Current Issue : Article / Volume 3, Issue 1
- Research Article | DOI:
- https://doi.org/10.58489/2836-8851/016
Astrogliosis and Neuron Specific Enolase Expression Following Tramadol and Coffee Co-administration on Rats Cerebellum
- Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
Samuel J. Umanah
Samuel J. Umanah, Idorenyin U. Umoh, Aquaisua N. Aquaisua, Innocent A. Edagha. (2024). Astrogliosis and Neuron-Specific Enolase Expression Following Tramadol and Coffee Co-administration on Rats Cerebellum. Neurons and Neurological Disorders. 3(1); DOI: 10.58489/2836-8851/016
© 2024 Samuel J. Umanah, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 20-03-2024
- Accepted Date: 05-04-2024
- Published Date: 08-04-2024
Astrocytes, GFAP, NSE, Cerebellum, Immunohistochemistry
Abstract
This study assessed the effects of co-administration of tramadol and coffee on the microstructural aspects of the cerebellum of adult Wistar rats as glial fibrillary acidic protein antibody for astrocyte expression and neuron specific enolase antibody for indication of brain injuries and damages, respectively, were the progenitors. Forty-five rats with an average weight of 190 g were randomly assigned into nine groups of five each. Group A rats served as control. Group B - D rats were given 36.23, 72.46 and 108.69 mg/kg of coffee daily respectively. Groups E - G rats were administered with 1.43, 2.86 and 5.71 mg/kg of tramadol daily respectively. Animals in group H were administered 72.46 mg/kg of coffee and 2.86 mg/kg of tramadol daily, and group I received 108 mg/kg of coffee and 2.86 mg/kg of tramadol daily. All administrations were via oral gavage and for twenty-eight days. On day 29, the rats were anaethesized with intraperitoneal injection of 50 mg of ketamine hydrochloride. The brain tissues were perfused with phosphate buffered saline and formalin, excised, fixed in 10 % buffered formalin, processed and stained for immunohistochemical studies. In immunohistochemical analysis, astrogliosis was more expressed in the combined groups H and I than in groups D and G compared to the control respectively. NSE staining also showed high staining intensity of neurons in combined groups H and I compared to the control respectively. It is concluded that 108.69 mg/kg of coffee and 5.71 mg/kg of tramadol caused immunohistochemical alterations. However, combined ingestion of these drugs caused a more deleterious effect on cerebellar tissues.
Introduction
Tramadol is an analgesic used for prevention and treatment of multiple pain conditions, such as postoperative pain, renal colic, dental pain, neuropathic pain, and pain from cancer (Abdel-Zaher et al., 2011; Elkhateeb et al., 2015). Tramadol, a member of the opioid family, acts as a weak mu opioid receptor agonist and a reuptake inhibitor of serotonin and noradrenaline (Close, 2005; García-López et al., 2022). This dual mechanism contributes to its analgesic properties. Tramadol has been demonstrated to be safe and effective in managing pain, including in conditions such as osteoarthritis in cats (Faria et al., 2018). However, tramadol is associated with certain drawbacks. One significant concern is the risk of intra-operative awareness when used in clinically effective analgesic doses for severe pain (Takhtfooladi et al., 2015). Additionally, tramadol may lead to nausea, and there is a potential for abuse and dependency, although the risk is considered low compared to other opioids (Radbruch et al., 2013). Studies have also demonstrated that Tramadol administration impairs memory function in rodent models by activation of μ-opioid receptors (Tashakori and Afshari, 2010; Hosseini-Sharifabad et al., 2016; Miotto et al., 2017). A recent Lancet world report highlights the opioid crisis in Africa and its use for non-medical purposes (SalmReifferscheidt, 2018). Studies in Nigeria indicate the use of Tramadol cuts across all parts of the country. A cross sectional study among ‘Almajiris’ (street children) in Borno State, North-Eastern Nigeria, reported a 7% prevalence of Tramadol misuse (Abdulmalik et al., 2009). In Kano, Northern Nigeria, another cross-sectional study amongst commercial bus drivers reported that 85.2% of respondents misuse Tramadol (Yunusa et al., 2017). In Owerri, South East Nigeria, a survey on the use of psychoactive substances amongst university students indicated that 53.4 admitted to the use of Tramadol (Duru et al., 2017).
