Archive : Article / Volume 2, Issue 3
Case Report | DOI: https://doi.org/10.58489/2836-2322/024
Effectiveness of manual cleaning in a line of oral suspensions
Company Oral Liquids Pharmaceutical Laboratory, Medilip, UEB Quality Control, Microbiology Laboratory, Bayamo, Cuba.
Correspondng Author: Leobel Fajardo Cedeño *
Citation: Leobel Fajardo Cedeño, (2023). Effectiveness of manual cleaning in a line of oral suspensions. Pharmacy and Drug Development. 2(3); DOI: 10.58489/2836-2322/024.
Copyright: © 2023 Leobel Fajardo Cedeño, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2023-04-17, Received Date: 2023-04-17, Published Date: 2023-09-19
Abstract Keywords: cleaning and disinfection; alert limits; action limits; poured plate; Rinsing method.
Abstract
The microbiological control of production equipment in the pharmaceutical industry is essential to achieve quality medicines. This is achieved through proper control of the cleaning and disinfection processes of the equipment. Samples were taken by the rinsing method and for the count of microorganisms, the poured plate method containing soy tryptone agar was used. The samples were incubated according to the physiological needs of the microorganisms for a period of 48h at a temperature of 35 ° C. Selective media and gram stain were used for the identification of microorganisms and the Poisson distribution was used to calculate the usual working interval and the alert and action limits respectively.The cleaning and disinfection of the equipment is done manually, associated with this, the large volumes of the same and the places of difficult access can promote microbial growth, the arithmetic means of isolate microorganisms ranged from 7.62 cfu / mL in the transfer pump to 69.28ufc / Ml in the plastic container, which was eliminated from the production process due to the formation of pores that can accommodate microcolonies. Alert, action, and work interval limits were determined for each individual computer taking into account the volume of each. The determination of these limits allows preventive and corrective actions to be taken to reduce the microbial load, thus avoiding the possibility of contamination of the finished product.
Introduction
Microbiological control of production equipment in the pharmaceutical industry is essential to achieve quality medicines. This is achieved through adequate control of the equipment cleaning and disinfection processes.
Method
A checklist was used to review the critical points that could affect the increase in the microbial load. The rinsing method was used for sampling and the poured plate method containing tryptone soy agar was used to count microorganisms. The samples were incubated according to the physiological needs of the microorganisms for a period of 48h at a temperature of 35°C. The Poisson distribution was used to calculate the usual work interval and the alert and action limits, respectively.
Results
The cleaning and disinfection of the equipment is carried out manually, associated with this, the large volumes of the same and the places of difficult access can promote microbial growth, the arithmetic means of isolated microorganisms ranged from 7.62 cfu/ mL in the transfer pump up to 69.28ufc/mL in the plastic container, which was eliminated from the production process due to the formation of pores that can accommodate microcolonies. The alert and action limits and the work interval were determined for each individual piece of equipment taking into account the volume of each one.
Conclusions
The determination of these limits allows taking preventive and corrective actions to reduce the microbial load, thus avoiding the possibility of contamination of the finished product.
The manufacture of pharmaceutical products is governed by Good Manufacturing Practices. These standards constitute international quality standards for the pharmaceutical industry, adopted in our country by the health authority, which ensure that the pharmaceutical product meets the necessary quality and safety requirements, [1] it is the basic standard that raises the essential aspects that must be met in the drug plants to obtain safe and effective products [2]. Pharmaceuticals and their active ingredients can be contaminated by other pharmaceuticals and active ingredients, by cleaning agents, by microorganisms or other materials such as lubricants, air particles, raw materials, intermediatesand auxiliaries. In many cases the same equipment can be used for the production of different subsequent products; It is essential then, not only a good cleaning procedure but also an adequate cleaning validation strategy [3].
