Archive : Article / Volume 1, Issue 1
- Review Article | DOI:
- https://doi.org/10.58489/2836-2284/003
Principles of Lung Cancer Metastasis to Brain
- College of Medicine, University of Florida, Gainesville, FL
- College of Osteopathic Medicine, Nova Southeastern University, Clearwater, FL
- University of Arizona, Phoenix, AZ
- College of Medicine, University of Central Florida, Orlando, FL
- Department of Radiology, University of Florida, Gainesville, FL
- Department of Neurosurgery, University of Florida, Gainesville, USA
Brandon Lucke-Wold
J. Goeckeritz, J. Cerillo, C. Sanghadia, Md. Hosseini, A. Clark, K. Pierre, B. Lucke-Wold, (2022). Principles of Lung Cancer Metastasis to Brain. Journal of Skeleton System. 1(1). DOI: 10.58489/2836-2284/003
© 2022 Brandon Lucke-Wold, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 06-09-2022
- Accepted Date: 19-09-2022
- Published Date: 28-10-2022
lung cancer, brain metastasis, stereotactic radiosurgery, whole-brain radiation therapy.
Abstract
Lung cancer is a disease associated with significant morbidity and mortality on a global setting. This form of cancer commonly gives raise to metastatic lesions the brain, which can further worsen outcomes. In this focused review, we discuss an overview of lung cancers that metastasize to the brain: known risk factors; means of detection and diagnosis; and options for treatment including a comparison between surgical resection, stereotactic radiosurgery, and whole-brain radiation therapy. These interventions are still being assessed by clinical trials and continue to be modified through evidence-based practice.
Introduction
Lung cancer is the most common form of malignancy and cause of cancer-related deaths in the world, and the second leading form of cancer-related deaths in the United States.[1,2] Several types of lung cancer exist and may roughly be grouped into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with the latter being broken down further into adenocarcinoma, squamous cell cancer, and large cell carcinoma.[3] Cases of respiratory cancers with metastases involving the brain have been associated with significantly higher levels of both morbidity and mortality.[4,5] An estimated 20% of patients who present with lung cancer will have a brain metastasis at the time of diagnosis, and up to 50% of lung cancer patients will develop brain metastases (BrMs) over the course of their illness.[6-8] The formation of BrMs is a complex, multistep process that involves the spread of cancerous cells from the initial site of neoplastic growth to the eventual colonization of the brain.[9,10] Genetic analyses have linked several driver mutations in the development of BrMs in varying lung malignancies: mutations in tumor suppressor LKB1 and KRAS are predictive of BrMs in NSCLCs;[11] lung adenocarcinomas with mutations in EGFR and ALK, and hyperactivity within the WNT signally pathway have demonstrated higher occurrence of BrMs;[12-14] and upregulation of ANGT4 and PDGFRB genes have been linked with SCLC BrMs.[15]
Central Nervous System Diagnosis
BrMs are often initially detected from imaging as part of a metastatic tumor workup, or following the advent of clinical symptoms; with a definitive diagnosis later being confirmed via biopsy.[16,17] BrMs, particularly with those presenting with neurologic symptoms, are associated with a poorer prognosis.[4,18,19] However, outcomes are greatly improved when metastases are detected earlier, and are thus smaller in size.[4,18] Early detection could allow for use of minimally invasive procedures such as LITT,[20] stereotactic radiosurgery,[21-23] gamma knife surgery,[24] whole brain radiotherapy, or chemotherapy.[23] As such, there is increasing research for the detection of early brain metastases, with a focus on identifying risk factors.
