Archive : Article / Volume 2, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-3558/005
Effects of Neuroglobin in Cerebral Ischemia and Introduction of Omega-3 Polyunsaturated Fatty Acids
Grodno State Medical University, 80, Gorkogo St., 230009, Grodno, Republic of Belarus
Correspondng Author: Lizaveta I. Bon
Citation: Lizaveta I. Bon, Maksimovich N.Ye., Zimatkin S.M., Karnyushko O.A., Bakush U.A., Gaiko D.V., (2023). Effects of Neuroglobin in Cerebral Ischemia and Introduction of Omega-3 Polyunsaturated Fatty Acids. Psychiatry and Psychological Disorders. 2(1).10.58489/2836-3558/005
Copyright: © 2023 Lizaveta I. Bon, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2023-01-09, Received Date: 2023-01-09, Published Date: 2023-01-23
Abstract Keywords: neuroglobin, cerebral ischemia, omega-3 polyunsaturated fatty acids
Abstract
The aim of the work was to evaluate the content of neuroglobin in the brain of rats with cerebral ischemia and under the conditions of administration of omega-3 polyunsaturated fatty acids.
The content of neuroglobin in the cytoplasm of neurons in the parietal cortex and hippocampus of rats under conditions of cerebral ischemia and administration of omega-3 polyunsaturated fatty acids was studied.
Against the background of the introduction of the drug Ï-3 PUFAs, there was an improvement in the indicators of antioxidant protection of brain neurons, which was manifested by an increase in the content of neuroglobin in hippocampal neurons.
Introduction
Neuroglobin (Ngb) is a metalloprotein of the globin family that contains protoporphyrin with an iron atom in the center forming six coordination bonds. Ngb is expressed mainly in the nervous system, retina and some endocrine structures [1,2,3]. The similarity of the Ngb structure with other globin proteins [4] suggests similar functions of providing oxygen homeostasis of cells [5]. But since the level of affinity of Ngb to oxygen is very high, and this is an obstacle to the release of oxygen, this metalloprotein is more often considered as an indicator of the oxygen level in mitochondria that oxidize organic substances and form ATP [6,38]. At the cellular level, Ngb is found in the cytoplasm, vesicular structures, neurotubules, nucleus and mitochondria [5,39,40].
Analysis of the subcellular localization of Ngb shows that Ngb mRNA and protein are constantly detected in the perikaryon and processes of neurons, which indicates the active participation of Ngb in metabolic processes [41].
Neuroglobin regulates the functioning of neurons in pathology: suppresses oxidative stress, blocks mitochondrial apoptosis factors, binds free radicals. Neuroglobin synthesis is promoted by hypoxia-inducible factor 1-alpha (HIF-1α), retinoid X receptor (RXR), zinc finger X-chromosomal protein (ZFX), nuclear transcription factor Y subunit alpha (NFYA) and TEA domain transcription factor 1 (TEAD1) and SOX-4 [42].
The role of neuroglobin in hypoxia / ischemia is still unclear. Some studies indicate its neuroprotective effect in cerebral ischemia due to increased expression of endothelial nitric oxide synthase [43]. Other data refute its significance for the survival of neurons in conditions of lack of oxygen, since Ngb deficiency apparently increases the expression of HIF-1α [41].
Due to its ability to bind oxygen, Ngb enhances the supply of oxygen to the mitochondria of metabolically active neurons. This hypothesis is confirmed by the existence of Ngb in metabolically active cells and subcellular compartments.
In addition, neuroprotective effects can be realized through the following mechanisms:
1. Neuroglobin is a free radical scavenger and has chelating activity;
2. It is able to utilize NO;
3. In mitochondria, it reduces the release of cytochrome C into the cytosol by inhibiting the caspase-9-dependent pathway of apoptosis;
4. Acts as an inhibitor of the dissociation of the guanine nucleotide of the heterotrimeric Gα protein, necessary for the normal functioning of intracellular signaling;
5. Inhibits Pak1 kinase and interacts with Rho GDP-dissociation inhibitor (RhoGDI) and members of the Rho GTPase family, suppressing the spread of hypoxia or death signal, induced by N-methyl-D-aspartic acid;
6. Performs the role of a metabolic regulator that increases cellular anabolism through inhibition of AMP-activated signaling.
