Archive : Article / Volume 2, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-2322/017
Formulation And In-Vitro Evaluation of Herbal Tablet Containing Withania Coagulans Extract
Mula Education Societyâs College of Pharmacy, Sonai, Tal- Newasa, Dist- Ahmednagar, Maharashtra 414105 and Savitribai Phule Pune University, Pune, Maharashtra, India
Correspondng Author: M.D. Sonawane
Citation: M.D. Sonawane, A. R. Pawar, S. V. Patwa, S. P. Pawar, (2023). Formulation And In-Vitro Evaluation of Herbal Tablet Containing Withania Coagulans Extract. Journal of Pharmacy and Drug Development. 2(1). DOI:10.58489/2836-2322/017
Copyright: © 2023 M.D. Sonawane, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-12-31, Received Date: 2022-12-31, Published Date: 2023-02-09
Abstract Keywords: Anti-diabetic, Withania coagulans fruits, Disintegration, Preformulation study, herbal formulation etc.
Abstract
Ayurvedic phytomedicines are an hour requirement and more probable than allopathic medicines, which are not only pricey about "lead" but also linked to several negative side effects. The fruits of Withania coagulans exhibit notable anti-diabetic action, according to the ethnopharmacological usage and a literature assessment. A formulation incorporating the plant material was created after a thorough analysis of the powdered ethanolic extract of Withania coagulans fruits, making it more palatable and appropriate for diabetics. Pharmaceutical dosage formulations with a special dry plant extract and other components like talc, starch, and microcrystalline cellulose have been shown to have statistically significant anti-diabetic action. All of the values were within permissible limits, according to the findings of preformulation studies. The formulation's notable hardness and friability revealed that the tablets were mechanically stable. The formulation's disintegration took more than a minute. Significant results from an in-vitro anti-diabetic examination of a tablet formulation are evidence that the traditional Indian medical systems assertions regarding the effectiveness of this plant in the treatment of diabetes are true. The last inference made from the facts stated above is that since natural medicines do not have the substantial side effects associated with synthetic drugs, it may be appropriate to reconsider using these cost-effective, non-toxic, and plant-safe alternatives. The results of the preformulation studies showed that all of the values were within acceptable ranges.
Introduction
One of the most prevalent diseases, diabetes mellitus (DM), is characterised by a variety of metabolic issues that lead to an increase in blood sugar levels. A significant global health danger is posed by diabetes mellitus (DM) and its severe and chronic consequences. In India, it is presently spreading at epidemic levels. In ancient Indian scriptures, the illness is referred to as "madhumeha"[1]. The use of paneer ke phool, also known as Indian rennet, Withania coagulans, or pannerdoda, is one among the natural treatments for diabetes control that health professionals advise [2]. The flower Withania coagulans, also known as "Paneer ke Phool," is indigenous to Afghanistan, Pakistan, and India [3]. The Charaka Samhita, under the headings Brihaniya Mahakashaya and Madhurskandha dravya, mentions the plant's antidiabetic properties. It is helpful for diabetics and is considered to have therapeutic benefits. The dried fruits of Withania coagulans Dunal (Solanaceae Family) have demonstrated resistance to diabetes. Economically significant species of the Withania genus are W. somnifera (Ashwagandha) and W. coagulans (Paneerbooti/Ashutoshbooti), which are grown in many parts of the world for their Ayurvedic uses [4]. Indian Rennet is the most well-known species of Withania coagulans (Paneer Dodi). As all components of the plant, including the flowers, fruits, seeds, leaves, stems, roots, and bark, are known to have significant health advantages, paneer dodi is referred as as a "health elixir". [5] It is used to treat diabetes, cancer, and stress. Additionally, it is used to treat intestinal infections, dyspepsia, chronic liver problems, and flatulence. It is used to treat biliousness, strangulation, and asthma. Additionally, it is used to treat pruritis, polyuria, giddiness, weakness, and limb and body aches [6]
Materials and Methods
Fruits of Withania coagulans plants were procured from a nearby market and verified by the Botanical Survey of India in Pune. The fruits were grounded into a powder, extracted with ethanol, concentrated using a Buchi Rota evaporator, and then kept dry for future research. Purchased from Hi-lab India: Glycine max, starch, sodium benzoate, gelatin, microcrystalline cellulose, talc, and magnesium stearate.
Preparation of plant Extract
The plant materials were washed dried in the shade, and ground. With increasing polarity, Soxhlet apparatus was used to extract withania coagulans (fruit) powder samples weighing around 27 g each. Using a Buchi rotary vacuum evaporator, the extract was filtered and concentrated after extraction at room temperature. By using a conventional process, the extract was submitted to a qualitative approach of preliminary phytochemical analysis. The process of the manufacture of herbal tablets, the active methanol extract was made based on the preliminary photochemical screening [7,8].