The cleaning of equipment is a requirement of Good Production Practices, but it was at the end of the 80s that it became more popular. With the continued rise of multipurpose industries, the potential risk of cross-contamination and adulteration of drugs subsequently produced on the same equipment has increased. To minimize these risks of contamination, the U.S. Food and Drug Administration (FDA) placed much more emphasis on cleaning equipment. In July 1993, a review of cleanliness validation appeared in the FDA's inspection guide. It required companies to have in writing the general procedure of the cleaning process that would be validated, which should also indicate the sampling procedure and the analytical method used in the quantification of the active ingredient residue, including the number of batches, the number of repetitions of the cleaning process and other requirements to complement the proposed objective [4]
The presence and growth of microorganisms in finished products can cause health risks, or alter the physical-chemical characteristics that would prevent their commercialization. For this reason, all production areas must be kept under very strict hygienic conditions, with minimum levels of micro-organisms and particles called "clean zones" [5]
Many factors can result in the introduction of microorganisms in production processes, some have a higher probability of microbial contamination. These manufacturing factors include: water used as an ingredient, pharmaceutical ingredients, processing equipment, manufacturing personnel manufacturing environment [6]
The concentration of microbial load on surfaces has been extensively studied in places such as hospitals, industries and laboratories, to evaluate the effectiveness of the cleaning and disinfection process and to establish the relationship between microorganisms on surfaces and contamination in machinery used for the manufacture of products [7]
To minimize the entry of microorganisms into the manufacturing environment, manufacturers must be able to implement a microbiological control program that includes the air present in the facilities, the surfaces at different points: warehouses, production areas and microbiological quality control laboratories [5,8].
The objective of these programs is to eliminate or reduce the microbial load present in the equipment, surfaces and environments where the different formulation and filling processes are carried out. The elimination or reduction of the microbial load is determined by factors such as: the nature of the surfaces that come into contact with the products being processed and the removal by mechanical action of the remains of raw materials embedded or adhered to surfaces and equipment. To achieve a good cleaning and disinfection in the facilities it is necessary to know the possible forms of contamination, the use of a disinfectant and its characteristics [9]
In addition to cleaning, the disinfection process allows to obtain an optimal quality of the products from the microbiological point of view, reducing to the minimum possible the microbial load, in the production areas, in equipment, surfaces and personnel [10]
In order to establish a limit for microbiological contamination, the route of administration of the pharmaceutical product and the nature or type of the contaminating microorganism must be taken into account. For example, the presence of enteric microorganisms such as E. coli or enterococus and Pseudomonas sp could be unacceptable. Parenteral and ophthalmological products must have a more rigorous control for the limit of microorganisms, in these cases it is also necessary to establish a limit for the number of endotoxins present on the contact surface of the equipment with the product (3).
An important component within this program corresponds to the establishment of microbiological limits of alert and action (9,11,12).
Therefore, we propose to establish the limits of alert and microbiological action in the production equipment of non-sterile oral suspensions
Counting of microorganisms. Isolation of microorganisms
Microbiological controls were carried out on the production equipment before starting the manufacture of 50 batches of an oral suspension, the rinsing method was used by taking 120mL of the last rinse water after the cleaning and disinfection processes. From the sample, aliquots of 1mL were taken and seeded in sterile 90mm Petri dishes to which was added, soy tryptone agar (poured plate) an aliquot was also poured into liquid medium tryptone soybean broth. The samples were incubated in Friocell incubator from 35 to 37ºC per periodof 24 to 48 hours. After the incubation period, the number of colony forming units (CFU) was counted using the digital colony counter S (13,14,15). Gram staining was performed and subcultures were performed in selective culture media. The culture media were prepared according to the manufacturer's instructions.
Isolation of microorganisms
Gram staining was performed on the most representative colonies of each sample and subcultures were performed in selective culture media from tryptone soybean broth. The culture media were prepared according to the manufacturer's instructions.
Statistical analysis for the determination of alert and action limits
According to the nature and way in which the microorganisms behave, the Poisson distribution was used for the calculation of the arithmetic mean and the standard deviation [16]
The usual work interval and internal limits for each team were calculated as follows:
- Usual Work Interval: from zero to the arithmetic mean increased in two standard deviations.
- Alert Limit: arithmetic mean increased by a standard deviation.
- Action Limit: for values above the highest value obtained for the Usual Work Interval.
In addition, the operation of the equipment, the material with which they were built and the capacity of each equipment or container were taken into account.
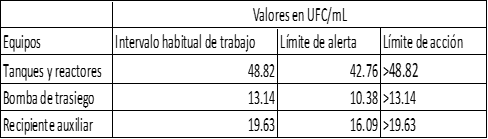
Table 1 Microbiological results of microorganism counts in processing equipment

The table shows the arithmetic mean of the number of colony-forming units isolated from each of the equipment involved in the manufacturing process of a non-sterile oral suspension.
