Article In Press : Article / Volume 3, Issue 1
- Case Report | DOI:
- https://doi.org/10.58489/2836-5070/013
Efficacy of the probiotic Bacillus subtilis DG101 in preventing overweight in healthy individuals: a double blind, placebo-controlled study
- Instituto de Nutrición Grupo Cardinali. 9 de Julio 1287, Rosario (2000), Argentina
- Universidad Nacional de Rosario, Facultad de Ciencias BioquÃmicas y Farmacéuticas, Departamento de MicrobiologÃa. Suipacha 531 (Sala 9 Hospital Provincial del Centenario), Rosario (2000), Argentina
- Actual address: Kyojin S.A., I & D Department. Castellanos 1335, Rosario (2000), Argentina
Roberto Ricardo Grau
Nestor Cardinali, Facundo Rodriguez Ayala, Cecilia Leñini, and Roberto Ricardo Grau. (2024). Efficacy of the probiotic Bacillus subtilis DG101 in preventing overweight in healthy individuals: a double blind, placebo-controlled study. Journal of Obesity and Fitness Management. 3(1); DOI: 10.58489/2836-5070/013
© 2024 Roberto Ricardo Grau, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 08-01-2024
- Accepted Date: 12-01-2024
- Published Date: 13-01-2024
Overweight, Obesity, Metabolic Diseases, Fitness, Probiotics, Natto, B. subtilis DG101
Abstract
At some point in life, most people have successfully tried to achieve an adequate body weight. But such achievement is difficult to maintain over time. A good balance between diet and lifestyle is beneficial to achieve a healthy personal fitness, and the intestinal microbiota, in particular probiotics, could play an important role on it. In this work, we demonstrate the proficiency of the human probiotic bacterium Bacillus subtilis DG101 to maintain body weight, total fat content and BMI of healthy individuals within optimal limits. We conducted a double blind, placebo-controlled study involving a total of 133 healthy participants meeting the inclusion criteria and randomized in two groups (intervention group, n = 66 participants who received the probiotic, and the placebo group, n = 67 participants). At each bi-monthly visit to our center over 12 months (study duration), the parameters measured in each participant were body weight, fat content and BMI. Linear regression (ANOVA) was used to test differences in response to treatments (probiotic and placebo). The participants (of both sexes) who incorporated the probiotic B. subtilis DG101 into their diets were able to maintain, and even improve (healthy decrease), the body weight, fat content and BMI compared to the placebo group after the 12 months. The participants did not manifest any adverse effect caused by consuming the probiotic. Overall, the present results show the human probiotic Bacillus subtilis DG101 as an effective strategy to prevent overweight in healthy individuals.
Introduction
Overweight and obesity are defined as abnormal or excessive body mass and fat accumulation that presents a risk to individual health and a burden from the social point of view [1]. According to the WHO, nearly 39% of people over 18 years of age are overweight, and almost 2.8 million deaths per year are a consequence of overweight and obesity [1,2]. Obesity is one side of the double problem of malnutrition, and today more people are obese than underweight in every region except sub-Saharan Africa and Asia. Once considered a problem only in high-income countries, overweight and obesity are now dramatically on the rise in low- and middle-income countries. The vast majority of overweight or obese children live in developing countries, where the rate of increase has been more than 30% higher than that of developed countries [1]. Body weight, body fat content, and the body mass index (BMI) value are quantifiable parameters often used on the routine medical practice to monitor overweight and obesity [3]. BMI is calculated as the ratio of body weight in kilograms divided by the individual's height in squared meters (kg/m2) [3]. A BMI between 18.5 kg/m2 to 24.9 kg/m2 corresponds to a person with an ideal or optimal (physiological fitness) body weight [3,4]. BMI in the range of 25 kg/m2 – 29.9 kg/m2 or greater than 30 kg/m2 correspond to overweight / obese people, respectively [3.4]. Different clinical studies have shown an inverse correlation between BMI and the risk of morbidities (for example, arterial hypertension, dyslipidemia, coronary heart disease, ischemic stroke, osteoarthritis, and type 2 diabetes mellitus) and mortality [4]. The etiology of overweight / obesity results from a complex, and still poorly understood, combination of genetic, cultural, socio-economic, and environmental factors [4,5]. The available approaching to prevent, combat or reverse overweight / obesity consist on the adoption of low-calorie diets; daily exercise routines; drugs and dietary supplements, prescribed by doctors or nutritionists, to reduce appetite or reduce the absorption of fats; and in extreme cases (e.g., morbid obesity with BMI greater than 40 kg/m2) bariatric surgery [2,5]. In general, to reverse and prevent overweight / obesity, a moderate physical activity, and a balanced diet low in fatty substances and rich in fiber, are recommended [6].