Thus, neuroglobin, according to the literature, can act as a neuroprotector in cerebral ischemia, but its content in brain neurons with ischemia of varying severity remains unexplored.
Omega-3 PUFAs affects many biological processes of the body [10], has the following effects:
1) improve endothelial function, which leads to a decrease in peripheral vascular resistance due to improved endothelium-dependent and independent vasodilation, an increase in the level of nitric oxide in the blood and a decrease in the level of endothelin-1;
2) reduce the predisposition to thrombosis as a result of a decrease in the aggregation activity of platelets, suppression of the adhesion of monocytes to the endothelium, a decrease in the expression of adhesion molecules VCAM-1, TLAM-1, ICAM-1;
3) reduce the activity of chronic nonspecific inflammation, which is manifested by a decrease in the content of tumor necrosis factor in the blood, interleukins 1 and 6, an increase in the formation of anti-inflammatory eicosanoids;
4) contribute to an increase in the formation of ATP, reduce oxygen consumption and calcium content, improve mitochondrial function;
5) increase the activity of calcium-magnesium ATPase, contribute to the inhibition of fast voltage-dependent sodium channels and L-type calcium channels [11,12,13,14,15,16,17,18,19].
Omega-3 PUFAs are competitors for arachidonic acid when binding the enzyme 5-lipoxygenase, since Omega-3 PUFAs replaces arachidonic acid in membrane phospholipids, reducing the production of eicosanoids [7,8,9,20]. In addition to the anti-inflammatory effect based on the interruption of the arachidonic acid cascade, Omega-3 PUFAs have an anti-inflammatory effect through a decrease in the activity of nuclear factor-κB (NF-KB), a powerful stimulator of the production of pro-inflammatory cytokines, including interleukin-6 and alpha-factor of tumor necrosis [21,22]. In general, the enrichment of Omega-3 PUFAs cell membranes disrupts dimeralization and the participation of toll-like receptor 4, which can contribute to the anti-inflammatory effect by suppressing the activation of NF-KB [23]. Thus, Omega-3 PUFAs inhibits lipogenesis and increases the production of resolvins and protectins – small lipid molecules that suppress the development of the inflammatory process [24,25,26].
In addition, the action of Omega-3 PUFAs includes the ability to increase the secretion of adiponectin, an anti-inflammatory adipokine [27]. Omega-3 PUFAs act as a modulator of the G-protein coupled receptor, and are also able to influence the direct regulation of gene expression through nuclear receptors and transcription factors, which, in turn, are modulated by intracellular lipid-binding proteins transporting these fatty acids to the nuclei. This effect leads to an increase in the synthesis of anti-inflammatory proteins associated with antioxidants [28,29,30,31].
Omega-3 PUFAs participate as coenzymes in the oxidative decarboxylation of pyruvic acid and alpha-keto acids, are an essential component in the recycling of of major endogenous antioxidants, such as vitamin E (alpha-tocopherol), vitamin C, glutathione, ubiquinone (coenzyme Q 10) [28,32,33,34]. In addition, they have their own antioxidant and lipotropic activity due to activation of coenzyme A formation, transport of acetate and fatty acids from the cytosol to the mitochondrial matrix, acceleration of fatty acid oxidation, stabilization of cell membranes [35,36,37].
The aim of the work was to evaluate the content of neuroglobin in the brain of rats with cerebral ischemia and under the conditions of administration of omega-3 polyunsaturated fatty acids.
Materials and methods
The experiments were performed on 60 male mongrel white rats weighing 260 ± 20 g in compliance with the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of 22.09.2010 on the protection of animals used for scientific purposes.
The choice of experimental animals is due to the similarity of the angioarchitectonics of the rat and human brains. CI modeling was performed under intravenous thiopental anesthesia (40-50 mg/kg).
Subtotal cerebral ischemia (SCI) was modeled by simultaneous ligation of both common carotid arteries (CCA). Subtotal cerebral ischemia (SCI) was modeled by simultaneous ligation of both common carotid arteries (CCA). To study the effects of ω-3 PUFAs, animals before CI were intragastrically injected with the preparation ω-3 PUFAs at a dose of 5 g/kg of body weight for a week.
