Archive : Article / Volume 1, Issue 1
Case Report | DOI: https://doi.org/10.58489/2836-3558/002
Neuroprotective Effect of Neuroglobin in Simultaneous Incomplete Ischemia
Grodno State Medical University, Grodno, Republic of Belarus.
Correspondng Author: E.I. BON
Citation: E.I. BON, MAKSIMOVICH N. Ye, S.M. ZIMATKIN, N.V. KOKHAN, D.V. GAIKO U.A. BAKUSH, (2022). Neuroprotective Effect of Neuroglobin in Simultaneous Incomplete Ischemia. Psychiatry and Psychological Disorders. 1(2). DOI: 10.58489/2836-3558/002
Copyright: © 2022 E.I. BON, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2022-12-01, Received Date: 2022-12-01, Published Date: 2022-12-27
Abstract Keywords: neuroglobin, cerebral ischemia, neurons.
Abstract
The role of neuroglobin in ischemia has not been studied in detail. A number of studies indicate its neuroprotective effect, other data refute its importance for the survival of neurons in conditions of lack of oxygen. In the course of the conducted studies, the neuroprotective role of neurooglobin in certain types of cerebral ischemia has been established. The results obtained resolve the contradiction noted in the literature data about the role of neuroglobin in the pathogenesis of cerebral ischemia and show that in certain models of cerebral ischemia, with sufficient time to implement compensatory mechanisms, neuroglobin participates in neuroprotection, while in forms of cerebral ischemia, where this time is insufficient, its content decreases and there is no neuroprotective effect.
Introduction
Neuroglobin is a metalloprotein of the globin family, which contains protoporphyrin with an iron atom in the center. It is present in the neurons of the central and peripheral nervous system, the retina of the eye, in some endocrine structures and organs – pancreatic islets, adenohypophysis, adrenal glands. Currently, neuroglobin is considered as a protein that provides oxygen to the mitochondria of metabolically active neurons [1,3,10].
The role of neuroglobin in ischemia / hypoxia has not been studied. A number of studies (conducted on transgenic mouse with increased expression of neuroglobin in neurons and astrocytes of the brain) indicate its neuroprotective effect [4-9,11,12], other data refute its importance for the survival of neurons in conditions of oxygen deficiency [2].
At the same time, the content of neuroglobin in the brain neurons of non-transgenic animals with ischemia remains unexplored.
Materials and Methods
The experiments were performed on 24 male mongrel white rats weighing 260 ± 20 g in compliance with the requirements of Directive of the European Parliament and of the Council No. 2010/63/EU of 22.09.2010 on the protection of animals used for scientific purposes. Permission of the Committee on Biomedical Ethics and Deontology of the Grodno State Medical University was obtained to conduct the study (extract from Protocol No. 1 of 05.01.2022).
Cerebral simulation was performed under intravenous thiopental anesthesia (40-50 mg/kg). The animals were removed from the experiment by decapitation under intravenous thiopental anesthesia.
The study used a model of stepwise subtotal cerebral ischemia (SSCI) with sequential ligation of both common carotid arteries (CCA) at intervals of 7 days (subgroup 1), 3 days (subgroup 2) or 1 day (subgroup 3).
The neuroglobin content was determined by immunohistochemistry using monoclonal antibodies. For this purpose, after decapitation, the brain was quickly extracted from rats, pieces of the cerebral cortex were fixed in zinc-ethanol-formaldehyde at +4°C (overnight), then enclosed in paraffin.
Paraffin sections with a thickness of 5 microns were prepared using a microtome, mounted on slides. The preparations were processed according to the protocol of immunocytochemistry for light microscopy, excluding the procedure of thermal unmasking of antigens. To determine the immunoreactivity of the molecular marker of neuroglobin, primary monoclonal mouse antibodies Anti-Ngb antibody from Abcam (UK, ab. 14748) were used at a dilution of 1:600 at +4°C, exposure 20 h in a wet chamber. To detect bound primary antibodies, the EXPOSE Mouse and Rabbit specific HRP/DAB detection IHC kit Abcam (UK, ab. 80436) was used. The content of neuroglobin was studied in the cytoplasm of neurons of the fifth layer of the parietal cortex and neurons of the hippocampal CA1 field in immunohistochemical preparations based on the optical density of the chromogen precipitate using an Axioscop 2 plus microscope (Zeiss, Germany), a digital video camera (LeicaDFC 320, Germany) and the ImageWarp image analysis program (Bitflow, USA).