During the recent years, the role of the intestinal microbiota (the trillions of microorganisms that habit the human gut) in the etiology of overweight / obesity is beginning to emerge [7]. There are profound differences between the microbiomes of thin and obese people, with a marked intestinal dysbiosis prevailing in the latter [7,8]. The transplantation of intestinal microbiota from obese animals to lean animals results in an increase in overweight not only in the transplanted animal but also in its offspring, which evidences the existence of a causal relationship between the gut microbiota and obesity, although the mechanism remains poorly understood [8,9]. There are already available combinations of intestinal bacteria known as Live Biotherapeutic Products (LBP) on the market, including some probiotics, associated with the reduction of body weight excess, and its maintenance within physiological anthropomorphic parameters [10,11]. During recent years, the incorporation of beneficial bacteria (i.e., probiotics) to a person's diet has been proposed as a new element to combat overweight and obesity [9-12]. Probiotics are defined as live microorganisms that, when administered in adequate quantities, and arriving alive at their site of action (for example, the intestine), produce a health benefit for the consumer [13]. Within the probiotic microorganisms, in particular probiotic bacteria, there are two broad categories: those non-spore-forming probiotic bacteria (for example, lactic acid bacteria such as lactobacilli and bifidobacteria) and those spore-forming probiotic bacteria of the Bacillus genus such as B. subtilis, B. coagulans and B. clausii [14]. In particular, the Japanese probiotic bacteria natto B. subtilis DG101, is capable to normalizing the level of serum lipids (total cholesterol, LDL, HDL, and triglycerides) in an animal model [15]. Interestingly, the intervention of overweight / obese diabetic patients with B. subtilis DG101 has been successful in the recovery of a normal body weight and BMI in such ill people [16].
Over the life time, a person has successfully tried to lose weight, but it is also highly probable that the ideal or optimal achieved weight has not been maintained for a long period of time, giving rise to a rebound effect [17]. In this work, we present the results of a randomized, double-blind controlled-trial on the effectiveness of the probiotic B. subtilis DG101 for maintaining optimal body weight, body fat content and BMI in healthy adults over time.
Results
Healthy individuals, 18 – 77 years of age, were recruited by personnel of the Instituto de Nutrición Grupo Cardinali (INGC) over a 3-month period by letter, electronic email or WhatsApp. The inclusion criteria were average height of 1.68 – 1.71 m, normal body weight and fat content; a body mass index (BMI) between 18.0 and 25.0 kg/m2; healthy determined by physical examination, clinical biochemistry, liver and kidney functions tests; no consumption of probiotics, postbiotics or prebiotics for 2 months before the start of the study. The exclusion criteria were pregnant and breast-feeding females; absence of a history of chronic diseases (i.e., intestinal disorders, diabetes, celiac disease, thyroid disorders, cancer; and any other condition that would affect the individual safety or ability to follow and complete the study. The work consisted of a double-blind, placebo-controlled study of 12 months of duration between July 2022 to June 2023. After confirming eligibility, the participants (n = 150, Figure 1) were randomized to receive B. subtilis DG101 (intervention group, 20 drops of probiotic, equivalent to 1 x 108 colony forming units, CFU) or a placebo (placebo group, 20 drops of distilled water) dissolved in ~ 100 – 200 ml of water, once a day in the morning. Subsequent visits to the nutritional clinic (i.e., INGC) were scheduled every two months to collect data and compliance with the regime. The participants were instructed to maintain a controlled diet (suggested by the medical and nutritional staff of INGC) and their current physical activity levels throughout the study. The probiotic B. subtilis DG101 (KyojinÒ Probiotic) and the placebo (distilled water) were provided by Kyojin S.A. (www.kyojin.com.ar) in indistinguishable dropper cap bottles of 180 ml of content. The bottles (intervention and placebo) were labeled with a code (in accordance with the Good Clinical Practice Guidelines) by members of the staff of the INGC who were blinded in conducting any phase of the study. A total of 3 bottles were used by each person in the intervention or placebo group throughout the one-year duration of the study. The sample size of this study was 150 healthy participants which were randomized equally in 2 groups of 75 participants in a double-blind manner. A total of 66 and 67 participants from the intervention and placebo groups, respectively, reached the end of the study successfully (Figure 1). At each bi-monthly visit to the INGC, the outcome measures were vital signs (resting heart rate and blood pressure), body weight, fat content and BMI. At these visits the participants expressed any problems or unexpected success that might have occurred. Urine and blood were analyzed at the star and the end of the study. No adverse effects attributed to the probiotic consumption were observed, and 12 and 5 participants voluntary dropped out or were excluded by the INGC staff, respectively, during the study. Linear regression (ANOVA) was used to test differences in response to treatments (probiotic and placebo). The obtained values are presented as means ± S.D. unless otherwise specified. A p-value of 0.05 was considered statistically significant. All analyzes were performed using the Statistical Analysis System (SAS 9.2; SAS Institute, Cary, NC, USA).