Previous research has identified several risk factors specific for the presence of BrMs in NSCLC: being the female gender; concurrent lymphatic metastases; specific microRNA signatures; a high neutrophil to lymphocyte (NLR) ratio; elevated levels of neurofilament light chain; presence of EGFR driver mutation; and elevated serum levels of CEA, S100B, ProApolipoprotein A1 (apo A-1), Ki-67, VEGF-C, caspace-3, and calcium.[25-37] Sun, et at., have even postulated that ProApolipoprotein A1 and S100B alone may be used for an independent and accurate diagnosis of metastatic brain tumors; which could allow a clinician performing metastatic work ups to administer prophylactic treatments, such as intracranial irradiation.[25]
Preclinical studies using rodent models have demonstrated early detection of BrMs by employing molecular MRI with contrast agents that highlight tumor vascular factors ALCAM21 and VCAM-1.[38-40] Routine pre-operative and post-operative imaging should also be considered: a study by Yokoi, et al., showed that CT and MRI detected brain metastases in 6.8% and 7.1% of 155 and 177 patients, respectively, during the perioperative period for patients with non-squamous cell lung cancer.[41] Preclinical rodent studies have also indicated that brain metastases can be diagnosed even at micrometastatic stages by screening for urine metabolites; however, these were not specific for lung cancer.[42]
The development of machine learning algorithms has also been shown as a promising method of early detection. Machine learning is performed by teaching a machine a dataset with known predictors and outcomes using algorithms. What the machine then “learns” can be used in diagnosis in datasets where the diagnosis is unknown.[43,44] Cho (2021) performed a systematic review and meta-analysis of 12 studies using classical machine learning and deep learning on MRI modalities, revealing pooled 88.7% and 90.1
Imaging Modalities
Several options in terms of imaging modalities are available in the diagnosing of BrMs. Magnetic Resonance Imaging (MRI) is the modality primarily used in the diagnosing and localization of brain tumors in patients with brain lesions, as high level of availability, comparatively high resolution, and excellent capabilities for the characterization of soft tissues are provided by this device; additionally, with specific sequences, supplementary biological information like apoptosis, cell density, or angiogenesis can be measured (diffusion-weighted MRIs or perfusion-weighted MRIs)[48,49] Certain paramagnetic contrast agents (CA) can also reveal impaired blood-brain barriers (BBBs).[50] The downside to the modality is the lack of specificity for neoplastic tissue, which makes it challenging to detect malignancies, monitor cancer progression, or detect potential lesion growth.[51] Additionally, MRI is unable to assess treatment response after surgery, chemotherapy, or radiotherapy nor the quantity of inflammatory, demyelinating, infarction, and ischemia.[48]
A molecular imaging technique called Positron Emission Tomography (PET), which detects emitted photons from radiotracers, is another advanced imaging technique widely used in brain cancer patients. Using PET imaging, metabolic processes, like glucose metabolism and amino acid uptake, can be measured noninvasively and quantitatively.[52] Despite this, PET is unable to distinguish between grey and white matter structural abnormalities. PET is also limited by its lower spatial resolution, and inability to detect rapid changes in brain activity.[53] However, PET does have the advantage of being able to co-register medical images with other imaging methods. In oncology, integrating these two techniques to develop simultaneous multimodal imaging is particularly relevant, as it allows clinicians to assess the tumor microenvironment with the help of several diagnostic biomarkers.[54,55]
Hybrid PET/MRI scanners enable high-resolution metabolic and anatomical imaging.[53,56] This method combines both the high sensitivity of PET and the high resolution of MRI to provide a comprehensive picture of anatomical details. These coupled PET and MRI examinations may prove to be significantly more advantageous than independent examinations when attempting to understand tumor characteristics and determining whether surgery or radiation therapy would be an more appropriate intervention.[54,57] However, there is no conclusive evidence that PET/MRI is superior to PET/CT in oncology, and hybrid PET/MRI systems typically require longer scanning times and are associated with higher costs when compared to PET/CT systems.[58]
