addis Ababa, Ethiopia.
- National Meteorology Service Agency of Ethiopia (NMSAE) (2012): Annual meteorological analysis and report.
- Njoroge E.M., Mbithi P.M.F., Gathuma J.M., Wachira T.M., Gathura P.B., Magambo J.K. and Zeyhle E. (2002). A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet. Parasitol. 104(1): 85–91. DOI: 10.1016/s0304-4017(01)00614-8.
- Ohiolei J.A., Hong B.Y., Li L., Abdullahi A.M., Joshua L., Guo Q.Z., Clement I., Manfred E.O., Yan T.W, Mughees A.A., Rosline J.M, Bao Q.F. and Wan Z.J (2019). Cystic echinococcosis in Nigeria: First insight into the genotypes of Echinococcus granulosus in animals. Parasites Vectors., 12: 392. https://doi.org/10.1186/s13071-019-3644-z.
- Omer R.A., Dinkel A., Romig T., Mackenstedt U., Elamin M., Elnahas A., Aradaib I.E., Ahmed M.E., Elmalik K.H. and Adam A. (2004). Strain Characterization of Human Hydatidosis in Sudan. Int. Arch. Hydatid., 35: 41.
- Omer R.A., Dinkel A., T. Romig T., Mackenstedt U., Elnahas A.A., Aradaib I.E., Ahmed M.E., Elmalik K.H., Adam A. (2010). A molecular survey of cystic echinococcosis in Sudan. Vet. par., 5149: 7. DOI: 10.1016/j.vetpar.2010.01.004.
- Osman O.M. and Abdalla H.S. (2013): Prevalence of Echinococcus granulosus in Stray Dogs and Hydatidosis in Camels in Tambool Area, Gezira State, Sudan. Sudan J. Vet. Res., 28: 15-18. sudanjvr.net.
- Parodi P., Mantovani A. and Seimenis A. (2001). Public health education in control programmes. In: Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS, editors. WHO/OIE manual on echinococcosis in humans and animals. Paris: World Health Organization for Animal Health; Pp. 219–24.
- Parry E., Godfrey D. and Gill M. (2004). Principles of Medicine in Africa. (3rdedn), Cambridge, USA, Pp. 406-408.
- Parsini H.R., Veer S. and Momin R.R. (2008). Common parasitic diseases of camels. Veterinary World., 1(10): 317-318.
- Pawlowski Z., Eckert J., Vuitton D.A., Ammann R.W., Kern P. and Craig P.S. (2001). Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert J., Gemmell M.A., Meslin F.X. and Pawlowski Z., editors. WHO/OIE Manual on Echinococcosis in humans and animals. Paris: Office International des E´pizooties. Pp. 20-71.
- Polydorou K. (1981). Animal health and economics, Case-study: Echinococcosis with a reference to Cyprus (dogs). Department of Veterinary services, Nicosia (Cyprus); Bulletin, OIE., 93(5-6): 981–992.
- Rashed A.A, Omer H.M, Fouad M.A.H and Al-Shareef A.M.F (2004). The effect of severe cystic hydatidosis on the liver of a Najdi sheep with special reference to the cyst histology and histochemistry. J. Egypt. Soc. Parasitol., 34(1): 297–304. PMID: 15125534.
- Regassa A., Nesibu A., Birhanu H., Yisehak Ts. And Teshale S. (2015). Internal and external parasites of camels (Camelus dromedarius) slaughtered at Addis Ababa Abattoir. Ethiopia. J. Vet. Med. Anim. Health., 6(7): 30-60. DOI: 10.5897/JVMAH2014.0346.
- Rokni M.B. and Aminian B. (2006). Evaluation of the Enzyme-linked ImmunoelectroTransfer Blot (EITB) technique using hydatid cyst antigens B/5 and total IgG antibodies in laboratory diagnosis of human hydatidosis. Pak. J. Med. Sci., 22(2): 127-131.
- Rokni M.B., Lesan S., Massoud J., Kia E.B. and Molawi G. (2006). Comparative evaluation of Fast enzyme linked immunosorbent assay (FastELISA) and standard-ELISA for the diagnosis of human hydatidosis. Iranian. J. Publ. Health., 35(2): 29-32.
- Romazanvoc F. (2001). Cestode zoonosis: Echinococcosis and Cysticercosis: An emergent and Global problem. Philip Craig and Zbigniew Pawlowski, editors IOS Press, Netherlands. Pp. 34-57.