To evaluate the contribution of the incorporation of the probiotic B. subtilis DG101 into the daily diet of a healthy person as an adjuvant against overweight / obesity, 3 related parameters (body weight, fat content and BMI) were measured bimonthly, and averaged over the 12 months (study duration). Average values lesser than 25 Kg/m2 of BMI, 75 kg or 65 kg of weight (for men and women with an average height of 1.68 – 1.71 m, respectively), and 20 -23 % of total body fat correspond to acceptable values for a healthy person, and the starting values of each of the 3 indicators measured in the population under study met these criteria as shown in Table 1. As shown in Figure 1 and Table 1, of the 150 healthy adults selected to start the study, 133 (50 men and 83 women) (~ 87 %) completed the study over the 12 months from July 2022 to June 2023. Also, during this time, no abnormalities in either renal or hepatic function, were observed in both groups.
Healthy subjects of both sexes who incorporated the probiotic B. subtilis DG101 into their diets (1 x 108 CFU/day) were able to maintain, and even improved, their body weight values (Figure 2). The change in body weight of men in the intervention and placebo groups (Figure 2 A and B, respectively) after the 12 months of study was – 1.01 kg and + 4.75 kg, respectively (data not shown, see below Table 2). For women, the annual change in weight in the intervention and placebo groups (Figure 2 C and D, respectively) was – 0.86 kg and + 4.44 kg, respectively (data not shown, see Table 2 below). These values indicate that subjects in the placebo group showed a significant increase in the average body weight value i.e., an increase of ~ 7.0 and ~ 8.0%, both men and women, respectively (Figure 2 B and D, yellow circles). On the contrary, participants in the treated group with the probiotic (intervention group) showed optimal maintenance of body weight throughout the year of the study, i.e., a decrease of body weight of ~ 1.4% for both sexes (Figure 2 A and C, yellow circles).
The analysis of the body fat percentage variation in both men and women after 12 months of treatment also showed interesting results (Figure 3). The percentage of annual variation in body fat content of healthy subjects of both sexes who incorporated the probiotic B. subtilis DG101 into their diets (1 x 108 CFU/day) showed a decrease in total fat of ~ 2% for both men and women (Figure 3 A and C, yellow circles). On the contrary, for the participants (men and women) in the placebo groups (Figure 3 B and D, respectively), a significant increase in total fat content of ~11% was evident for both sexes (Figure 3 B and D, yellow circles). The analysis of the BMI change in men in the groups treated with the probiotic (Figure 4A) or the placebo (Figure 4B), showed a decrease of nearly 2% and an average increase of 11% in BMI, respectively (yellow circles in Figure 4 A and B, respectively). Similarly, for the female group, the average change in BMI after the 12 months of study for the treated (Figure 4C) and placebo (Figure 4D) groups was – 1.7% and + 11%, respectively.


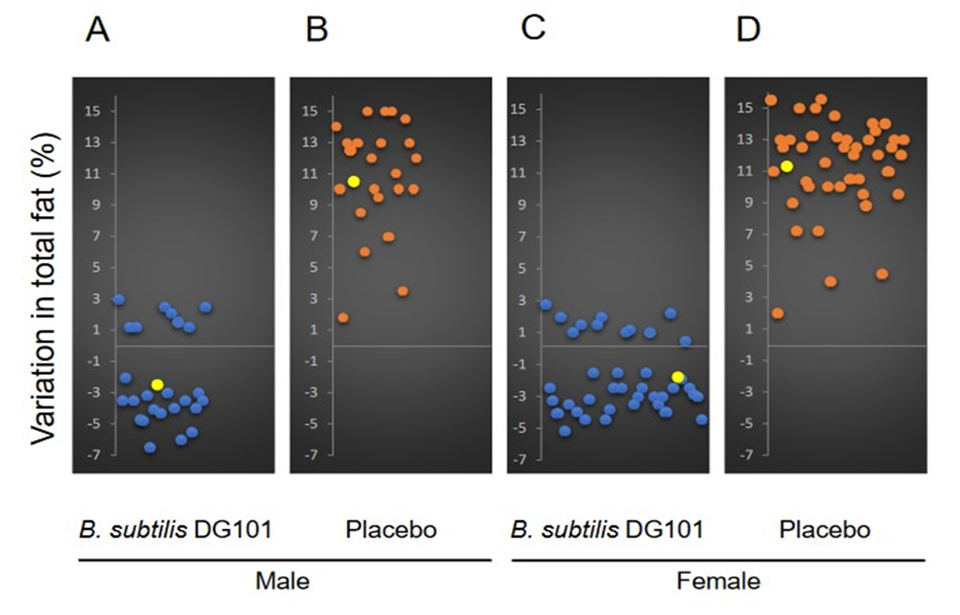

Table 1: Participant features at the start of the study. Average values of height (m), corporal weight (kg), total body fat (%) and BMI (kg/m2) at the start of the study. Data are means ± S.D.; p 0.005.