For electron microscopic studies, immediately after decapitation and rapid extraction of the brain, sections of parietal cortex and hippocampus were cut out with a blade and placed in a 1% osmium fixator on a Millonig’s buffer (pH = 7.4) for 2 hours at a temperature of 4°C.
Next, the slices were washed in a mixture of Millonig’s buffer (20 ml) and sucrose (900 mg), dehydrated in alcohols of increasing concentration, a mixture of alcohol and acetone, pure acetone; passed through a mixture of resins (araldite M + araldite H + dibutyl phthalate + DMR-30) and acetone and enclosed in a mixture of resins.
Semi-thin slices (about 350 nm thick) were made on an ultramicrotome M-7000 (RMC, USA), stained with methylene blue and cut out the areas necessary for the study with a blade.
Ultrathin sections (about 35 nm thick) were made on the same ultramicrotome, assembled on support meshes, contrasted with uranium acetate and lead citrate. To do this, the mesh sections were lowered into a drop of uranyl acetate and kept for 20 minutes in the dark at room temperature, then washed in 3 portions of double distilled water for 5 seconds and contrasted with lead citrate for 8 minutes, washed in 3 portions of double distilled water for 5 seconds.
The obtained preparations were studied under the electron microscope JEM-1011 (JEOL, Japan), photographed with the digital camera Olympus MegaView III (Olympus Soft Imaging Solutions, Germany).
To prevent systematic measurement errors, brain samples from the compared control and experimental groups of animals were studied under the same conditions.