Radiopharmaceuticals should be selected based on the characteristics of the tumor being examined. PET tracers like [18F] fluorodeoxyglucose (FDG) are most used because tumor cells exhibit a higher glucose metabolism than healthy tissues[54,59] In cancer cells, [18F] FDG is trapped after crossing the BBB. Beta-emitting 14Cdeoxyglucose (DG) was demonstrated as a BBB crosser in the early 1970s.[60] By the hexokinase system, [18F] FDG is phosphorylated by glucose transporters and transported into cells. As a result, it persists in tissues for a long time since it cannot be metabolized.[61] [18F] FDG has a low specificity and shows a high background uptake by the normal brain despite its widespread use in clinical practice. A PET tracer based on amino acids was developed in response to these limitations.[54,62] It can be demonstrated that these amino acid tracers are elevated in malignant tumors because of unregulated protein synthesis, a symptom of increased cell proliferation.[49] The most common examples are 3'-deoxy-3'-[18F] fluorothymidine ([18F] FLT), 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine ([18F] FDOPA), and [11C] methionine ([11C] MET).[54,59,62,63]
Treatment
Current treatments for lung cancer patients with BrMs include supportive care, surgical resection, radiotherapies.[64] The integration of palliative care in the management of BrMs should also be considered as it has been shown to greatly improve the quality of life, appetite, and mood; and is correlated with better survival rates, despite less aggressive treatment.[65] Supportive medications, such as steroids and antiseizure drugs, have also demonstrated increased survival rates when coupled with traditional radiotherapies.[6,66-68]
Surgical Management
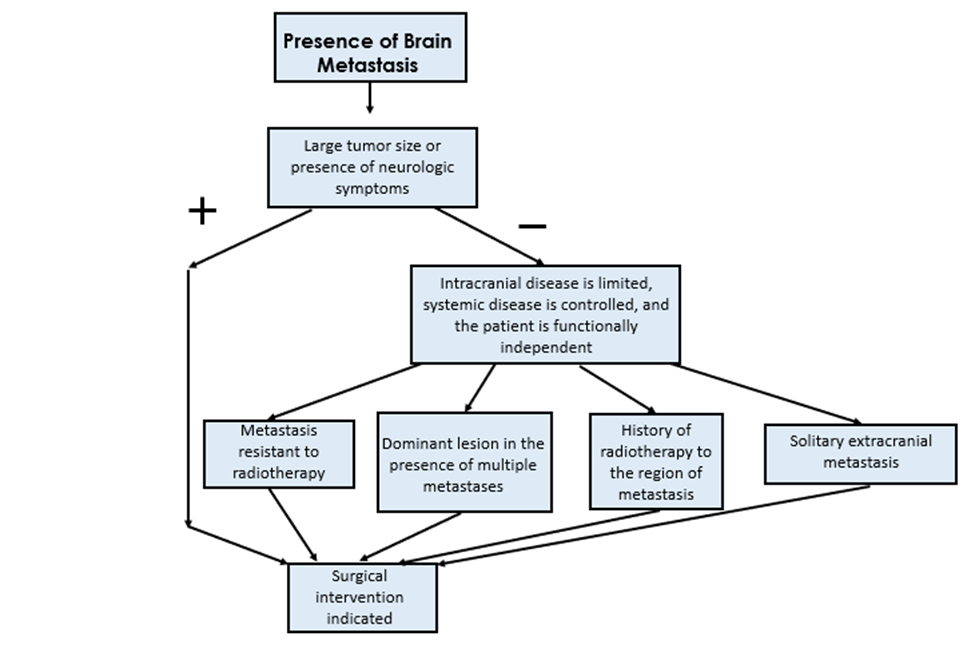
The discussion of surgically resecting BrMs is best understood by first outlining the distinct subsets of clinical presentations. One subset is when the tumor size is large and causing severe neurologic symptoms, such as mass effect.[69,70] The former subset often falls under the category of necessarily more urgent or emergent in nature, often requiring hastened neurosurgical intervention.[71,72] The mechanism behind this presentation may be either due to direct pressure on the brain tissue from the tumor itself, or from uncontrolled cerebral edema that secondarily increases intracranial pressure and can lead to acute herniation syndrome.[73] Given that some evidence points to the capacity for brain metastases to cause even more cerebral edema than primary tumors, this may further increase the importance of considering expedited neurosurgical intervention in these patients via resection of the tumor.[74,75]
Another subset of patients who meet criteria for surgical resection are those with a brain metastasis that is not large or causing severe neurologic symptoms, but in patients in which the intracranial disease is limited, systemic disease is controlled, and the patient is functionally independent.[70,76] Furthermore, surgery is often preferred with an extracranial metastasis from lung cancer if there is only one single lesion.[77] Given that radiation therapy is a commonly used treatment modality for brain metastases from lung cancer, it is also important to describe when surgery is preferred in these instances. This is often the case with BrMs that arise from a primary lung cancer that is resistant to radiotherapy.[78,79] Surgery for patients comprised in this subset is also often indicated in patients who have had radiation therapy in the past, as this is often necessary to definitively distinguish between radiation-related tissue necrosis and presence of tumor metastasis.[80-82] Lastly, in patients with multiple brain metastases, surgical resection is commonly indicated for the dominant lesion.[83] A summary of the above indications is outlined in Figure 1.
Radiation
Radiotherapies in the treatment of metastatic brain lesions are primarily groups into Stereotactic radiosurgery (SRS), and whole brain radiation therapy (WBRT).[84,85] SRS is a non-invasive form of radiation therapy that uses concentrated, multi-focal beams of radiation to destroy tissues.[84,86] There are multiple radiation delivery methods of SRS: Gamma Knife, Linear accelerator (LINAC), and Proton beam therapy. Gamma knife surgery uses gamma radiation in a very small operating field, while LINAC uses x-ray radiation with greater operating flexibility. Proton beam therapy is like Gamma knife and LINAC; however, it uses protons or neutrons instead of photons and has been thought to prevent some deleterious side effects related to conventional therapy. In contrast to the concentrated nature of SRS, whole brain radiation therapy (WBRT) is an exposure of the entire cranium to radiation.[87] WBRT is the current standard for treatment of BrMs from NSCLC in patients with multiple metastases.[88] Although WBRT is being replaced with SRS for other forms of BrMs, it remains the standard for NSCLC and SCLC metastases.[89]
SRS and WBRT may be used exclusively as well as in conjunction with other modalities.[88] Literature reports that WBRT in addition to SRS has a negative impact on cognitive function post treatment, but also shows lower cancer recurrence rates overall.[85-87,90-92] Aoyama et al. reported tumor reoccurrence rate of about 45% in SRS + WBRT and about 75% in SRS alone.[86] Brown et al. found that adult patients with 1-3 lung cancer metastasis who underwent SRS+WBRT (n=48) treatment had worse post-operative cognitive scores and neurological deterioration compared to those treated with SRS alone (n=63).[90] These studies suggest that using SRS in conjunction with WBRT could lead to worse cognitive outcomes, but lower rates of tumor recurrence compared to exclusive SRS treatment.