Coffee is a popular beverage enjoyed worldwide, made from roasted coffee beans which are the seeds of berries from the Coffea plant, and its sensory profile including its aroma, flavor, and aftertaste, plays a crucial role in consumer perception and acceptance (Stokes et al., 2016). Its consumption was consistently associated with a lower risk of Parkinson’s disease, even after adjustment for smoking, and across all categories of exposure (Noyce et al., 2012). Decaffeinated Coffee was associated with a lower risk of Parkinson’s disease, which did not reach significance. Consumption had a consistent association with lower risk of depression (Wang et al., 2015; Grosso et al., 2016) and cognitive disorders, especially for Alzheimer’s disease in meta-analyses of cohort studies (Liu et al., 2016). The cerebellum is a vital component in the human brain as it plays a role in motor movement regulation and balance control. The cerebellum coordinates gait and maintains posture, controls muscle tone and voluntary muscle activity but is unable to initiate muscle contraction. Damage to this area in human results in a loss in the ability to control fine movements, maintain posture, and motor learning (Manto et al., 2012; Roostei et al., 2014; Witter and DeZeeuw, 2015).
Astrocytes are the most abundant brain cells and become reactive in virtually all pathological situations in the brain. Although the characteristics of astrocytes vary from one region of the brain to another, they all have a large number of processes that ramify into branches and then secondary branches. Hence, protoplasmic astrocytes are large, bushy-shaped cells with diameters of ~40 - 60 µm and volumes of ~104 µm (Chever et al., 2014). Astrocytes are involved in neuro-inflammation, and become reactive in virtually all pathological situations in the brain. Astrocyte reactivity is characterized by GFAP overexpression and subsequent morphological changes, such as process hypertrophy and remodeling (Hol and Pekny, 2015). Neuron specific enolase (NSE) is a glycolytic enzyme present almost exclusively in neurons and neuroendocrine cells. It is useful in estimation of neuronal injury and clinical outcome of patients with serious clinical problems like dementia, tumors, stroke and encephalitis (Lima et al., 2004). This study was carried out using immunohistochemical techniques to ascertain the level of damage caused by tramadol and coffee co-administration on the cerebellum.
Materials and Methods
Animal Handling
Forty-five (45) adult male Wistar rats with an average weight of 190 g were procured from the Animal House, College of Health Sciences of the University of Uyo and allowed to acclimatize for two weeks under standard housing conditions (ventilated room with 12/12 hour light/dark cycle at 24 ± 20C). The rats were fed with standard rat chow and water given ad-libitum.
Ethical Approval
Ethical approval was obtained from the Faculty of Pharmacy Ethics Committee on the Use of Laboratory Animals, University of Uyo, Uyo, Nigeria. All procedures were carried out in accordance with the National Academy of Science’s Guide for Care and Use of laboratory animals (National Research Council, 2011). Consent for the provision/ purchase of Tramadol was obtained from the National Drug Law Enforcement Agency, Akwa Ibom State Command.
Preparation and Administration of Tramadol and Coffee
Tramadol was obtained from Ranbaxy Nigeria Limited, Lagos State, Nigeria. The tablets were ground to powder and dissolved in physiological saline solution (Carrillo-Mingua et al., 2015) and appropriate doses given.
Coffee (Nescafé ®) was purchased from a grocery store in Uyo, South-South Nigeria. Every 100 g of Nescafe contains 4.72 g of caffeine (Nmaju et al., 2014). Fifty grammes of the Coffee powder was mixed with 500 mL of distilled water and allowed to soak for 48 hours under room temperature. The mixture was filtered twice using Whatman filter paper and the filtrate was left to evaporate under room temperature until it is totally dried. 10 g of each of the powder (Tramadol and Coffee) was dissolved in 100 ml of phosphate buffered saline, which served as the vehicle of administration. Treatment was administered orally via an orogastric tube.
Determination of Median Lethal Dose (LD50) of Coffee
To investigate the LD50 of Coffee, an ethanol extract of Coffee was obtained by Soxhlet extraction as described by Kumar et al. (2012), using 99% ethanol as solvent. The LD50 was calculated using probit kill of the dose according to the method of Lorke (1983).
Experimental Design
The rats were randomly divided into nine groups (five rats per group) as follows (Table 3.1)
Table 1: The Experimental Design
Groups | Description/Dosage | Duration |
A | Normal Control | 28 days |
B | 36.23 mg/kg of Coffee | 28 days |
C | 72.46 mg/kg of Coffee | 28 days |
D | 108.69 mg/kg of Coffee | 28 days |
E | 1.43 mg/kg of Tramadol | 28 days |
F | 2.86 mg/kg of Tramadol | 28 days |
G | 5.71 mg/kg of Tramadol | 28 days |
H | 72.46 mg/kg of Coffee + 2.86 mg/kg of Tramadol | 28 days |
I | 108.69 mg/kg of Coffee + 2.86 mg/kg of Tramadol | 28 days |
Termination of Experiment and Sample Collection
On the 29th day, the animals were sacrificed. The animals were anaesthesized through injection of 50 mg/kg of ketamine intraperitoneally, and the animals were perfused with phosphate buffered saline (for cleansing) and phosphate buffered formalin (for fixing) and the brain tissues excised. The cerebellum was isolated, weighed and fixed immediately in Bouin's fluid and processed for routine microtomy and immunohistochemical analysis using glial fibrillary acidic protein (GFAP) Chever et al., 2014) and neuron specific enolase (NSE) (Duraiyan et al., 2012) antibodies.