As a result of the studies, quantitative continuous data were obtained. Since the experiment used small samples that had an abnormal distribution, the analysis was carried out using nonparametric statistics with the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). The data is presented in the form Me (LQ; UQ), where Me is the median, LQ is the boundary of the lower quartile; UQ is the boundary of the upper quartile. The differences between the groups were considered significant at p<0>
In the course of previous studies, it was found that SSCI with an interval between ligation of the CCA for 7 days is manifested by the least pronounced morphological (Fig. 1) and energetic changes, which indicates the effectiveness of compensatory mechanisms when using this model of cerebral ischemia.
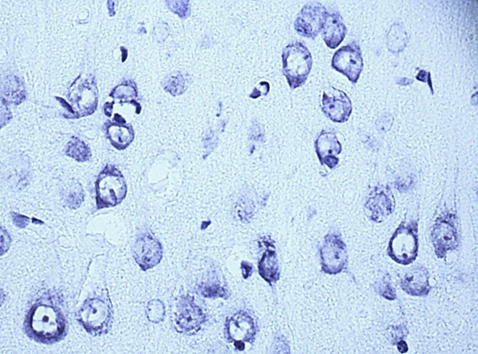
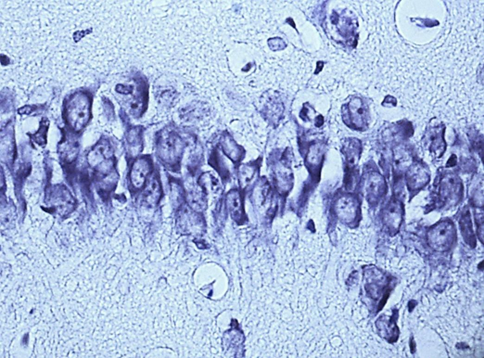
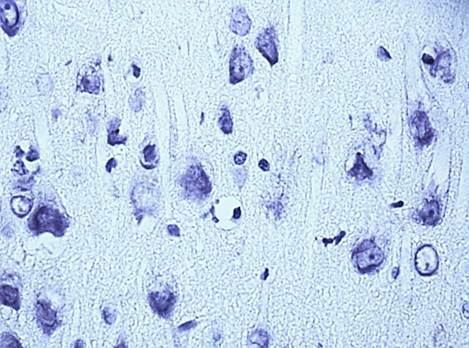
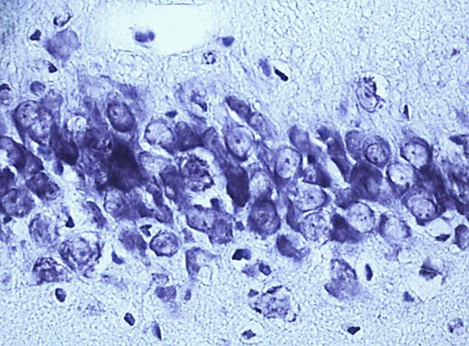
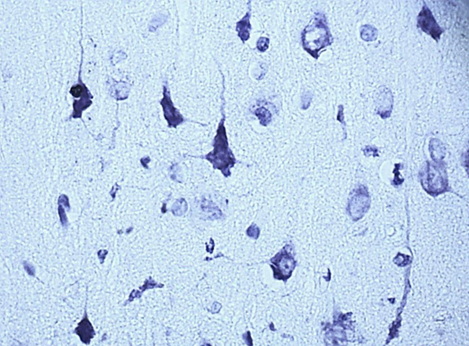
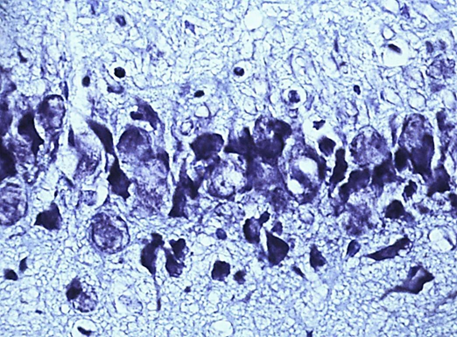
Nissl staining. Digital microphotography. Magnification 40
Fig. 1. Neurons of the fifth layer of the parietal cortex (A,C,E) and the pyramidal layer of the hippocampus (B,D,F).
A, B – control, C, D – subgroup 1, 7 days after ligation of the second CCA, E, F – subgroup 3, 1 days after ligation of the second CCA
Results
According to studies, changes in the content of neuroglobin in the neurons of the parietal cortex and hippocampus of the rat brain in the 1st, 2nd and 3rd subgroups of SSCI were multidirectional: in the 1st subgroup with a maximum interval between ligations of the CCA of 7 days it increased, while as the time interval between ligations of the CCA decreased, its content was less than in the control group, indicating the absence of involvement of neuroglobin in the development of compensation mechanisms in more severe forms of cerebral ischemia (subgroup 2 and 3), fig. 2,3, tab. 1.