Treatment via SRS and WBRT differs in dosage and number of fractions. A fraction refers to dividing up the total radiation dosage into multiple treatments and maximizes the effectiveness of radiotherapy. This is accomplished by administering radiation on regularly time intervals which correlate with radiosensitive stages in the cell cycle of cancer cells.[93] SRS treatment typically consists of one fraction at a dose of 15-24 Gray (Gy).[93-95] However, new therapies like hypo-fractioned SRS (HF-SRS), that deliver multiple fractions, have recently shown to increase outcomes and decrease toxicity for large (>3cm) tumors.[93] A limitation to this approach is the possibility of tumor cell regrowth between fractioned doses.[93] In contrast to single dose SRS, WBRT is administered in multiple fractions. WBRT irradiates the entire cranium and is typically administered in 10 fractions of 3 Grays (Gy).[84,88,96,97] Literature shows that fraction dosages greater than 3 Gy may be associated with WBRT-related neurotoxic effects.[96-98] WBRT may cause cognitive decline, but it may also treat micro-metastases that have gone undetected on imaging.
When ionizing radiation is introduced to tissues, a large quantity of free radicals is created, and these free radicals combined with oxygen in the blood and destroy surrounding tissues.[99-101] Studies have demonstrated hypoxia to decrease radiation therapy results because of free oxygen able to radicalize.[101,102] As such, hypoxic tumors need 2.5-3 times the radiation dosage to reach the same efficacy as non-hypoxic tumors.[99,103] Fractioning schedules allow time for blood to return to tumor cells, increasing the amount of oxygen available to be ionized and the overall effectiveness of radiotherapy.
Conclusion
The development of brain metastases in lung cancer patients continues to be a major health concern on a global scale. These metastatic tumors significantly increase both morbidity and mortality rates among patients. Despite advances in medical technology, no treatment yet exists without adverse effects, or low remission rates: surgical resection alone leaves concern for untreated micrometases; and radiotherapies are associated with gross cognitive decline. Optimum dosing and fractioning in both stereotactic radiosurgery and whole brain radiation therapy have been investigated to find an optimal approach, but results are not without there drawbacks. Ultimately, the most promising option for improving mortality and morbidity rates lies in the detection of brain metastases as early as possible; thereby minimizing the intensity of treatment—and therefore adverse consequences—needed.
Tables and Figures
Known risk factors | Advanced tumor grade, stage, and size |
Elevated serum CEA, S100b, Apo A-1, Ki-67, VEGF-C, caspace-3, calcium, NLR, NFL | |
Female gender | |
Lymphatic spread | |
Adenocarcinoma, EGFR mutation | |
mRNA-378, hsa-miR-210, has-miR-214, hsamiR-15a | |
Pre-clinical studies | Molecular MRI (contrast specific for ALCAM and VCAM) |
Machine learning models using MRI | |
Routine perioperative imaging during lung resections | |
Machine learning models using miRNA expression | |
Machine learning models using demographic and patient factors | |
Urine metabolites |
Table 1. Summary of known risk factors and pre-clinical studies with early detection of BrMs in lung cancer.
Therapy Type | Treatment | Dosage/Fraction | Benefits | Drawbacks |
|---|---|---|---|---|
WBRT | Radiation to the entire cranium | Multiple small dose fractionated treatments 3 Gy x 10 Fractions | May treat micro-metastasis not seen on imaging | Greater radiation dosage to healthy tissue |
SRS | Multifocal beams of radiation concentrated on the tumor only | Single or multiple high dosage fractions/treatments 15-24 Gy x 1 Fraction | Decreased tumor toxicity >3cm Limits radiation dosage to healthy tissues | Higher probability of tumor resurgence |
Table 2. Summary of comparison between Whole Brain Radiation Therapy (WBRT), and Stereotactic Radiation Surgery (SRS).
References
- Cancer Facts & Figures 2021. Published online 1930:72.