The control group consisted of falsely operated rats of similar sex and weight.
After decapitation of the animal, the right hemisphere was used to study the indicators of the pro-oxidant-antioxidant balance, and the left hemisphere was used to determine the content of neuroglobin.
A method for studying the content of neuroglobin in neurons of the parietal cortex and hippocampus of the brain
Determination of the content of metalloproteins of the globin family neuroglobin was carried out by immunohistochemistry using monoclonal antibodies. For this purpose, after decapitation, the brain was quickly extracted from rats, pieces of the cerebral cortex were fixed in zinc-ethanol-formaldehyde at +4°C (overnight), then enclosed in paraffin.
Paraffin sections with a thickness of 5 microns were prepared using a microtome, mounted on slides. The preparations were processed according to the protocol of immunocytochemistry for light microscopy, excluding the procedure of thermal unmasking of antigens. To determine the immunoreactivity of the molecular marker of neuroglobin, primary monoclonal mouse antibodies Anti-Ngb antibody from Abcam (UK, ab. 14748) were used at a dilution of 1:600 at +4°C, exposure 20 h in a wet chamber [44,47]. To detect bound primary antibodies, the EXPOSE Mouse and Rabbit specific HRP/DAB detection IHC kit Abcam (UK, ab. 80436) was used. The content of neuroglobin was studied in the cytoplasm of neurons of the fifth layer of the parietal cortex and neurons of the hippocampal CA1 field in immunohistochemical preparations based on the optical density of the chromogen precipitate using an Axioscop 2 plus microscope (Zeiss, Germany), a digital video camera (LeicaDFC 320, Germany) and the ImageWarp image analysis program (Bitflow, USA).
To prevent systematic measurement errors, brain samples from the compared control and experimental groups of animals were studied under the same conditions.
Results
As a result of the studies, quantitative continuous data were obtained. Since the experiment used small samples that had an abnormal distribution, the analysis was carried out using nonparametric statistics with the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). The data is presented in the form Me (LQ; UQ), where Me is the median, LQ is the boundary of the lower quartile; UQ is the boundary of the upper quartile. The differences between the groups were considered significant at p<0>
The introduction of Omega-3 PUFA had no effect on the level of neuroglobin in the cytoplasm of neurons of the parietal cortex in rats with SCI compared with the SCI group without the introduction of Omega-3 PUFAs (р>0,05), (table 3.5.2, figure 3.5.1, 3.5.2).
Table 3.5.2 – The content of neuroglobin in the cytoplasm of pyramidal neurons of the parietal cortex and the СА1 field of the hippocampus of the brain of rats with SCI and the introduction of Omega-3 PUFAs, Me (LQ; UQ)
Groups | The content of neuroglobin / units of optical density | |
parietal cortex | hippocampus | |
Control | 0,167(0,162;0,172) | 0,165(0,163;0,165) |
SCI | 0,114(0,108;0,116)* | 0,117(0,107;0,126)* |
SCI+Omega-3 PUFAs | 0,119(0,114;0,121)* | 0,162(0,156;0,168) # |
Note – * – р<0>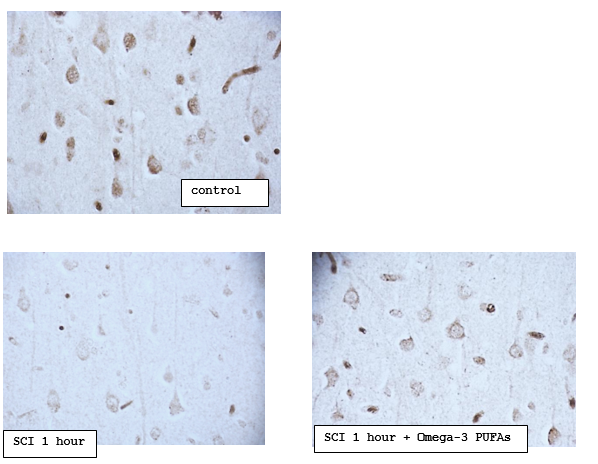
Digital microphotography. Magnification 40