Immunohistochemistical Assessment
Glial Fibrillary Acidic Protein Immunohistochemistry
Tissues were deparaffinized in two changes of xylene, then hydrated through graded ethyl alcohol to distilled water. Antigen retrieval was performed using citric acid solution (pH 6.0) in microwave of power 100 watt for 15 minutes (Duraiyan et al., 2012). The lot number for the antibody is 5566XKC11, Santa Barbara, CA 93117, USA. The sections were equilibrated by gently displaying hot citric acid with running tap water for 3 minutes. Peroxidases in tissue sections were blocked using 3 % hydrogen peroxide for 15 minutes before the tissues were washed for 2 minutes with phosphate buffered solution (PBS) mixed with Triton X - 100. Blocking of protein was performed with Novocastra protein block for 15 minutes. The sections were then washed for 2 minutes with PBS, incubated with anti-GFAP in 100 dilutions for one hour, washed in PBS for 3 minutes, and incubated in secondary antibody for 30 minutes. Tissues were washed twice with PBS. Chromogen was diluted 1 in 100 with the PBS substrate and was allowed for 15 minutes. Sections were washed with water and counter-stained for 2 minutes in haematoxylin. Sections were washed, dehydrated, cleared and mounted in DPX (Chever et al., 2014).
Neuron Specific Enolase Immunohistochemistry
Peroxidase method was used. Tissue sections were deparaffinised using two changes of xylene (5 minutes each) followed by dehydration twice in absolute alcohol, 95 % alcohol, and 50 % alcohol respectively for 5 minutes each and then rinsed in tap water. Antigen retrieval was performed using citric acid solution (pH 6.0) in a microwave power of 100 watt from 5 minutes, thereafter sections were equilibrated by gently displacing hot citric acid with water for 3 minutes (Duraiyan et al., 2012).
The tissue sections were then pre-treated with 3 % hydrogen peroxide for 10 minutes to inhibit endogenous peroxidase activity. Sections were then washed in phosphate buffered saline triton X-100 (PBS-Tx) solution. To eliminate the non-specific binding, sections were pre-treated with normal rabbit serum, and incubated with anti-NSE primary antibodies (Zymed, Carlton Court, San Francisco, USA with lot number 21160JACOA) overnight at 4 ˚C in a humidified chamber. Following washing in PBS-Tx, biotinylated anti-immunoglobulin-G secondary antibodies were applied for 2 hours at room temperature. Sections were washed with PBS-Tx, and streptavidin-peroxidase conjugate was applied to the sections for 15 minutes at room temperature. Then, 0.6 % hydrogen peroxide and 0.02 % diaminobenzidine were applied until developed. Sections were washed in tap water, counter stained in haematoxylin for 2 minutes, washed, dehydrated, cleared and mounted on DPX (Lima et al., 2004).
Results
Acute Toxicity Test (LD50) of Coffee
According to Lorke’s (1983), LD50= √A+B=3622.843. 10%, 20% and 30% of the score were low, middle and high doses. Therefore, Low dose = 36.23 mg/kg, Middle dose = 72.46 mg/kg and High dose = 108.69 mg/kg.
Immunohistochemical Analysis of Astrocytes using Glial Fibrillary Acid Protein (GFAP) Staining on Effect of Tramadol and Coffee
The control section of the cerebellar cortex of rats stained with glial fibrillary acidic protein revealed normal cellular architecture of molecular layer, Purkinje cell layer, granular layer, white matter, and a few cell bodies and dendrites of astrocytes at dormant state (Figure 1). Low and middle doses of Coffee i.e 36.23 mg/kg bodyweight and 72.46 mg/kg bodyweight of Coffee administered groups (group B and C) revealed normal cytoarchitecture to mild distortions of the molecular, granular and Purkinje cell layers, and low to moderate expression of astrocytes (Figure 1). The high dose (108.69 mg/kg bodyweight) of Coffee administered group (group D) showed vacuolations of the Purkinje cells, and high expression of astrocytes. The group administered with 1.43 mg/kg bodyweight of Tramadol (group E) of the cerebellar cortex showed normal histological features with a low expression of astrocytes. The group administered with 2.86 mg/kg bodyweight of Tramadol (group F) of the cerebellar cortex revealed mild histological distortions in the cerebellar architecture with low expression of astrocytes (Figure 1). The group administered with 5.71 mg/kg bodyweight of Tramadol (group G) of the cerebellar cortex showed high expression of astrocytes in the molecular, granular, and Purkinje cell layers, as well as in the white matter (Figure 1). The cerebellar cortex of rats in group H and I administered with 72.46 mg/kg bodyweight (middle dose) of Coffee + 2.86mg/kg bodyweight of Tramadol and 108.69 mg/kg bodyweight (high dose) of Coffee + 5.71 mg/kg bodyweight of Tramadol revealed severe histological distortions with high expression of astrocytes (Fig 1).