- Ferlay J, Soerjomataram I, Dikshit R, et al. (2012), Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN. International Journal of Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
- Types of lung cancer | Cancer Research UK. Accessed August 28, 2022. https://www.cancerresearchuk.org/about-cancer/lung-cancer/stages-types-grades/types
- Sperduto PW, Kased N, Roberge D, et al. (2012), Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. JCO.30(4):419-425. doi:10.1200/JCO.2011.38.0527
- Sacks P, Rahman M. (2020), Epidemiology of Brain Metastases. Neurosurgery Clinics of North America.31(4):481-488. doi:10.1016/j.nec.2020.06.001
- Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. (1988), Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. JCO.6(9):1474-1480. doi:10.1200/JCO.1988.6.9.1474
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69(1):7-34. doi:10.3322/caac.21551
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. JCO. 2004;22(14):2865-2872. doi:10.1200/JCO.2004.12.149
- Valastyan S, Weinberg RA. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011;147(2):275-292. doi:10.1016/j.cell.2011.09.024
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):1-26. doi:10.1038/s41572-018-0055-y
- Zhao N, Wilkerson MD, Shah U, et al. Alterations of LKB1 and KRAS and risk of brain metastasis: comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung Cancer. 2014;86(2):255-261. doi:10.1016/j.lungcan.2014.08.013
- Hayes DN, Monti S, Parmigiani G, et al. Gene expression profiling reveals reproducible human lung adenocarcinoma subtypes in multiple independent patient cohorts. J Clin Oncol. 2006;24(31):5079-5090. doi:10.1200/JCO.2005.05.1748
- Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543-550. doi:10.1038/nature13385
- Nguyen DX, Chiang AC, Zhang XHF, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138(1):51-62. doi:10.1016/j.cell.2009.04.030
- Ilhan-Mutlu A, Siehs C, Berghoff AS, et al. Expression profiling of angiogenesis-related genes in brain metastases of lung cancer and melanoma. Tumour Biol. 2016;37(1):1173-1182. doi:10.1007/s13277-015-3790-7
- Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl 4):S209-S219. doi:10.4103/2152-7806.111298
- Posner JB. Management of brain metastases. Rev Neurol (Paris). 1992;148(6-7):477-487.
- Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20(4):e300-e306. doi:10.3747/co.20.1481
- Sánchez de Cos J, Sojo González MA, Montero MV, Pérez Calvo MC, Vicente MJM, Valle MH. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63(1):140-145. doi:10.1016/j.lungcan.2008.04.013
- Bastos DC de A, Fuentes DT, Traylor J, et al. The use of laser interstitial thermal therapy in the treatment of brain metastases: a literature review. International Journal of Hyperthermia. 2020;37(2):53-60. doi:10.1080/02656736.2020.1748238
- Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45-68. doi:10.1007/s11060-009-0073-4
- Swinson BM, Friedman WA. Linear accelerator stereotactic radiosurgery for metastatic brain tumors: 17 years of experience at the University of Florida. Neurosurgery. 2008;62(5):1018-1031; discussion 1031-1032. doi:10.1227/01.neu.0000325863.91584.09
- Chi A, Komaki R. Treatment of Brain Metastasis from Lung Cancer. Cancers (Basel). 2010;2(4):2100-2137. doi:10.3390/cancers2042100
- Sansur CA, Chin LS, Ames JW, et al. Gamma Knife Radiosurgery for the Treatment of Brain Metastases. SFN. 2000;74(1):37-51. doi:10.1159/000056462
- Sun DS, Hu LK, Cai Y, et al. A systematic review of risk factors for brain metastases and value of prophylactic cranial irradiation in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(3):1233-1239. doi:10.7314/apjcp.2014.15.3.1233
- An N, Jing W, Wang H, et al. Risk factors for brain metastases in patients with non–small‐cell lung cancer. Cancer Med. 2018;7(12):6357-6364. doi:10.1002/cam4.1865
- Chen S, Hua X, Jia J, et al. Risk factors for brain metastases in patients with non-small cell lung cancer: a meta-analysis of 43 studies. Annals of Palliative Medicine. 2021;10(4):3657672-3653672. doi:10.21037/apm-20-1722
- Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumor Biol. 2012;33(4):1065-1073. doi:10.1007/s13277-012-0344-0