Preparation of Herbal Tablet
The excipients and plant extract were combined before being compacted into tablets. Table No. 1 provided the composition's specifics.
Table 1: Formulation of anti-diabetic tablet
Ingredients | Batch No. | |||||
Quantity Per tablet (mg) | ||||||
F1 | F2 | F3 | F4 | F5 | F6 | |
Plant Extract | 300 | 300 | 300 | 300 | 300 | 300 |
Carbopol | 20 | 30 | 40 | - | - | - |
Ethyl cellulose | - | - | - | 20 | 30 | 40 |
Microcrystalline cellulose | 40 | 40 | 40 | 40 | 40 | 40 |
Dibasic calcium phosphate | 30 | 20 | 10 | 30 | 20 | 10 |
PEG 4000 | 10 | 10 | 10 | 10 | 10 | 10 |
Methyl Paraben | 0.1 % | 0.1 % | 0.1 % | 0.1 % | 0.1 % | 0.1 % |
Weight per tablet | 400 | 400 | 400 | 400 | 400 | 400 |
Preformulation Studies
Evaluation of powder blend
The produced grains for all test batches passed the evaluation criteria since they displayed proper flow characteristics and did not click in the hopper. The powder mixture was put through a number of tests, including bulk and tapped densities, Carr's index, Hauser's ratio, and angle of repose. The results are shown in table 2.
Table 2: Preformulation of Powder Blend
Batch | Bulk density (gm/ml) | Tapped density (gm/ml) | Carr’s index (%) | Hausner’s ratio | Angle of repose (o) |
F1 | 0.4285 | 0.48 | 15.18 | 1.12 | 29.68 |
F2 | 0.44 | 0.49 | 15.12 | 1.20 | 27.47 |
F3 | 0.43 | 0.51 | 14.16 | 1.31 | 32.10 |
F4 | 0.45 | 0.50 | 16.78 | 1.42 | 33.57 |
F5 | 0.42 | 0.52 | 13.52 | 1.15 | 30.95 |
F6 | 0.44 | 0.50 | 17.18 | 1.18 | 31.21 |
Bulk density
Having a general notion of the drug substance's true and bulk densities is very helpful in determining the size of the final dosage form. This parameter obviously plays a crucial role for medications with modest potency, which may make up the majority of the final granulation or table. When a densities issue is found, it is frequently quickly fixed by milling, slugging, or formulation. Bulk density of a product varies significantly with the method of crystallization, milling, or formulation. The properties of powder flow may be impacted. It has an impact on the size of high-dose capsule products or the homogeneity of a low-dose formulation where there are significant variations in the densities of the medication and excipients.
Tapped Density
The mass-to-volume ratio of a powder after it has been tapped for a specific amount of time is known as the powder's "tapped density." The random dense packing of a powder is represented by its tapped density. Tapped density can be calculated using Eq.
Tapped Density = m/vf
where M= mass in grams, and Vf= the tapped volume in milliliters.
Carr’s index (%) & Hausner ratio
Hausner ratio Depending on the material, the compressibility index can be determined using V10 instead of V0. If V10 is used, it is clearly stated in the results. The Carr index (Carr's index or Carr's Compressibility Index) is an indication of the compressibility of a powder. It is named after the scientist Ralph J. Carr, Jr. The Carr index is calculated by the formula=100 [ρT‒ρB/ρB], where ρB is the freely settled bulk density of the powder, and ρT is the tapped bulk density of the powder after "tapping down". It can also be expressed as s C=100[1‒ρB/ρT] In pharmaceutics, the Carr index is widely employed as a measure of a powder's flow ability. The bulk density and tapped density of a free-flowing powder would be same. The Carr index would be small as a result. On the other hand, the discrepancy between the measured bulk and tapped densities would be greater in a poorly flowing powder where there are more inter particle interactions, leading to a bigger Carr index. A Carr index above 25 is seen as a sign of poor flow ability, and one below 15 as a sign of high flow ability. The Hausner ratio, denoted by the symbol ρT/ρB., is another way to gauge a powder's flow.
Angle of repose (o)
The plane of contact between two bodies produces an angle with the horizontal when the upper body is just about to slide; the angle's tangent is the coefficient of friction between the two bodies. This angle is known as the angle of repose. The steepest angle of fall or dip in relation to the horizontal plane that a material may be stacked to without collapsing is known as the angle of repose, or critical angle of repose, of a granular material. The material on the slope face is almost ready to slide at this angle.