Measure |
B. subtilis DG101 (n=66) |
Placebo (n=67) | ||
| MALE | FEMALE | MALE | FEMALE | |
| Sex (n) | 26 | 40 | 24 | 43 |
| Height (m) | 1.72 ± 0.08 | 1.68 ± 0.07 | 1.73 ± 0.09 | 1.69 ± 0.06 |
| Body weight (Kg) | 70.3 ± 0.8 | 61.0 ± 0.6 | 70.8 ± 0.8 | 60.8 ± 0.8 |
| Total fat (%) | 21.5 ± 0.6 | 23.0 ± 0.5 | 22.0 ± 0.6 | 22.5 ± 0.5 |
| BMI (Kg/m2) | 23.76 ± 0.1 | 21.61 ± 0.2 | 23.66 ± 0.1 | 21.11 ± 0.2 |
Table 2: Summary of the variation of weight control parameters produced by B. subtilis DG101 consumption. Average variation of weight expressed in kg and %, body fat content in %, and BMI in after the 12 months of study, in men and women with or without probiotic intervention. Data are means ± S.D.; p 0.005.
Measure |
B. subtilis DG101 |
Placebo | ||
MALE (n=26) | FEMALE (n=40) | MALE (n=24) | FEMALE (n=43) | |
| Body weight (Kg) | 69.29 ± 0.07 | 60.14 ± 0.05 | 75.55 ± 0.08 | 65.24 ± 0.07 |
| Body weight variation (%) | - 1.43 | - 1.41 | + 6.71 | + 8.20 |
| Total fat (%) | 20.99 ± 0.5 | 22.58 ± 0.5 | 24.38 ± 0.5 | 25.05 ± 0.4 |
| Total fat variation (%) | - 2.37 | - 1.82 | + 10.82 | + 11.34 |
| BMI (Kg/m2) | 23.27 ± 0.3 | 21.24 ± 0.2 | 26.16 ± 0.2 | 23.46 ± 0.3 |
| BMI variation (%) | - 2.07 | - 1.72 | + 10.57 | + 11.13 |
Discussion
Table 2 summarizes the results related to the changes in BMI, body fat and weight in healthy participants of both sexes divided into the placebo and intervention groups after the 12 months of study. The results demonstrate the effectiveness of the probiotic B. subtilis DG101 in maintaining, and improving, parameters linked to the person's good physical condition (fitness). Among the participants who did not consume the probiotic, there was a significant increase in all variables, in some cases reaching values far from optimal or desirable values (for example, a BMI > 25 kg/m2 and a % of total body fat > 25% for men and women, respectively, in the placebo group), (Table 2). The participants (of both sexes) in the treated group showed maintenance and even improvement (healthy decrease) in body weight, fat content and BMI (Table 2) without manifestation of any adverse effect caused by consuming the probiotic (data not shown). There is a significant amount of scientific evidence that supports the existence of a physiological relationship between the intestinal microbiota and the person efficiency in maintaining weight and BMI within healthy ranges, preventing overweight or obesity [7-11]. This cause – effect association, places probiotics as a potential tool of interest for the prevention or treatment of overweight and obesity together with existing therapies (i.e., low-calorie diets, physical exercise, pharmacotherapy, surgery) [4,5,11,12]. There is an emerging scenario for the probiotic consumption that might represent a sustainable strategy to maintain over time an optimal body weight, fat content and BMI that might help to prevent the appearance of diseases such as type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver (NAFL), dyslipidemia, cardiovascular and cerebral (stroke) accidents, all of which would result in a better life quality [10-12]. In a recent work, we demonstrated the effectiveness of the probiotic B. subtilis DG101 for the reduction of overweight and obesity in diabetic patients [16]. Now, the results presented here expand the property of B. subtilis DG101 to prevent overweight and obesity to healthy individuals [18].