- Choi H, Puvenna V, Brennan C, et al. S100B and S100B autoantibody as biomarkers for early detection of brain metastases in lung cancer. Transl Lung Cancer Res. 2016;5(4):413-419. doi:10.21037/tlcr.2016.07.08
- Winther-Larsen A, Hviid CVB, Meldgaard P, Sorensen BS, Sandfeld-Paulsen B. Neurofilament Light Chain as A Biomarker for Brain Metastases. Cancers (Basel). 2020;12(10):2852. doi:10.3390/cancers12102852
- Gaebe K, Li AY, Das S. Clinical Biomarkers for Early Identification of Patients with Intracranial Metastatic Disease. Cancers (Basel). 2021;13(23):5973. doi:10.3390/cancers13235973
- Chen L tao, Xu S dong, Xu H, Zhang J feng, Ning J feng, Wang S fa. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol. 2012;29(3):1673-1680. doi:10.1007/s12032-011-0083-x
- Naresh G, Malik PS, Khurana S, et al. Assessment of Brain Metastasis at Diagnosis in Non–Small-Cell Lung Cancer: A Prospective Observational Study From North India. JCO Glob Oncol. 2021;7:GO.20.00629. doi:10.1200/GO.20.00629
- Milano MT, Bates JE, Budnik J, et al. Risk of brain metastases in T1–3N0 NSCLC: a population-based analysis. Lung Cancer Manag. 9(1):LMT25. doi:10.2217/lmt-2019-0010
- Ji Z, Bi N, Wang J, et al. Risk Factors for Brain Metastases in Locally Advanced Non-Small Cell Lung Cancer With Definitive Chest Radiation. International Journal of Radiation Oncology*Biology*Physics. 2014;89(2):330-337. doi:10.1016/j.ijrobp.2014.02.025
- Marchi N, Mazzone P, Fazio V, Mekhail T, Masaryk T, Janigro D. ProApolipoprotein A1. Cancer. 2008;112(6):1313-1324. doi:10.1002/cncr.23314
- He J, Wang X, Xiao R, Zuo W, Zhang W, Yao H. Risk factors for brain metastases from non-small-cell lung cancer. Medicine (Baltimore). 2021;100(9):e24724. doi:10.1097/MD.0000000000024724
- Cheng VWT, de Pennington N, Zakaria R, et al. VCAM-1–targeted MRI Improves Detection of the Tumor-brain Interface. Clinical Cancer Research. 2022;28(11):2385-2396. doi:10.1158/1078-0432.CCR-21-4011
- Cheng VWT, Soto MS, Khrapitchev AA, et al. VCAM-1–targeted MRI Enables Detection of Brain Micrometastases from Different Primary Tumors. Clinical Cancer Research. 2019;25(2):533-543. doi:10.1158/1078-0432.CCR-18-1889
- Serres S, Soto MS, Hamilton A, et al. Molecular MRI enables early and sensitive detection of brain metastases. Proceedings of the National Academy of Sciences. 2012;109(17):6674-6679. doi:10.1073/pnas.1117412109
- Yokoi K, Kamiya N, Matsuguma H, et al. Detection of Brain Metastasis in Potentially Operable Non-small Cell Lung Cancer: A Comparison of CT and MRI. Chest. 1999;115(3):714-719. doi:10.1378/chest.115.3.714
- Larkin JR, Dickens AM, Claridge TDW, et al. Early Diagnosis of Brain Metastases Using a Biofluids-Metabolomics Approach in Mice. Theranostics. 2016;6(12):2161-2169. doi:10.7150/thno.16538
- Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine Learning for Medical Imaging. Radiographics. 2017;37(2):505-515. doi:10.1148/rg.2017160130
- Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence—what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
- Cho SJ, Sunwoo L, Baik SH, Bae YJ, Choi BS, Kim JH. Brain metastasis detection using machine learning: a systematic review and meta-analysis. Neuro Oncol. 2020;23(2):214-225. doi:10.1093/neuonc/noaa232
- Wang KJ, Makond B, Wang KM. Modeling and predicting the occurrence of brain metastasis from lung cancer by Bayesian network: A case study of Taiwan. Computers in Biology and Medicine. 2014;47:147-160. doi:10.1016/j.compbiomed.2014.02.002
- Zhao S, Yu J, Wang L. Machine Learning Based Prediction of Brain Metastasis of Patients with IIIA-N2 Lung Adenocarcinoma by a Three-miRNA Signature. Translational Oncology. 2018;11(1):157-167. doi:10.1016/j.tranon.2017.12.002
- Marner L, Henriksen OM, Lundemann M, Larsen VA, Law I. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clinical and translational imaging. 2017;5(2):135-149.
- Lopci E, Franzese C, Grimaldi M, et al. Imaging biomarkers in primary brain tumours. European journal of nuclear medicine and molecular imaging. 2015;42(4):597-612.
- Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. Journal of nuclear medicine. 2012;53(12):1916-1925.
- Lohmann P, Werner JM, Shah NJ, Fink GR, Langen KJ, Galldiks N. Combined amino acid positron emission tomography and advanced magnetic resonance imaging in glioma patients. Cancers. 2019;11(2):153.