Figure 3.5.1 – The content of neuroglobin in the cytoplasm of pyramidal neurons of the parietal cortex of rats with subtotal cerebral ischemia (SCI) and introduction of Omega-3 polyunsaturated fatty acids (Omega-3 PUFAs)
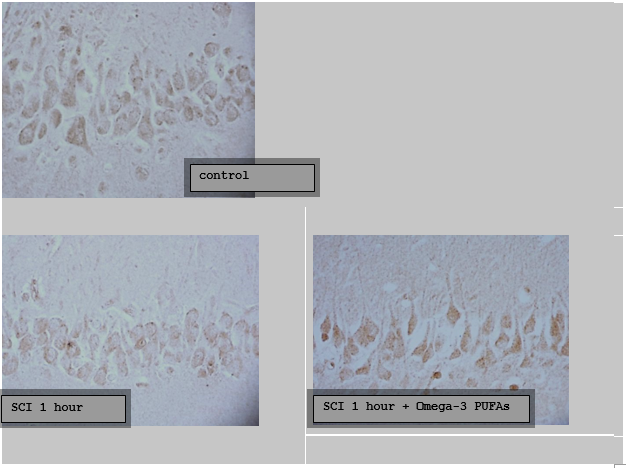
Digital microphotography. Magnification 40
Figure 3.5.2 – The content of neuroglobin in the cytoplasm of pyramidal neurons of the CA1 field of the hippocampus of the brain of rats with subtotal cerebral ischemia (SCI) and introduction of Omega-3 polyunsaturated fatty acids (Omega-3 PUFAs)
However, in the hippocampal neurons there was an increase in the content of neuroglobin, compared with the group SCI, by 28(21;33) %, р<0>0.05).
Discussion
The conducted studies revealed the presence of antioxidant properties in Omega-3 polyunsaturated fatty acids, which is consistent with the literature data. The favorable effect of polyunsaturated fatty acids on the state of neurons in the cerebral cortex under conditions of subtotal cerebral ischemia may be due to the normalization of blood rheological properties due to a decrease in the production of thromboxane A by platelets and an increase in the level of tissue plasminogen activator, as well as an improvement in neuronal membrane fluidity, a decrease in blood viscosity. Omega-3 polyunsaturated fatty acids also have anti-inflammatory effects due to their incorporation into the phospholipid layer of cell membranes. In addition, polyunsaturated fatty acids, affecting the synthesis of prostaglandins, regulate vascular tone and prevent vascular vasoconstriction under the influence of catecholamines, which causes a moderate vasodilatory effect [17,18,19,20].
Omega-3 polyunsaturated fatty acids are able to reduce the activity of oxidative stress, as they are a necessary component in the recycling of the main endogenous antioxidants. In addition, they have their own antioxidant activity due to the activation of the formation of coenzyme A, the transport of acetate and fatty acids from the cytosol to the mitochondrial matrix, and the stabilization of cell membranes [20].
Conclusion
Thus, against the background of the introduction of the drug ω-3 PUFAs, there was an improvement in the indicators of antioxidant protection of brain neurons, which was manifested by an increase in the content of neuroglobin in hippocampal neurons.
References
- E.I. Bon, N.Ye. Maksimovich Comparative analysis of morphological disorders of neurons of the parietal cortex and hippocampus of rats in various types of experimental cerebral ischemia // Orenburg Medical Bulletin. – 2021. – № 2. – С. 29–36.
- Butin А.А. Patterns of changes in the vascular-capillary network of the cerebral cortex in response to acute cerebral ischemia // Omsk Scientific Bulletin. – 2004. – № 26. – С. 46–57.
- Chen H., Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia // Neurol. Res. 2005. – V. 27, N 3. – P. 280–286.
- Clemens J.A. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants // Free Radic. Biol. Med. – 2000. – V. 28. – P. 1526–1531.
- Bon L.I., Maksimovich N.E., Zimatkin S.M. Morphology of rat brain neurons in subtotal ischaemia and introduction of L-NAME and omega-3 polyunsaturated fatty acids // Journal of Medical Science. – 2020. – Р. 1–8.