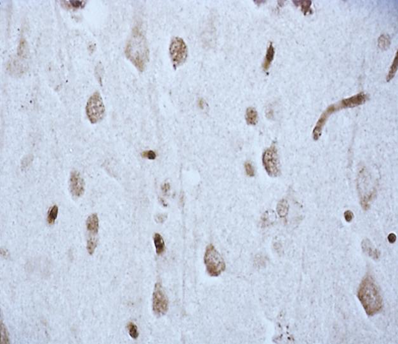
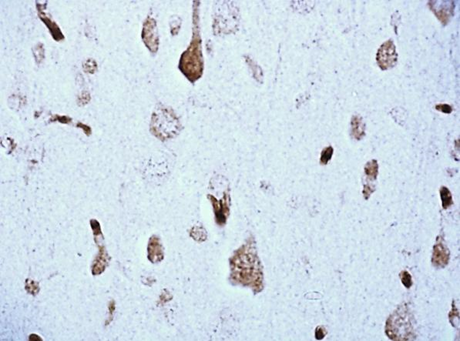
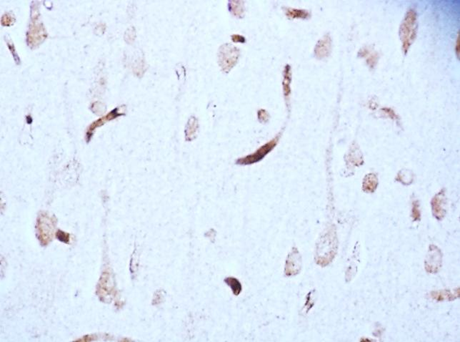
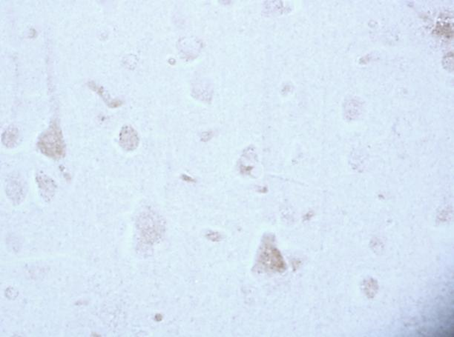
Fig. 2. The content of neurooglobin in the cytoplasm of pyramidal neurons of the parietal cortex of rats with stepwise subtotal cerebral ischemia (SSCI). Immunohistochemistry using monoclonal antibodies × 40.
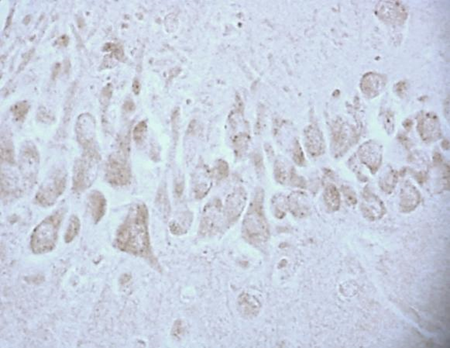
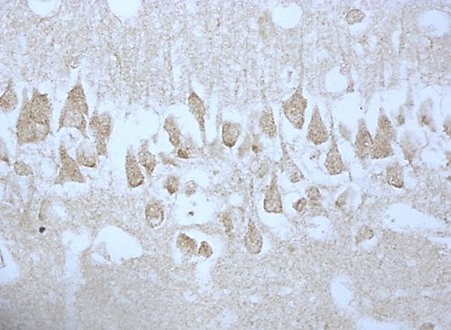
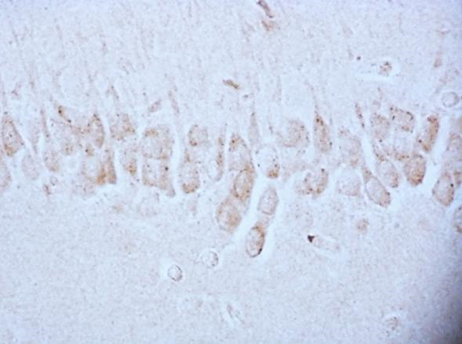

Fig. 3. The content of neuroglobin in the cytoplasm of pyramidal neurons of the CA1 field of the hippocampus of rats with stepwise subtotal cerebral ischemia (SSCI). Immunohistochemistry using monoclonal antibodies × 40.
Tab. 1. The content of neuroglobin in the cytoplasm of pyramidal neurons of the parietal cortex and the CA1 field of the hippocampus of the brain of rats with stepwise subtotal cerebral ischemia (SSCI), Me (LQ; UQ)
Groups | The content of neuroglobin / units of optical density | ||
parietal cortex | hippocampus | ||
Control | 0,167(0,162;0,172) | 0,165(0,163;0,165) | |
SSCI | Subgroup 1 | 0,191(0,186;0,193)* | 0,192(0,191;0,216)* |
Subgroup 2 | 0,145(0,142;0,152)* + | 0,153(0,149;0,158)* + | |
Subgroup 3 | 0,115(0,111;0,123)* +# | 0,111(0,108;0,117)* +# | |
Note – * – р<0>
In addition, giant mitochondria were observed in the cytoplasm of neurons of the cerebral cortex during SSCI with CCA ligation at 7-day intervals in the cytoplasm of neurons of the cerebral cortex (Fig. 5), which indicates their hypertrophy as one of the mechanisms of compensation for the resulting hypoxia.