- Mier W, Mier D. Advantages in functional imaging of the brain. Frontiers in human neuroscience. 2015;9:249.
- Overcast WB, Davis KM, Ho CY, et al. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Current Oncology Reports. 2021;23(3):1-15.
- Ferda J, Ferdová E, Hes O, Mraček J, Kreuzberg B, Baxa J. PET/MRI: Multiparametric imaging of brain tumors. European Journal of Radiology. 2017;94:A14-A25.
- Rosenkrantz AB, Friedman K, Chandarana H, et al. Current status of hybrid PET/MRI in oncologic imaging. AJR American journal of roentgenology. 2016;206(1):162.
- Heiss WD. The potential of PET/MR for brain imaging. European journal of nuclear medicine and molecular imaging. 2009;36(1):105-112.
- Rausch I, Rischka L, Ladefoged CN, et al. PET/MRI for oncologic brain imaging: a comparison of standard MR-based attenuation corrections with a model-based approach for the Siemens mMR PET/MR System. Journal of Nuclear Medicine. 2017;58(9):1519-1525.
- Mayerhoefer ME, Prosch H, Beer L, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. European journal of nuclear medicine and molecular imaging. 2020;47(1):51-60.
- Puttick S, Bell C, Dowson N, Rose S, Fay M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug discovery today. 2015;20(3):306-317.
- Quartuccio N, Laudicella R, Vento A, et al. The additional value of 18F-FDG PET and MRI in patients with glioma: a review of the literature from 2015 to 2020. Diagnostics. 2020;10(6):357.
- Verberne S, Temmerman O. Imaging of prosthetic joint infections. In: Management of Periprosthetic Joint Infections (PJIs). Elsevier; 2017:259-285.
- Nandu H, Wen PY, Huang RY. Imaging in neuro-oncology. Therapeutic advances in neurological disorders. 2018;11:1756286418759865.
- Glaudemans AW, Enting RH, Heesters MA, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. European journal of nuclear medicine and molecular imaging. 2013;40(4):615-635.
- Mehta MP, Rodrigus P, Terhaard C, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. Journal of Clinical Oncology. 2003;21(13):2529-2536.
- Temel JS, Greer J. muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin Cm, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with non-metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. Journal of Clinical Oncology. 2005;23(25):6207-6219.
- Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18(3):298-306.
- Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. Journal of Clinical Oncology. 2006;24(8):1295-1304.
- Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res. 2018;7:F1000 Faculty Rev-1772. doi:10.12688/f1000research.15903.1
- Hatiboglu MA, Akdur K, Sawaya R. Neurosurgical management of patients with brain metastasis. Neurosurg Rev. 2020;43(2):483-495. doi:10.1007/s10143-018-1013-6
- BRUZZANITI P, LAPOLLA P, D’AMICO A, et al. En Bloc Resection of Solitary Brain Metastasis: The Role of Perilesional Edema. In Vivo. 2022;36(3):1274-1284. doi:10.21873/invivo.12827
- Wen PY, Loeffler JS. Brain metastases. Curr Treat Options Oncol. 2000;1(5):447-458. doi:10.1007/s11864-000-0072-3
- Esquenazi Y, Lo VP, Lee K. Critical Care Management of Cerebral Edema in Brain Tumors. J Intensive Care Med. 2017;32(1):15-24. doi:10.1177/0885066615619618
- Baris MM, Celik AO, Gezer NS, Ada E. Role of mass effect, tumor volume and peritumoral edema volume in the differential diagnosis of primary brain tumor and metastasis. Clin Neurol Neurosurg. 2016;148:67-71. doi:10.1016/j.clineuro.2016.07.008
- Yaltirik Bilgin E, Unal O, Ciledag N. Vasogenic Edema Pattern in Brain Metastasis. J Coll Physicians Surg Pak. 2022;32(8):1020-1025. doi:10.29271/jcpsp.2022.08.1020
- Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):33-43. doi:10.1007/s11060-009-0061-8
- Nemeth AJ, Henson JW, Mullins ME, Gonzalez RG, Schaefer PW. Improved detection of skull metastasis with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2007;28(6):1088-1092. doi:10.3174/ajnr.A0501
- Tirinato L, Onesto V, Garcia-Calderon D, et al. Human lung-cancer-cell radioresistance investigated through 2D network topology. Sci Rep. 2022;12(1):12980. doi:10.1038/s41598-022-17018-0
- Yao Y, Fareed R, Zafar A, et al. State-of-the-art combination treatment strategies for advanced stage non–small cell lung cancer. Frontiers in Oncology. 2022;12. Accessed August 29, 2022. https://www.frontiersin.org/articles/10.3389/fonc.2022.958505
- Ozcan G, Singh M, Vredenburgh JJ. Leptomeningeal Metastasis from Non-Small Cell Lung Cancer and Current Landscape of Treatments. Clin Cancer Res. Published online August 16, 2022:CCR-22-1585. doi:10.1158/1078-0432.CCR-22-1585
- Park SJ, Lim SH, Kim YJ, et al. The Tumor Control According to Radiation Dose of Gamma Knife Radiosurgery for Small and Medium-Sized Brain Metastases from Non-Small Cell Lung Cancer. J Korean Neurosurg Soc. 2021;64(6):983-994. doi:10.3340/jkns.2021.0165
- Devan SP, Jiang X, Luo G, et al. Selective cell size MRI differentiates brain tumors from radiation necrosis. Cancer Res. Published online July 25, 2022:CAN-21-2929. doi:10.1158/0008-5472.CAN-21-2929
- Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56(5):1021-1034; discussion 1021-1034.