- Belaeva L.E., Pavlyukevich А.N. Early programming of human diseases and the use of nutraceuticals for preventive purposes: focus on fish oil // Bulletin of the VSMU. – 2019. – № 5. – С. 12–25.
- Gao Q. Oxidative Stress and Autophagy // Adv Exp Med Biol. –2019. – V. 1206. P. 179–198. doi:10.1007/978-981-15-0602-4_9
- Adipose oxidative stress and protein carbonylation / A.K. Hauck [et al.] // J. Biol Chem. – 2019. – V. 294, N 4. – P. 1083–1088. doi:10.1074/jbc.R118.003214
- O'Grady S.M. Oxidative stress, autophagy and airway ion transport // Am J. Physiol Cell Physiol. – 2019. – V. 316, N1. – P. 16–32. doi:10.1152/ajpcell.00341.2018
- LaManna J.С., Vendel L.M., Farrell R.M. Brain adaptation to chronic hypobaric hypoxia in rats // J. Appl. Physiol. – 1992. – V. 72. – P. 2238–2243.
- Kremer J.M., Lawrence D.A., Petrillo G.F. Effects of high-dose oil on rheumatoid arthritis after Stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates // Arthritis Rheum. – 1995. – V. 38. – P. 1107–1114.
- Kremer J.M. Effects of modulation of inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids // lipids. – 1996. – V. 31. – P. 243–247.
- Kromhout D., Bosschieter E.B., Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease // N. Engl. J. Med. – 1985. – V. 312. – P. 1205–1209.
- Kromhout D. The importance of n-6 and n-3 fatty acids in carcinogenesis // Med. Oncol. Tumor Pharmacother. – 1990. – N 7. – P. 173–176.
- Lewis R., Lee T., Austen K. Effects of omega-3 fatty acid on the generation of products of 5-lipooxygenase pathway // Simopoulos A., Kifer R., Martin R., eds. Health effects of polyunsaturated fatty acid in seafoods. – Orlando, FL: Academic press, 1986. – P. 227–238.
- Morris M.C., Sacks F., Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials // Circulation. – 1993. – V. 88. – P.523–533.
- Simopoulos A.P., Kifer R.R., Martin R.E. Health effects of omega-3 polyunsaturated fatty acid in seafood's // World Rev. Nutr. Diet. – 1991. – V. 66, N 1. – P. 592.
- Thien F.C., Mencia-Huerta J.M., Lee T.H. Dietary fish oil effects on seasonal hay fever and asthma in pollen-sensitive subjects // Am. Rev. Respir. Dis. – 1993. – V. 147. – P. 1138–1143.
- Weber P., Fischer S., von Schaky C. Dietary omega-3 polyunsaturated fatty acid and eicosanoids formation in man // Academic press. – 1986. – P. 227– 238.
- Relationship of Oxidative Stress as a Link between Diabetes Mellitus and Major Depressive Disorder / G.Z. Réus [et al.] // Oxid Med Cell Longev. – 2019. 2019:8637970. Published 2019 Mar 3. doi:10.1155/2019/8637970
- Antiarrythmic effects of fish oils. Health effects of omega-3 polyunsaturated fatty acid in seafcods / J.S. Charnock [et al.] // World Rev. Nutr. Diet. – 1991. – V. 66. – P. 278–291.
- Drevon C.A. N-6 and n-3 fatty acids - how much and which balance? // Scand. J. Nutr. – 1990. – V. 34. – P. 56–61.
- DiPietro V., Lazzarino G., Amorini A. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat // Free Radic. Biol. Med. – 2014. – V. 69. – P. 258–264.
- Kaliannan K., Li X. Y., Wang B. Multiomic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease // Commun Biology. – 2019. – V. 2, N 1. – P. 276–280. 25.
- Khunt D., Shrivas M., Polaka S. Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: a Comparative Study // Pharmacology Sciencific Technology. – 2020. – V. 21, N 2. – P. 45–50. 26.