LEGEND: NC= Normal Control, LC= Low dose of Coffee, MC= Middle dose of Coffee, HC= High dose of Coffee, T1= 1.43 mg/kg of Tramadol, T2= 2.86 mg/kg of Tramadol, T3= 5.71 mg/kg of Tramadol, MC+T2= Middle dose Coffee + 2.86mg/kg Tramadol, HC+T2= High dose Coffee + 5.71mg/kg Tramadol
Immunohistochemical Effect of Tramadol and Coffee on Neurons using Neuron Specific Enolase (NSE) Staining
The control section of the cerebellar cortex of rats stained with neuron specific enolase revealed normal cellular architecture of molecular layer, Purkinje cell layer, granular layer, with low staining intensity of neurons estimating no brain injury (Figure 4.58). The group administered with 36.23 mg/kg bodyweight and 72.46 mg/kg bodyweight of Coffee (group B and C) revealed normal cytoarchitecture to mild distortions of the molecular, granular and Purkinje cell layers, with moderate staining intensity of neurons (Figure 4.59 and 4.60). The high dose (108.69 mg/kg bodyweight) of Coffee administered group (group D) showed vacuolations of the Purkinje cells, with slightly high staining intensity of neurons indicating neuronal injury (Figure 4.61).
The group administered with 1.43 mg/kg bodyweight of Tramadol (group E) of the cerebellar cortex showed normal histological features with low staining intensity of neurons (Figure 4.62). The group administered with 2.86 mg/kg bodyweight of Tramadol (group F) of the cerebellar cortex revealed mild histological distortions in the cerebellar architecture with moderate staining intensity of neurons (Figure 4.63). The group administered with 5.71mg/kg bodyweight of Tramadol (group G) of the cerebellar cortex showed high staining intensity of neurons (Figure 4.64). The cerebellar cortex of rats in group H and I administered with 72.46 mg/kg bodyweight (middle dose) of Coffee + 2.86 mg/kg bodyweight of Tramadol and 108.69 mg/kg bodyweight (high dose) of Coffee + 5.71 mg/kg bodyweight of Tramadol revealed severe histological distortions with high staining intensity of neurons (Figure 4.65 and 4.66).
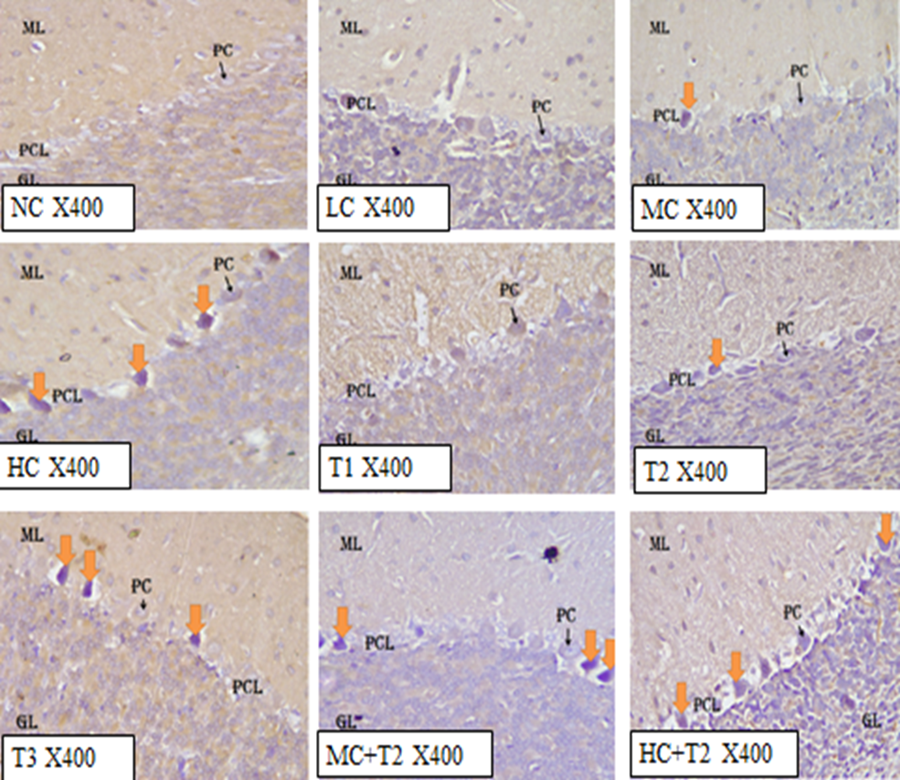
LEGEND: NC= Normal Control, LC= Low dose of Coffee, MC= Middle dose of Coffee, HC= High dose of Coffee, T1= 1.43 mg/kg of Tramadol, T2= 2.86 mg/kg of Tramadol, T3= 5.71 mg/kg of Tramadol, MC+T2= Middle dose Coffee + 2.86mg/kg Tramadol, HC+T2= High dose Coffee + 5.71mg/kg Tramadol
Discussion
In the modern society, people rely more intensively on stimulant beverages and drugs, and this makes the substances popular (Wolska et al., 2017). Combining different analgesics that act by different mechanisms (multimodal analgesia) to enhance clinical outcome is a common strategy in pain management (Raffa et al., 2012; Wolska et al., 2017). Today, Tramadol is increasingly being used worldwide, since it is believed to have fewer side effects than the other opioids (Loughrey et al., 2003; Zhuo et al. 2012). In addition to this, Tramadol is absorbed rapidly and almost entirely when it is orally administered (Grond and Sablotzki, 2004). It has also been reported that Tramadol easily crosses the blood-brain barrier and affects the tissues and functions of the central nervous system (Bertaina-Anglade et al., 2006; Hosseini-Sharifabad et al., 2016). Coffee, one of the most popular drinks in the world, is among those beverages that can have a stimulating effect on humans. About 40 % of the world’s population starts a day with a cup of Coffee (Mussatto et al., 2011; Oliveira et al., 2012).