The equipment used in the production of medicines, are susceptible to microbial contamination, because remains of pharmaceutical products can be adhered to the surface of the equipment serving as nutrients to the microorganisms that are introduced into the production process for different reasons, among which we can cite the environment of the manufacturing and filling area, the water used in the cleaning and disinfection processes and the personnel who carry out sampling or carry out the manufacturing process; it is therefore essential to evaluate the quality of the cleaning and disinfection processes of the equipment used, thus guaranteeing a pharmaceutical product with quality. Oral suspensions are more difficult to clean than solutions due to the intrinsic nature of the product, therefore remains of the pharmaceutical product can be adhered to the surface of the equipment so the cleaning and disinfection process must be more intense emphasizing the places of difficult access of the equipment; to achieve the final quality of the cleaning and To all the above raised is added that the cleaning process for this line of oral suspensions is done manually, so it becomes more rigorous and necessary the microbiological control of the equipment to avoid any microbial contamination that can bring with it quality failures in the final product.
Table 1 shows the mean values of the isolated colony-forming units in all the equipment involved in the manufacture of the batches of oral suspensions for one year.
Of all the containers and equipment used, the plastic tank was where greater insulation was obtained, although it has a volume of 220L, the plastic material with which it is manufactured has pores and they could ag range or even form new Due to the erosion caused by the cleaning and disinfection processes, which can result in the growth of microcolonies that become resistant to disinfectants and later form which are difficult to eliminate and can contaminate the final product, this container being a risk of microbial contamination.
The storage tanks and reactors follow in descending order of the number of isolated colony forming units after the cleaning and disinfection processes, values ranging from 23.18 cfu/mL in reactor number 1 to 40.24 cfu/mL in storage tank number 2. Although they are built in stainless steel, a material that is resistant to the formation of pores and prevents the adhesion of microorganisms, they present intrinsic risks such as large volumes of capacity; The cleaning and disinfection process is done manually and there are points of difficult access that hinder the elimination of waste, resulting in microbial growth in these equipment. It was found that storage tanks have higher counts than manufacturing reactors, this may be due to the fact that the manufacturing process in the reactors is more aggressive due to the operations that are carried out in them and can decrease the number of viable microorganisms in these containers.
Another important factor that can affect the number of isolated microorganisms is the time of permanence of the product in the equipment, being the longer exposure time in the storage tanks than in the reactors, so that the microorganisms introduced to the manufacturing process could use these remains for their growth, affecting the increase of the microbial load of these containers.
The auxiliary container of 50 liters having little volume and built-in stainless steel, has little risk of microbiological contamination, since it is very easy to carry out the cleaning and disinfection processes; because it can reach the entire interior of the container making both processes effective making it possible for cleaning and disinfection to be reproducible between different operators.
The transfer pump is where the least number of microorganisms was isolated at 7.62 cfu / mL; Because, during its operation, the generation of turbulence at high speeds prevents the adhesion of microorganisms within the equipment, thus avoiding the formation of biofilms due to the decreased in the number of viable cells within it.
The arithmetic means of the isolates determined showed results less than 50 cfu/mL, although in certain production batches isolates above this value were made, the microbiological quality of the final product was not affected. An exhaustive analysis of the primary data verified that the increase of microorganisms above 50 cfu/mL occurred when the equipment was clean but inactive on two consecutive days, probably due to the residual humidity of the cleaning and disinfection processes together with the temperature of the premises and inside the equipment during the shutdown period. , allowed the growth of certain bacterial colonies; So we define to carry out a cleaning and disinfection before starting the manufacture of the product even if it had been done at the end of the previous product.
When making comparisons of the isolates made when drugs were manufactured in the form of solutions and oral syrups, it was verified that the arithmetic means determined decreased drastically since the colony forming units were 10 or less, which shows that oral suspensions are the most difficult case to perform cleaning and disinfection procedures, demonstrating that if we achieved effectiveness of the process implemented for oral suspensions we could perform the same cleaning procedure for solutions and syrups achieving similar or better results after sanitization of the equipment.
It is important to note that in none of the isolates carried out pathogenic microorganisms were isolated and the microorganisms mostly isolated were sporulated aerobic gram-positive bacilli, probably present in the aire of the formulation room or carried there by the air currents present in the installation or by personnel working in them. None of the manufactured batches presented microbiological quality failures, so the level of microorganisms in each of the containers was accepted except for the plastic container.
Although the microbial counts obtained did not affect the quality of the final product, it is also necessary to establish alert levels that allow preventive or corrective actions to be taken to reduce the microbial load before it reaches dangerous limits for the quality of the pharmaceutical product, in addition to providing an adequate tool for evaluating the cleaning and disinfection processes. Table 2 shows the alert and action limits defined according to the isolations performed.