- Bjorneboe A., Soyland E., Bjorneboe G.E. Effect of dietary supplementation licosapentaenoic acid in the treatment of atopic dermatitis // Br. J. Dermatol. – 1987. - V. 117. – P. 463–469.
- Oxidative stress and aging / A.D. Romano [et al.] // J. Nephrol. – 2010. – V. 15. – P. 29–33.
- Dyerberg J. Coronary heart disease in Greenland Inuit: A paradox. Implication for Western diet patterns // Artic. Med. Res. – 1989. – V. 48. – P. 47–54.
- Ernst E. Effects of n-3 fatty acids on blood rheology // J. Internal Med. – 1989. – V. 225 (Suppl. 1). – P. 129–132.
- GISSI-Prevenzione Investigators Dietary Supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of GISSI-Prevenzione trial // Lancet. – 1999. – V. 354. – P. 447–455.
- Godley P.A., Campbell M.K., Gallagher P. Biomarkers of essential fatty acids consumption and risk of prostatic carcinoma // Cancer Epidemiol. Biomarkers Prev. – 1936. – N 5. – P. 889–895.
- Hirai A., Terano T., Saito H. Clinical and epidemiological studies of eicosapentaenoic acid in Japan // Lands WEM, ed. Proceedings of the AOCS short course on polyunsaturated fatty acids and eicosanoids. – Champaign, IL: American Oil Chemists' Society, 1987. – P. 9–24.
- Kaizer L., Boyd N.F., Krinkov V. Fish consumption and breast cancer risk: an ecological study // Nutr. Cancer. – 1989. – N 12. – P. 61–68.
- Knapp H.R. N-fatty acids and human hypertension // Curr. Opin. Lipidol. – 1996. – V. 7. – P. 30–33.
- Gupta A.K., Ellis C.N., Tellnes D.C. Double blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis // Br. J. Dermatol. – 1989. – V. 120. – P. 801–807.
- Harris W.S. N-3 fatty acids and serum lipoproteins: human studies // Am. J. Clin. Nutr. – 1997. – V. 65 (5 Suppl). – P. 1645–1654.
- Heemskerk J.W., Vossen R.C., van Dam-Mieras M.C. Polyunsaturated fatty acids and function of platelets and endothelial cells // Curr. Opin. Lipidol. – 1996. – Vol. 7. – P. 24–29.
- Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease / K. Kaliannan [et al.] // Commun Biology. – 2019. – V. 1. – P. 276–280. 39.
- Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury / Y. Sun [et al.] // Proc Natl Acad Sci U S A. – 2001. – V. 98. – P. 15306–15311.
- Lipton P. Ischemic cell death in brain neurons // Physiol. Rev. 1999. – V. 79. – P. 1431–1568.
- Taylor J.M. Neuroglobin overexpression improves sensorimotor outcomes in a mouse model of traumatic brain injury // Neurosci Lett. – 2014. – V. 577. – Р. 125–129.
- Li R., Guo S., Lee S. Neuroglobin protects neurons against oxidative stress in global ischemia // J. Cereb. Blood Flow Metab. — 2010. — V. 30. – P. 1874–1882.
- Avella G., Ardiccioni C., Scaglione A. Engineering the internal cav¬ity of neuroglobin demonstrates the role of the haem-sliding mechanism // Acta Crystallogr. D. Biol. Crystallogr. – 2014. – V. 70 – P. 1640–1648.
- Korzhevsky D.E., Gilerovich E.G., Kirik O.V. Immunohistochemical examination of the brain // Saint-Petersburg: SpecLit, 2016. – 143 с.
- The Naked Mole Rat: A Unique Example of Positive Oxidative Stress / F. Saldmann [et al.] // Oxid Med Cell Longev. – 2019. – Р. 5258 - 5265.
- Rebrova O.Y. Statistical analysis of medical data. Application of the Statistica application software package. – M.: Мedia. Sphera, 2003. – 312 с.
- Bon L.I., Maksimovich N. Yе. Vasoprotective Effects of Omega-3 Polyunsaturated Fatty Acids in Cerebral Ischemia // International Journal of Cardiology and Cardiovascular Disorder. – 2021. – V. 2 – P. 1–5.