Evaluation of Tablets
Disintegration Time
This test was a time required for the tablet to separate into particles, the disintegration test measure only of the time required under a given set of conditions for a group of tablets to disintegrate into particles. This test was performed to identify the disintegration of tablet in a specific time period.
Hardness and Friability of Tablet
A particular amount of hardness, strength, and resistance to friability are needed for the tablet. A friabilator of the ROCHE type was used to determine the friability.
Weight variation test
Twenty tablets were taken, and the weights of each one and the group were recorded on a digital weighing scale in order to analyze the weight variation. The total weight was used to calculate the average weight of one tablet. The weight variation test would be an effective way to avoid mining the consistency of the drug content. Neither one nor more than two of the individual weights vary from the average weight by a percentage greater than that displayed in the following table. The variation and mean were calculated. The following formula was used to determine the percentage variation.
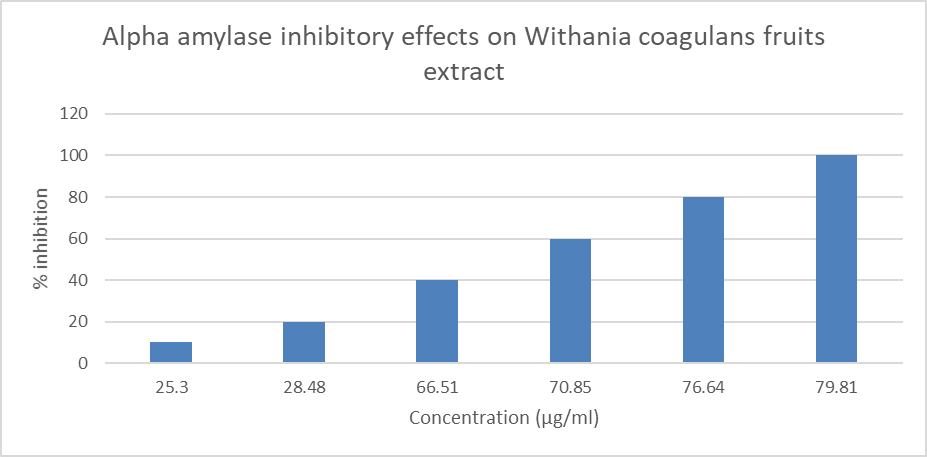
Results and discussion Anti-diabetic herbs play a significant part in reducing inflammation and blood sugar levels, protecting people from hyperglycemia. By keeping in mind that this research was done to assess the in-vitro anti-diabetic effectiveness of tablets containing Withania coagulans extract. Using increasing polarity solvents such as petroleum ether, acetone, alcohol, and water, the extract's preliminary phytochemical screening was conducted. (Table 3 & 4) Table 4: Percentage yield of the Withania coagulans fruit extracts Plant Part used % Yield Withania coagulans Fruits Pet. Ether Acetone Alcohol Distilled water 2.50 3.6 3.8 17.2 Table 4: Preliminary Phytochemical Evaluation of Withania coagulans fruit extracts Name of Extract Pet. Ether Acetone Alcohol Distilled Water Carbohydrate + + + + Alkaloid - + + - Protein + - + - Amino acid + - + - Glycoside + + + + Saponin + + - + Tannin - + + + Phenolic Compound + + + + Essential Oil + + + + + = Presence; - = Absence Preliminary phytochemical research indicates that alcohol extract contains more carbohydrates, alkaloids, proteins, amino acids, glycosides, tannins, phenolic compounds, and essential oils than other extracts. Consequently, the alcohol extract was chosen for the tablet formulation. In-vitro anti-diabetic Activity Withania coagulans fruit alcohol extract was utilized to decrease alpha amylase activity. The human body has several enzymes that help with food digestion. In order for complex sugar to be efficiently absorbed from the GIT, the alpha amylase catalyses the breakdown of complex sugar to simple sugar. Alpha amylase activity was inhibited by acarbose (100 g/ml) (Table 7 & Figure 1). At a concentration of 100 g/ml, the alcoholic extract of Withania coagulans fruits exhibited pronounced inhibitory action. [Table 8] Table 5: Alpha amylase inhibitory effects of Acarbose Drug Concentration (µg/ml) % of inhibition Acarbose 10 17.80 20 22.50 40 28.90 60 39.17 80 47.28 100 58.85 Figure 1: Alpha amylase inhibitory effects of Acarbose Table 6: Alpha amylase inhibitory effect on Withania coagulans fruits extract Treatment Concentration (µg/ml) % of inhibition Methanolic extract of Withania coagulans fruits 10 25.30 20 28.48 40 66.51 60 70.85 80 76.64 100 79.81 Figure 2: Alpha amylase inhibitory effect on Withania coagulansfruits extract Discussion Many plants have been shown to have anti-diabetic properties, and Ayurveda