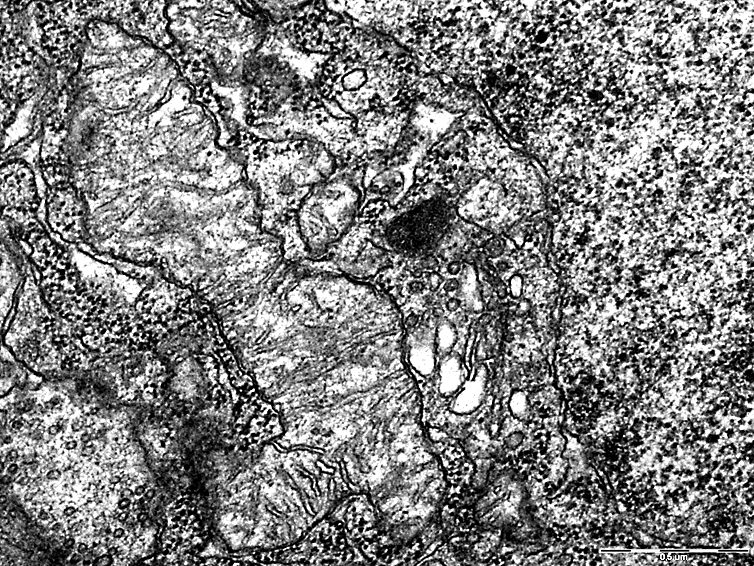
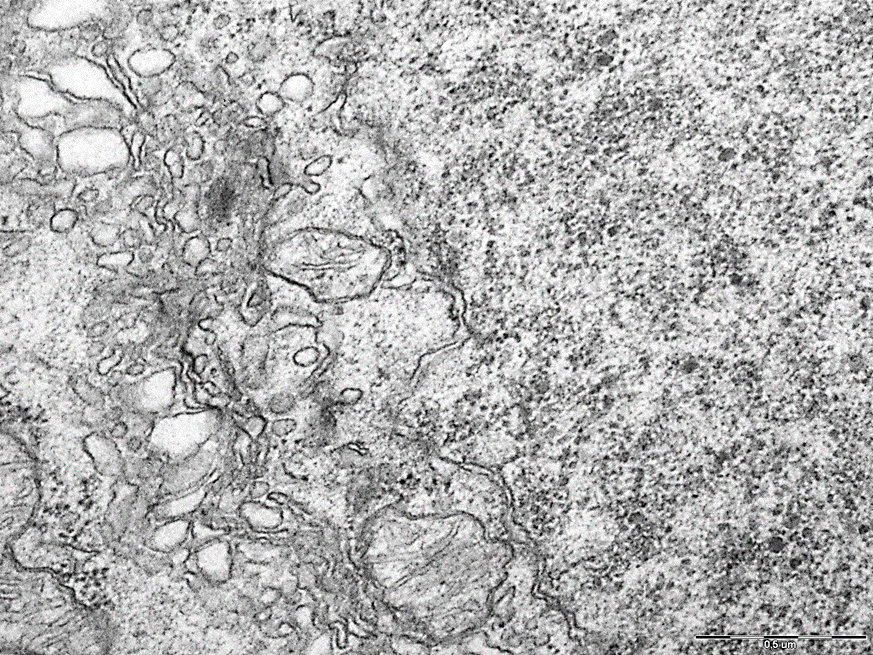
These changes were not observed in the 2nd and 3rd subgroups with a low content of neuroglobin (Fig. 6).