Astrocytes are the major glial cell population within the central nervous system, and they play important roles in brain functions which include; healing and recovery of neurons in various system pathologies (Kimelberg, 2010). Their activities can be expressed immunohistochemically by the structural protein called glial fibrillary acidic protein (GFAP), which is an intermediate filament protein of the astrocytes (Venkatesh et al., 2017). Astrocytes reactivity is heterogeneous with differential phenotypes dependent upon the inducing stimulus (Zamarian et al., 2012). Therefore, changes in GFAP levels have been proposed as an index of toxicant-induced reactive gliosis (O’Callaghan et al., 1995). Using GFAP to assess immunohistochemical changes, high level of astrocyte expression with proliferated dendrites was observed in the cerebellum of rats administered with the high dose of Coffee, 5.71 mg/kg of Tramadol and combination of Coffee and Tramadol, respectively indicating damages and alterations. In support, study showed that increased GFAP expression is indicative of reactive astrogliosis (Ekong and Nwakanma, 2017). The control sections, low and middle doses of Coffee, 1.43 mg/kg and 2.86 mg/kg of Tramadol, showed low to moderate expression of astrocytes in the cerebellum. However, it is reported that apparent decreased glial fibrillary acidic protein content reflects a decrease in glial fibrillary acidic protein expression, but may not be a decrease in the number of astrocytes (Bondon et al., 2013; Ekong and Nwakanma, 2017).
Nueron specific enolase or enolase 2 is the Y-dimer of the protein enolase 2-phospho-D-glyceride hydrolase (Cooper, 1994). It is an acidic, soluble cytosolic protein and glycolytic isoenzyme, with a total molecular weight of approximately 80,000 daltons, and found predominantly in the central and peripheral neurons, and neuroendocrine cells (Marangos and Paul, 1981). It serves as a marker of neuronal functional metabolic activity and synaptic connections (Butterfield and Lange, 2009), and also promotes survival of neurons (Cooper, 1994). Increased expression of neuron specific enolase indicates neuronal damage (Bharosay et al., 2012). Immunohistochemical analysis using neuron specific enolase (NSE) revealed high staining intensity of the neurons in the cerebellum of rats administered with the high dose of Coffee, 5.71 mg/kg of Tramadol and combinations of Coffee and Tramadol respectively estimating neuronal injuries, an indication of damages to the brain. The control sections, low and middle doses of Coffee, 1.43 mg/kg and 2.86 mg/kg of Tramadol, showed mild staining density of the neurons in the cerebellum.