All the containers and equipment used in the manufacturing lines complied during the isolations with less than 50 cfu / mL and the alert and action limits are in correspondence with the difficulty of cleaning the containers and the time spent in each of them. The alert and action limits of the table correspond to non-objectionable microorganisms, in the case of detecting the presence of objectionable microorganisms it is necessary to verify the cleaning and disinfection process, as well as all aspects related to the manufacturing processes of the product.
Conclusion
The cleaning and disinfection processes are the most critical point to control during the manufacture of the drug, due to the difficulties of places of difficult access and that is done manually, which is why the surveillance of this aspect is crucial to keep the number of microorganisms in the production equipment reduced. The final microbiological quality of the non-sterile products depends on the effectiveness of the cleaning and disinfection processes of the equipment used, which is strictly complied with by the personnel who perform it. Alert and microbiological action levels were established to ensurepreventive and corrective actions that could mitigate failures in these processes, both are indispensable points of review in the risk management of the formulation and packaging plant. The plastic tank was replaced by a stainless-steel container, thus avoiding the risk of biofilm formation in it that could contaminate the final product.
References
- Araújo J, Falero M, Laulhé S. Validation of cleaning in the pharmaceutical laboratory of the National Directorate of Health of the Armed Forces. Health Mil 2018; 37(1):32-38.
- Quintana Esquivel Marisel Guadalupe, Apezteguía Rodríguez Isabel. The Good Practices in the Production of biological agents and the Quality Mangement Systems. Rev Cubana Farm [Internet]. 2010 Dic [citado 2023 Ene 30] ; 44( 4 ): 547-557.
- LOPEZ M. Establishment of the acceptable limit for the cleaning residue in the production equipment of the pharmaceutical industry. Rev Cubana Farm. Online [accessed January 2011]2005, vol.39, n.3, pp. 0-0. ISSN 0034-7515.6
- Deza E, Elizabeth R. Validation of the cleaning procedure of semisolid manufacturing equipment for dexamethasone acetate. National University of Trujillo; 2011.
- Del M, De C, Rosa LA, Ullán C, Ma P, Prieto Y, et al. Microbiological air quality of a clean area in a pharmaceutical industry [Internet]. Core.ac.uk. [cited 13 January 2023.
- United States Pharmacopeia 40, National Formulary 35. Test 1115 Control de la Biocarga, Rockville, Md., USA. The United States Pharmacopeial Convention, Inc. 2017. (versión electrónica.
- Charry, N., & Gómez, S. L. (2016). Determination of the limits of microbial contamination present on surfaces of a district reference laboratory of pharmaceutical microbiology. Journal of Pharmacy & Pharmacognosy Research, 4(3), 115-12
- ICIDCA. About Sugarcane Derivatives [Internet]. Redalyc.org. [cited 13 January 2023.
- Ronner AB, Wong ACL. Biofilm Development and Sanitizer Inactivation of Listeria monocytogenes and Salmonella typhimurium on Stainless Steel and Buna-n Rubber. J Food Prot. 1993;56(9):750-758. doi: 10.4315/0362-028X-56.9.750.
- Fajardo Cedeño L. Evaluation of disinfectants for use in the pharmaceutical environment. Ars Pharm. 2021;62(2):175–81.
- Sánchez Álvarez KS Álvarez, Quintana Cantillo AQC, Mho González M, Tuñón MA, Porrero Martín F. Comparison of statistical methods used for the establishment of microbiological control limits in clean areas. Rev. CENIC Cienc. Biol. [Internet]. 6 April 2022 [cited 1 February 2023];32(3):153-61
- James Wilson - “Environmental Monitoring: Misconceptions and Misapplications”, PDA Journal of Pharmaceutical Science Technology, 185-190, Volume 55, No. 3, May/June 2001.
- Delgado, M., Escamilla, L., Pérez, A., & Arias, J. (2004). Determination of parameters of microbial contamination present in a manufacturing area of sterile drugs based on ß-lactam antibiotics. Universitas Scientiarum, 9(2), 23-33.
- Charry N, Gomez S. L. Determination of the limits of microbial contamination present on surfaces of a district reference laboratory of pharmaceutical microbiology. Journal of Pharmacy & Pharmacognosy Research [Internet]. 2016;4(3):115-121.
- United States Pharmacopeial 40. National Formulary 35Rockville, Md., USA. Convention, Inc. 2017. (electronic version). Test 1116. Microbiological control and monitoring of aseptic processing environments. Pp. 1571-1572.
- Bottale A, Ceferina-Riera L. Establishment of internal microbiological limits for areas classified Grade D. Revista Cubana de Farmacia [Internet]. 2016 [cited 31 Jan 2023]; 50 (1).