Declarations
Author Contributions
NC: conceptualization, methodology, data collection, and interpretation of the results.
FRA and CL: methodology, data collection and statistical analysis.
RG: conceptualization, methodology, interpretation and writing of the manuscript.
All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank the staff (secretaries, technicians, biochemists, nutritionists, doctors) of Instituto de Nutrición Grupo Cardinali and Kyojin S.A. for their technical assistance.
Conflict Of Interest Statement
NC and RG declare that they have no conflict of interest regarding the publication of this article. FRA and CL are employees of Kyojin S.A.
Funding Information
This study was funded by Kyojin S.A.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This study was conducted in accordance with the ethical principles indicated in the Declaration of Helsinki, its subsequent amendments, and the ethical recommended guidelines of the National University of Rosario and the Hospital Provincial del Centenario, Rosario - Santa Fe, Argentina. Agencia Santafesina de Seguridad Alimentaria (ASSAL) approved probiotic Bacillus subtilis DG101 use for human beings (RNPA 21-1194829).
Consent
Written informed consent was obtained from each participant of the present study in accordance with the journal’s patient consent policy.
References
- WHO. Obesity and overweight. World Health Organization: 2021 Jun 9. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016 Jul;22 Suppl 3:1-203. [PMID: 27219496].
- Peterson, C. M., Thomas, D. M., Blackburn, G. L., & Heymsfield, S. B. (2016). Universal equation for estimating ideal body weight and body weight at any BMI. The American journal of clinical nutrition, 103(5), 1197-1203.
- Lin, X., & Li, H. (2021). Obesity: epidemiology, pathophysiology, and therapeutics. Frontiers in endocrinology, 12, 706978.
- Heymsfield, S. B., & Wadden, T. A. (2017). Mechanisms, pathophysiology, and management of obesity. New England Journal of Medicine, 376(3), 254-266.
- Locke, A., Schneiderhan, J., & Zick, S. M. (2018). Diets for health: goals and guidelines. American family physician, 97(11), 721-728.
- Gomes, A. C., Hoffmann, C., & Mota, J. F. (2018). The human gut microbiota: Metabolism and perspective in obesity. Gut microbes, 9(4), 308-325.
- Cuevas-Sierra, A., Ramos-Lopez, O., Riezu-Boj, J. I., Milagro, F. I., & Martinez, J. A. (2019). Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Advances in nutrition, 10(suppl_1), S17-S30.
- Aron-Wisnewsky, J., Clement, K., & Nieuwdorp, M. (2019). Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Current Diabetes Reports, 19, 1-9.
- Schütz, F., Figueiredo-Braga, M., Barata, P., & Cruz-Martins, N. (2021). Obesity and gut microbiome: review of potential role of probiotics. Porto biomedical journal, 6(1).
- Cerdó, T., García-Santos, J. A., G. Bermúdez, M., & Campoy, C. (2019). The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients, 11(3), 635.
- Olle, B. (2013). Medicines from microbiota. Nature biotechnology, 31(4), 309-315.
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., ... & Sanders, M. E. (2014). Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Nature Reviews Gastroenterology and Hepatology, 11(8), 506.
- Cutting, S. M. (2011). Bacillus probiotics. Food microbiology, 28(2), 214-220.
- Leñini, C., Ayala, F. R., Goñi, A. J., Rateni, L., Nakamura, A., & Grau, R. R. (2023). Probiotic properties of Bacillus subtilis DG101 isolated from the traditional Japanese fermented food nattō. Frontiers in Microbiology, 14.
- Ayala, F. R., Cardinali, N., & Grau, R. (2022). Efficient Weight Loss and Type II Diabetes Control in Overweight and Obese Patients Consuming the Probiotic Bacillus Subtilis DG101: A Randomized Double-Blind Placebo-Controlled Study. Asploro Journal of Biomedical and Clinical Case Reports, 5(1), 51.
- Strohacker, K., Carpenter, K. C., & Mcfarlin, B. K. (2009). Consequences of weight cycling: an increase in disease risk? International journal of exercise science, 2(3), 191.
- Ayala, F. R., Bauman, C., Cogliati, S., Leñini, C., Bartolini, M., & Grau, R. (2017). Microbial flora, probiotics, Bacillus subtilis and the search for a long and healthy human longevity. Microbial Cell, 4(4), 133.
- Rodriguez Ayala, F., Francisco, M. G., Argarañaz, F., Crespo, C., Clementi, V., & Grau, R. R. (2021). Healthy Aging, Neuroprotection and Decreased Risk of Cardiovascular Death Associated with the Consumption of Probiotic Bacillus Subtilis. Geront Geratric Stud. 7(3): GGS 000662.