Conclusion
The low dose (36.23 mg/kg) and middle dose (72.46 mg/kg) of Coffee, as well as 1.43 mg/kg bodyweight and 2.86 mg/kg bodyweight of Tramadol, had mild to moderate astrogliosis and tumor expression in the cerebellum of adult Wistar rats, the high dose (108.69 mg/kg) of Coffee and 5.71 mg/kg of Tramadol caused high astrogliosis and tumor expression in rat cerebellum. However, combined ingestion of these drugs caused more deleterious effects as it damaged the cerebellar tissues of adult Wistar rats.
Contribution to Knowledge
This research study has revealed the detrimental effect of co-administration of Tramadol and Coffee on the microstructure of the cerebellum which however, can be a causative factor to some or all neurodegenerative disorders.
Recommendations
The effect of these combined drugs should be studied on other areas of the central nervous system. The kidney and liver are organs that are susceptible to adverse effects and toxicity of drugs, therefore these organs should also be investigated on combined administration of Tramadol and Coffee given this duration of study. Further investigation should also be done on the possible correlation between the combined ingestion of Tramadol and Coffee, and infertility and heart problems.
References
- Abdel-Zaher, A. O., Abdel-Rahman, M. S., & ELwasei, F. M. (2011). Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology, 32(6), 725-733.
- Abdulmalik, J., Omigbodun, O., Beida, O., & Adedokun, B. (2009). Psychoactive substance use among children in informal religious schools (Almajiris) in northern Nigeria. Mental Health, Religion and Culture, 12(6), 527-542.
- Bagheri-Abassi, F., Alavi, H., Mohammadipour, A., Motejaded, F. and Ebrahimzadeh-Bideskan, A. (2013). The effect of silver nanoparticles on apoptosis and dark neuron production in rat hippocampus. Iranian Journal of Baisc Medical Sciences, 18(7): 644- 648.
- Bharosay, A., Bharosay, V. V., Varma, M., Saxena, K., Sodani, A., & Saxena, R. (2012). Correlation of brain biomarker neuron specific enolase (NSE) with degree of disability and neurological worsening in cerebrovascular stroke. Indian Journal of Clinical Biochemistry, 27, 186-190.
- Bondan, E. F., Martins, M. D. F. M., & Viani, F. C. (2013). Decreased astrocytic GFAP expression in streptozotocin-induced diabetes after gliotoxic lesion in the rat brainstem. Arquivos Brasileiros de Endocrinologia & Metabologia, 57, 431-436.
- Butterfield, D. A., & Lange, M. L. B. (2009). Multifunctional roles of enolase in Alzheimer’s disease brain: beyond altered glucose metabolism. Journal of neurochemistry, 111(4), 915-933.
- Carrillo-Munguía, N., González-Trujano, M. E., Huerta, M., Trujillo, X., & Díaz-Reval, M. I. (2015). Tramadol and tramadol+ caffeine synergism in the rat formalin test are mediated by central opioid and serotonergic mechanisms. BioMed Research International, 2015.
- Chever, O., Lee, C. Y., & Rouach, N. (2014). Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. Journal of Neuroscience, 34(34), 11228-11232.
- Close, B. R. (2005). Tramadol: does it have a role in emergency medicine?. Emergency Medicine Australasia, 17(1), 73-83.
- Cohen, N. J. (2015). Navigating life. Hippocampus, 25(6), 704-708.
- Cooper, E. H. (1994). Neuro-specific enolase. International Journal of Biological Markers, 9(4): 205-210.
- Cui, K., Luo, X., Xu, K., & Murthy, M. V. (2004). Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28(5), 771-799.
- Daulatzai, M. A. (2013). Neurotoxic saboteurs: straws that break the hippo’s (hippocampus) back drive cognitive impairment and Alzheimer’s disease. Neurotoxicity research, 24(3), 407-459.
- Duraiyan, J., Govindarajan, R., Kaliyappan, K. and Palanisamy, M. (2012). Applications of immunohistochemistry. Journal of Pharmacy and Bioallied Sciences, 4(2): 307-309.
- Duru, C. B., Oluoha, U. R., Okafor, C. C., Diwe, K. C., Iwu, A. C., Aguocha, C. M., ... & Nwaigbo, E. (2017). Socio-demographic determinants of psychoactive substance use among students of tertiary institutions in Imo State, Nigeria. J Addict Res Ther, 8(5), 1-9.
- Eichenbaum, H., Amaral, D. G., Buffalo, E. A., Buzsáki, G., Cohen, N., Davachi, L., ... & Witter, M. (2016). Hippocampus at 25. Hippocampus, 26(10), 1238-1249.