has long used them to treat diabetes. Different plant extracts are employed today in numerous inventive compositions. In this study, herbal tablets were manufactured and their in-vitro anti-diabetic potential was tested. The normal Acarbose only showed a 58.85% alpha amylase inhibition at a concentration of 100 g/ml, whereas the methanolic extract had a strong alpha amylase inhibitory effect at 100 g/ml, or 79.81%. The percentage of inhibition increased as extract concentration rose. It can be because the extract contains the active ingredients. Conclusion Since the beginning of civilization, medicinal plants have been used to treat illnesses. It has been discovered that Withania coagulans has a wide variety of chemically varied and physiologically active chemicals with a significant therapeutic potential. Although there are medicinal uses for crude extracts from various Withania coagulans parts, particularly the fruits, modern drugs can only be created after thorough research into the bioactivity, mechanism of action, pharmacotherapeutics, toxicity, and after proper standardization and clinical trials. On the basis of the outcomes of the powder preformulation study and the formulated tablet's standardization parameter, it is concluded that all of the parameters assessed fell within an acceptable range. The formulation produced results in colour, appearance, weight variation, thickness, hardness, friability, and disintegration time, according to physical testing. There were noticeable alpha amylase inhibitory effects of the methanol extracts. Long-term toxicity studies and more research into the formed tablet's underlying mechanism of action are both required. Moreover, in-vivo anti-diabetic activity needs to be studied.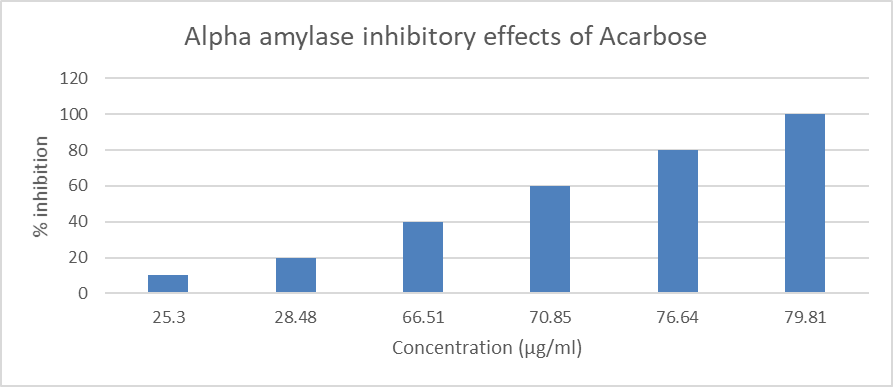
References
- Srinivas, P., Devi, K. P. & Shailaja, B. (2014). Diabetes mellitus (Madhumeha)-an Ayurvedic review. International Journal of Pharmacy and Pharmaceutical Sciences vol. 6 107–110.
- Khan, M. I. et al. (2021). Phytochemistry, food application, and therapeutic potential of the medicinal plant (Withania coagulans): A review. Molecules 26.
- Khattak, Z. F. et al. (2021), Anticonvulsant activity of methanolic extract of Withania cogulans in mice. Metab. Brain Dis.36, 2437–2443.
- Gupta, R., Sonawane, T. & Pai, S. (2021). An overview on pharmaceutical properties and biotechnological advancement of Withania coagulans. Advances in Traditional Medicine at https://doi.org/10.1007/s13596-021-00558-7.
- Panneerselvam, G. et al. (2020). Phytochemical screening, invitro antidiabetic activity of muntingia calabura leaves extract on alpha-amylase and alpha-glucosidase enzymes. Int. J. Res. Pharm. Sci.11, 1210–1213.
- Maurya, R. (2010). Chemistry and pharmacology of Withania coagulans : an Ayurvedic remedy . J. Pharm. Pharmacol.62, 153–160.
- Abubakar, A. R. & Haque, M. (2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy and Bioallied Sciences vol. 12 1–10 at https://doi.org/10.4103/jpbs.JPBS_175_19.
- Gayatri, S. & Angel Seslin Monica, V. (2020). Preliminary phytochemical investigation and in-vitro antidiabetic activity of an ayurvedic formulation. Bull. Pharm. Sci. Assiut43, 135–139.
- Majumder, P. & Paridhavi, M. (2016). Physiocochemical Standardization and Formulation Development of Poly-herbal Tablet for Diabetes. Br. J. Pharm. Res.12, 1–17.
- Sharma, D. C., Kumar, P., Hussain, I. & Sharma, S. K. (2021). An in-silico and in-vitro comparative study of compounds from phoenix sylvestris roxb. For alpha-amylase enzyme inhibition involved in diabetes mellitus. Biointerface Res. Appl. Chem.11, 13347–13358.