- Desai R, Rich KM. Therapeutic Role of Gamma Knife Stereotactic Radiosurgery in Neuro-Oncology. Mo Med. 2020;117(1):33-38.
- Goldberg SB, Contessa JN, Omay SB, Chiang V. Lung Cancer Brain Metastases. Cancer J. 2015;21(5):398-403. doi:10.1097/PPO.0000000000000146
- Abe E, Aoyama H. The Role of Whole Brain Radiation Therapy for the Management of Brain Metastases in the Era of Stereotactic Radiosurgery. Curr Oncol Rep. 2012;14(1):79-84. doi:10.1007/s11912-011-0201-0
- Perlow HK, Dibs K, Liu K, et al. Whole-Brain Radiation Therapy Versus Stereotactic Radiosurgery for Cerebral Metastases. Neurosurgery Clinics of North America. 2020;31(4):565-573. doi:10.1016/j.nec.2020.06.006
- Sas-Korczynska B, Rucinska M. WBRT for brain metastases from non-small cell lung cancer: for whom and when?—Contemporary point of view. J Thorac Dis. 2021;13(5):3246-3257. doi:10.21037/jtd-2019-rbmlc-06
- Rusthoven CG, Yamamoto M, Bernhardt D, et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases. JAMA Oncol. 2020;6(7):1028-1037. doi:10.1001/jamaoncol.2020.1271
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316(4):401-409. doi:10.1001/jama.2016.9839
- Tallet AV, Azria D, Barlesi F, et al. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiation Oncology. 2012;7(1):77. doi:10.1186/1748-717X-7-77
- Lee J, Ahn MJ. Brain metastases in patients with oncogenic-driven non-small cell lung cancer: Pros and cons for early radiotherapy. Cancer Treat Rev. 2021;100:102291. doi:10.1016/j.ctrv.2021.102291
- Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro Oncol. 2017;19(Suppl 2):ii38-ii49. doi:10.1093/neuonc/now301
- Badiyan SN, Regine WF, Mehta M. Stereotactic Radiosurgery for Treatment of Brain Metastases. JOP. 2016;12(8):703-712. doi:10.1200/JOP.2016.012922
- Gondi V, Bauman G, Bradfield L, et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Practical Radiation Oncology. 2022;12(4):265-282. doi:10.1016/j.prro.2022.02.003
- Rades D, Haatanen T, Schild SE, Dunst J. Dose escalation beyond 30 grays in 10 fractions for patients with multiple brain metastases. Cancer. 2007;110(6):1345-1350. doi:10.1002/cncr.22906
- Trifiletti DM, Ballman KV, Brown PD, et al. Optimizing Whole Brain Radiation Therapy Dose and Fractionation: Results From a Prospective Phase 3 Trial (NCCTG N107C [Alliance]/CEC.3). Int J Radiat Oncol Biol Phys. 2020;106(2):255-260. doi:10.1016/j.ijrobp.2019.10.024
- Jairam V, Chiang VL, Bond J, Yu JB. Equivalent whole brain dose for patients undergoing gamma knife for multiple lesions. J Radiosurg SBRT. 2015;3(3):187-191.
- Sorensen BS, Horsman MR. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front Oncol. 2020;10:562. doi:10.3389/fonc.2020.00562
- Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674-687. doi:10.1038/nrclinonc.2012.171
- Overgaard J. Hypoxic Radiosensitization: Adored and Ignored. JCO. 2007;25(26):4066-4074. doi:10.1200/JCO.2007.12.7878
- Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy. Biomed Res Int. 2013;2013:685308. doi:10.1155/2013/685308
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26(312):638-648. doi:10.1259/0007-1285-26-312-638