- Ekong, M. B., & Nwakanma, A. A. (2017). Rauwolfia vomitoria and Gongronema latifolium extracts influences cerebellar cortex. Alzheimer’s, Dement Cogn Neurol, 1(3), 1-6.
- Elkhateeb, A., El Khishin, I., Megahed, O., & Mazen, F. (2015). Effect of Nigella sativa Linn oil on tramadol-induced hepato-and nephrotoxicity in adult male albino rats. Toxicology reports, 2, 512-519.
- Fisone, G., Borgkvist, A., & Usiello, A. (2004). Caffeine as a psychomotor stimulant: mechanism of action. Cellular and Molecular Life Sciences CMLS, 61, 857-872.
- Faria, J., Barbosa, J., Moreira, R., Queirós, O., Carvalho, F., & Dinis‐Oliveira, R. J. (2018). Comparative pharmacology and toxicology of tramadol and tapentadol. European Journal of Pain, 22(5), 827-844.
- García-López, C., Gómez-Huertas, C., Sánchez-González, J. M., Borroni, D., Rodríguez-Calvo-de-Mora, M., Romano, V., ... & Rocha-de-Lossada, C. (2022). Opioids and ocular surface pathology: a literature review of new treatments horizons. Journal of clinical medicine, 11(5), 1424.
- Grosso, G., Micek, A., Castellano, S., Pajak, A., & Galvano, F. (2016). Coffee, tea, caffeine and risk of depression: A systematic review and dose–response meta‐analysis of observational studies. Molecular nutrition & food research, 60(1), 223-234.
- Hol, E. M., & Pekny, M. (2015). Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current opinion in cell biology, 32, 121-130.
- Hosseini-Sharifabad, A., Rabbani, M., Sharifzadeh, M., & Bagheri, N. (2016). Acute and chronic tramadol administration impair spatial memory in rat. Research in pharmaceutical sciences, 11(1), 49-57.
- Houghton, V., Du Preez, A., Lefèvre-Arbogast, S., De Lucia, C., Low, D. Y., Urpi-Sarda, M., ... & Thuret, S. (2020). Caffeine compromises proliferation of human hippocampal progenitor cells. Frontiers in cell and developmental biology, 8, 806.
- Kimelberg, H. K. (2010). Functions of mature mammalian astrocytes: a current view. The Neuroscientist, 16(1), 79-106.
- Kumar, S., Dhankhar, S., Arya, V. P., Yadav, S., & Yadav, J. P. (2012). Antimicrobial activity of Salvadora oleoides Decne. against some microorganisms. Journal of Medicinal Plants Research, 6(14), 2754-2760.
- Lima, J. E., Takayanagui, O. M., Garcia, L. V., & Leite, J. P. (2004). Use of neuron-specific enolase for assessing the severity and outcome of neurological disorders in patients. Brazilian Journal of Medical and Biological Research, 37, 19-26.
- Liu, H., Hu, G. H., Wang, X. C., Huang, T. B., Xu, L., Lai, P., ... & Xu, Y. F. (2015). Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutrition and cancer, 67(3), 392-400.
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Archives of toxicology, 54, 275-287.
- Marangos, P. J., & Paul, S. M. (1981). Brain Levels of Neuron‐Specific and Nonneuronal Enolase in Huntington's Disease. Journal of Neurochemistry, 37(5), 1338-1340.
- Miotto, K., Cho, A. K., Khalil, M. A., Blanco, K., Sasaki, J. D., & Rawson, R. (2017). Trends in tramadol: pharmacology, metabolism, and misuse. Anesthesia & Analgesia, 124(1), 44-51.
- Mohammadpour, A., Ashkezari, M. D., Farahmand, B., & Shokrzadeh, M. (2019). Demographic characteristics and functional performance of the kidneys and hearts of patients with acute tramadol toxicity. Drug research, 69(04), 207-210.
- Mussatto, S. I., Machado, E. M., Martins, S., & Teixeira, J. A. (2011). Production, composition, and application of coffee and its industrial residues. Food and bioprocess technology, 4, 661-672.
- Nmaju, A. U., Bisong, S. A., Nwankwo, A. A., Joshua, I. E., & Osim, E. E. (2013). Comparative effects of Garcinia kola and coffee diets on learning and memory in mice. British Journal of Medicine and Medical Research, 4(2), 731-746.
- Noyce, A. J., Bestwick, J. P., Silveira‐Moriyama, L., Hawkes, C. H., Giovannoni, G., Lees, A. J., & Schrag, A. (2012). Meta‐analysis of early nonmotor features and risk factors for Parkinson disease. Annals of neurology, 72(6), 893-901.