- Romig T., Omer R.A., Zeyhle E., Hüttner M., Dinkel A., Siefert L., Elmahdi I.E, Magambo J., Ocaido M., Menezes C.N., Ahmed M.E., Mbae C., Grobusch M.P. and Kern P. (2011). Echinococcosis in sub-Saharan Africa: emerging complexity. Vet. Parasitol., 181(1): 43-47. DOI: 10.1016/j.vetpar.2011.04.022. Epub 2011 Apr 19. PMID: 21561714.
- Sadjjadi S.M., Abidi H., Sarkari B., Izadpanah A. and Kazemian S. (2007). Evaluation of Enzyme Linked Immunosorbent Assay, Utilizing Native Antigen B for Serodiagnosis of Human Hydatidosis. Iran. J. Immunol., 4(3): 167-72. PMID: 17767016.
- Santivanez S. and Garcia H.H. (2010). Pulmonary cystic echinococcosis. Curr. Opin. Pulm. Med., 16(3): 257-261. DOI: 10.1097/MCP.0b013e3283386282.
- Sariozkan S. and Yalcin C. (2009). Estimating the production losses due to cystic echinococcosis in ruminants in Turkey. Vet. Parasitol., 163(4): 330-334. DOI: 10.1016/j.vetpar.2009.04.032.
- Sarkar M., Pathenia R., Jhobta A., Thakur B.R and Chopra R. (2016). Cystic pulmonary hydatidosis. Lung India., 33: 179-191. DOI: 10.4103/0970-2113.177449.
- Sazmand A. and Joachim A. (2017). Parasitic diseases of camels in Iran (1931 – 2017): A literature review. Parasite, 24:21. https://doi.org/10.1051/parasite/2017024.
- Scala A., Garippa G., Varcasia A., Tranquillo V. and Genchi C. (2005). Cystic echinococcosis in slaughtered sheep in Sardinia (Italy). Vet. Parasitol., 135(1): 33-8. DOI: 10.1016/j.vetpar.2005.08.006.
- Seimenis A. (2003). Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta. Trop., 85(2): 191–95. DOI: 10.1016/s0001-706x(02)00272-3.
- Seimenis A., Morelli D. and Mantovani A. (2006). Zoonoses in the Mediterranean Region. Ann. Ist. Super. Sanità., 42(4): 437–45. PMID: 17361068.
- Setotaw F., Yigezu A.Y., Seid K. and Aden Aw-H. (2014): Trends in Global and National Grain Legume Production and Trade: Implications on Local Chickpea and Lentil production Dynamics: The Case of Gimbichu and Minjar-Shenkora Districts of Ethiopia. ICARDA. Science for better livelihood in Dry areas. Pp. 1-80.
- Singh B.B., Sharma R., Sharma J.K. and Juyal P.D. (2010). Parasitic zoonoses in Gh B.B., Sharma R., Sharma J.K. and Juyal P.D. (2010). Parasitic zoonoses in India: An overview. Rev. Sci. Tech. Off. Int. Epiz., 29 (3): 629–637. DOI: 10.1007/978-81-322-1551-6.
- Smego R.A., Bhatti S., Khalij A.A. and Asim Beg M. (2003). Percutaneous aspiration-injection-reaspiration-drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin. Infect. Dis., 27: 1073—83.
- Somali Regional State, Ethiopia (2004). Livelihood Zone. Field Surveys conducted by SCUK/DPPB. Food Security Monitoring and Early Warning Programme.
- Soule C. (1994). The hydatid disease of ruminants. Veterinary Point. Maisond’Alfort. France. 26 (special number). pp.930–933.
- Tefera M. (2012). Camel in Ethiopia. College of Veterinary Medicine, Haramaya University. Ethiopia Veterinary Association. ISBN 9789994498192. Pp. 1-181.
- Tenaw M., Feyera T. and Abera B. (2015). Major causes of organ condemnation in camels slaughtered at Akaki Abattoir, Addis Ababa, Ethiopia. J. Anim. Health Prod., 3(1): 14-20. http://dx.doi.org/10.14737/journal.jahp/2015/3.1.14.20.
- The Center for Food Security and Public Health (CFSPH) (2012). Echinococcosis: Echinococciasis, Hydatidosis, Hydatid Disease. College of Veterinary Medicine.Lowa State University, Ames Lowa 50011. Pp. 4-14.
- Thrusfield M. and Christley R. (2018). Veterinary epidemiology, 4th ed., John Wiley and Sons Ltd., Oxford, UK. Pp. 275-276. ISBN: 978-1-118-28028-7.