Thus, in the course of immunohistochemistry and electron microscopy, the neuroprotective role of neuroglobin in certain types of cerebral ischemia has been established [3].
The results obtained resolve the contradiction noted in the references about the role of neuroglobin in the pathogenesis of cerebral ischemia and show that in certain models of cerebral ischemia, with sufficient time to implement compensatory mechanisms, neuroglobin participates in neuroprotection due to an increase in the efficiency of oxygen utilization and oxidation substrates in the mitochondria of neurons, while in forms of cerebral ischemia, where this time is insufficient, its content decreases and there is no neuroprotective effect.
References
- Зиматкин С.М. Роль нейроглобина при церебральной ишемии/гипоксии и другой нейропатологии / С.М. Зиматкин, Е.И. Бонь, Н.Е. Максимович // Журнал ГрГМУ. – 2018. – Т. 16, № 6. – С. 643–647.
- Avella G., Ardiccioni C., Scaglione A. Engineering the internal cavity of neuroglobin demonstrates the role of the haem-sliding mechanism // Acta Crystallogr. D. Biol. Crystallogr. – 2014. – V. 70 – P. 1640–1648
- Bon, L. I. Changes in the Content of Neuroglobin in the Neurons of the Cerebral Cortex of Rats with Ischemia / L.I. Bon, N.Ye. Maksymovich, O.A. Karnyushko, S.M. Zimatkin, I.N. Burak, V.F. Lazarev // Journal of Cytology & Histology Research. – Volume 1(1). –2022. – P. 1-5.
- Gunnar P H Dietz. Protection by neuroglobin and cell-penetrating peptide-mediated delivery in vivo: a decade of research. Comment on Cai et al: TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Experemebtal Neurology 2011;227(1):224-231. DOI: 10.1016/j.expneurol.2011.05.010.
- Haixia Wen, Liu Liu, Lixuan Zhan, Donghai Liang, Luxi Li, Dandan Liu et al. Neuroglobin mediates neuroprotection of hypoxic postconditioning against transient global cerebral ischemia in rats through preserving the activity of Na+/K+ ATPases. Cell Death & Disease volume. 2018.635:1-18. DOI: 10.1038/s41419-018-0656-0.
- Li R, Guo S, Lee S. Neuroglobin protects neurons against oxidative stress in global ischemia. Journal of Cerebral Blood Flow and Metabolism. 2010; 30:1874–1882. DOI: 10.1038/jcbfm.2010.90.
- Marco Fiocchetti, Patrizio Cracco, Emiliano Montalesi, Virginia Solar Fernandez, Jeffrey A Stuart, Maria Marino. Neuroglobin and mitochondria: The impact on neurodegenerative diseases. Archives of Biochemistry and Biophysics. 2021; 701:159-167. DOI: 10.1016/j.abb.2021.108823.
- Sun Y. Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury. Proceedings of the National Academy of Sciences. 2018; 98:15306–15311. DOI: 10.1073/pnas.251466698.
- Taylor J.M. Neuroglobin overexpression improves sensorimotor outcomes in a mouse model of traumatic brain injury // Neurosci Lett. – 2014. – V. 577. – Р. 125–129
- Uzlova, L.V. ATP Synthase and Neuroglobin as Factors Determining the Path of Neuronal Destruction in Cerebral Ischemia / L.V. Uzlova, S.M. Zimatkin, L.I. Bon // Biomed J Sci& Tech Res 46(3)-2022. –P. 37521-37533.
- Xin Xin Xiong, Feng Pan, Ruo Qiao Chen, Dian Xing Hu. Neuroglobin boosts axon regeneration during ischemic reperfusion via p38 binding and activation depending on oxygen signal. Cell Death and Disease. 2018;(9):163. DOI: 10.1038/s41419-017-0260-8.
- Zhanyang Yu, Xiang Fan, Eng H Lo, Xiaoying Wang. Neuroprotective roles and mechanisms of neuroglobin. Neurological Research. 2009; 2:122-127. DOI: 10.1179/174313209X389866.