- O'Callaghan, J. P., Jensen, K. F., & Miller, D. B. (1995). Quantitative aspects of drug and toxicant-induced astrogliosis. Neurochemistry international, 26(2), 115-124.
- Oliveira, M., Casal, S., Morais, S., Alves, C., Dias, F., Ramos, S., ... & Oliveira, M. B. P. (2012). Intra-and interspecific mineral composition variability of commercial instant coffees and coffee substitutes: Contribution to mineral intake. Food Chemistry, 130(3), 702-709.
- Radbruch, L., Glaeske, G., Grond, S., Münchberg, F., Scherbaum, N., Storz, E., ... & Cremer-Schaeffer, P. (2013). Topical review on the abuse and misuse potential of tramadol and tilidine in Germany. Substance abuse, 34(3), 313-320.
- Raffa, R. B., Tallarida, R. J., Taylor Jr, R., & Pergolizzi Jr, J. V. (2012). Fixed-dose combinations for emerging treatment of pain. Expert opinion on pharmacotherapy, 13(9), 1261-1270.
- Roostaei, T., Nazeri, A., Sahraian, M. A., & Minagar, A. (2014). The human cerebellum: a review of physiologic neuroanatomy. Neurologic clinics, 32(4), 859-869.
- Salm-Reifferscheidt, L. (2018). Tramadol: Africa's opioid crisis. The Lancet, 391(10134), 1982-1983.
- Stokes, C. N., O'Sullivan, M. G., & Kerry, J. P. (2016). Assessment of black coffee temperature profiles consumed from paper‐based cups and effect on affective and descriptive product sensory attributes. International Journal of Food Science & Technology, 51(9), 2041-2048.
- Takhtfooladi, H. A., Asl, A. H. K., Shahzamani, M., Takhtfooladi, M. A., Allahverdi, A., & Khansari, M. (2015). Tramadol alleviates myocardial injury induced by acute hindlimb ischemia reperfusion in rats. Arquivos brasileiros de cardiologia, 105, 151-159.
- Tashakori, A., & Afshari, R. (2010). Tramadol overdose as a cause of serotonin syndrome: a case series. Clinical Toxicology, 48(4), 337-341.
- United Nations Office on Drugs and Crime. (2017). World Drug Report 2017. Vienna, Austria: United Nations, 273p.
- Venkatesh, H. S., Tam, L. T., Woo, P. J., Lennon, J., Nagaraja, S., Gillespie, S. M., ... & Monje, M. (2017). Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature, 549(7673), 533-537.
- Wang, L., Shen, X., Wu, Y., & Zhang, D. (2016). Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Australian & New Zealand Journal of Psychiatry, 50(3), 228-242.
- Wang, R., Xue, J., Meng, L. and Lee, J. (2019). Caffeine improves the performance and thermal stability of perovskite solar cells. Joule, 2542(19):30173-30174.
- Witter, L., & De Zeeuw, C. I. (2015). Regional functionality of the cerebellum. Current opinion in neurobiology, 33, 150-155.
- Wolska, J., Janda, K., Jakubczyk, K., Szymkowiak, M., Chlubek, D., & Gutowska, I. (2017). Levels of antioxidant activity and fluoride content in coffee infusions of arabica, robusta and green coffee beans in according to their brewing methods. Biological Trace Element Research, 179, 327-333.
- World Health Organization (2014). Tramadol update review report. Thirty-sixth meeting of Expert Committee on Drug Dependence (ECDD) Agenda item 6.1, Geneva, Switzerland, 39 p.
- Yu, E. Y. W., Wesselius, A., van Osch, F., Stern, M. C., Jiang, X., Kellen, E., ... & Zeegers, M. P. (2019). The association between coffee consumption and bladder cancer in the bladder cancer epidemiology and nutritional determinants (BLEND) international pooled study. Cancer Causes & Control, 30, 859-870.
- Yunusa, U., Bello, U. L., Idris, M., Haddad, M. M., & Adamu, D. (2017). Determinants of substance abuse among commercial bus drivers in Kano Metropolis, Kano State, Nigeria. American Journal of Nursing Science, 6(2), 125-130.
- Zamarian, L., Sinz, H., Bonatti, E., Gamboz, N., & Delazer, M. (2008). Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology, 22(5), 645.
- Zhuo, H. Q., Huang, L., Huang, H. Q., & Cai, Z. (2012). Effects of chronic tramadol exposure on the zebrafish brain: a proteomic study. Journal of proteomics, 75(11), 3351-3364.