- Torgerson P. (2002). Transmission dynamics of taeniid parasites in animal hosts. In Cestode zoonoses; Echinococcosis and cysticercosis, an emergent and global problem. Philip Craig and Zbigniew Pawlowski, editors. Amsterdam, the Netherlands: IOS press, pp. 221–235.
- Torgerson P. and C. Budke C. (2003). Echinococcosis–an international public health challenge. Res.Vet. Sci., 74(3): 191-202.
- Torgerson P., Karaeva R., Corkeri N., Abdyjaparov T., Kuttubaev O. and Shaikenov B. (2003). Human cystic echinococcosis in Kyrgystan: an epidemiological study. Acta. Tropica., 85(1): 51-61.
- Torgerson P.R. (2003). The economic effects of echinococcosis. Acta. Trop. 85((2):113–118. https://doi.org/10.1016/s0001-706x(02)00228-0. PMID: 12606088.
- Toulah F.H., El-Shafaeis A.A. and Alsolami M.N. (2012). Prevalence of Hydatidosis among slaughtered animals in Jeddah, Kingdomof Saudi Arabia. J. Egypt. So.c Parasitology. 42(3): 563-572.
- Watson E.E., Kochore H.H. and Dabasso B.H. (2016). Camels and climate resilience: Adaptation in Northern Kenya. Hum. Ecol., 44: 701–713. https://doi.org/10.1007/s10745-016-9858-1.
- Wei-Hsin Y., Rheun-Chuan L., Yi-Hong Ch., Jen-Huey Ch., Yuh-Kuen Ch. and Hui-Chen Hs. (2005). Hydatid cyst of the liver: A case report and literature review. Kaohsiung. J. Med. Sci., 21(9): 418–23.
- Woldemeskel M., Issa A., Mersie A. and Potgieter L.N.D. (2001). Investigation of parasitic disease of one-humped camel (Camelus dromedarius) in eastern Ethiopia. J. Camel Pract. Res.,8(1): 77-81.
- Worku A. M, Feyisa L.G. and Beketie T. K. (2022). Climate trend analysis for a semi-arid Borana zone in southern Ethiopia during 1981–2018. Environ. Syst. Res., 11: 22. https://doi.org/10.1186/s40068-022-00247-7.
- World Health Organization (2015). Neglected zoonotic Diseases.
- World Health Organization/World Organization for Animal Health (WHO/OIE) (2001). Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris, France. World Organization for Animal Health. Ed. By J. Eckert J., Gemmell M.A., Meslin F.X. and Z.S. Pawłowski Z.S. 12, rue de Prony, 75017. http://www.oie.int. ISBN 92-9044-522-X. Pp. 1-286.
- Yifat D., Gedefaw D. and Desie S. (2011). Major Causes of Organ Condemnation and Financial Significance of Cattle Slaughtered at Gondar Elfora Abattoir, Northern Ethiopia. Glob. Vet., 7(5): 487-490. ISSN 1992-6197.
- Yildiz K. and Gurcan S. (2003). Prevalence of hydatidosis and fertility of hydatid cysts in sheep in Kirikkale, Turkey. Acta. Vet. Hung., 51: 181-187. DOI: 10.1556/AVet.51.2003.2.6.
- Zeleke M. and Bekele T. (2000). Camel herd health and productivity in Eastern Ethiopia selected semi-nomadic households. Revue Ele. Med. Vet. Pays trop., 53 (2): 213-217.
- Zhang W., Jun L., Renyong L., Hao W. and Donald P.M. (2012). Recent Advances in the Immunology and Serological Diagnosis of Echinococcosis. In: Al-Moslih M (ed). Serological Diagnosis of Certain Human, Animal and Plant Diseases. DOI: 10.5772/37133. Pp. 113–150.
Quick linksss
- Abstract
- Abbreviations
- 1. Introduction
- 2. Materials and Methods
- 2.2. Study Animal
- 2.3. Study Design
- 2.4. Sampling Method and Sample Size Determination
- 2.5. Study Methodology
- 2.6. Data Management and Analysis
- 2.7. Ethical Considerations
- 3. Results
- 3.2. Antemortem Examined Dromedary Camels
- 3.3. Gross Pathology
- 3.4. Microscopic Examination
- 3.5. Assessment of Direct Annual Financial Loss
- 4. Discussion
- 5. Conclusion and Recommendations
- References


