Current Issue : Article / Volume 3, Issue 1
- Review Article | DOI:
- https://doi.org/10.58489/2836-2330/016
A New Era for Cholangiocarcinoma Therapy â Targeting Metabolic Enzymes
- Finckensteinalle 137, 12205 Berlin, Germany.
Antonis G. Tsamaloukas
Antonis G. Tsamaloukas. (2024). A New Era for Cholangiocarcinoma Therapy â Targeting Metabolic Enzymes. Journal of Clinical and Medical Reviews. 3(1); DOI: 10.58489/2836-2330/016
© 2024 Antonis G. Tsamaloukas, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 22-01-2024
- Accepted Date: 25-01-2024
- Published Date: 27-01-2024
cholangiocarcinoma (CCA), metabolism, mitochondrion, Tricarboxylic Acid cycle (TCA), Isocitrate dehydrogenase (IDH), oncometabolite, ClarIDHy trial
Abstract
Cholangiocarcinoma (CCA) is a malignancy of the biliary tract epithelium and is the second most common primary liver tumor following hepatocellular carcinoma (HCC): it accounts for ~15% of primary liver cancers and ~3% of gastrointestinal malignancies. Due to late diagnoses, poor prognoses, and limited treatment strategies, CCA shows a dismal outcome with a less than 5% overall 5-year survival and only a median overall survival of ~7months. Moreover, epidemiological data show that the incidence (0.3â6 per 100,000) and mortality (1 -6 per 100,000) of CCA is increasing globally over recent decades.
Cholangiocarcinoma (CCA) is associated with approximately 3,500 new cases, the second most
common primary liver tumor in Germany. Cholangiocarcinoma (CCA) is classified into anatomic subtypes including intrahepatic CCA (iCCA), perihilar CCA (pCCA) and extrahepatic CCA (eCCA).
Introduction
Given a virtual explosion of published manuscripts in the last decade (>6,000 publications), guidelines for diagnosis and treatment of CCA( [1-3]and the many comprehensive and excellent reviews ([1-14]
I am unable to provide a complete review of all relevant studies here and point those who wish to obtain additional information to other resources (see Fig. 1):
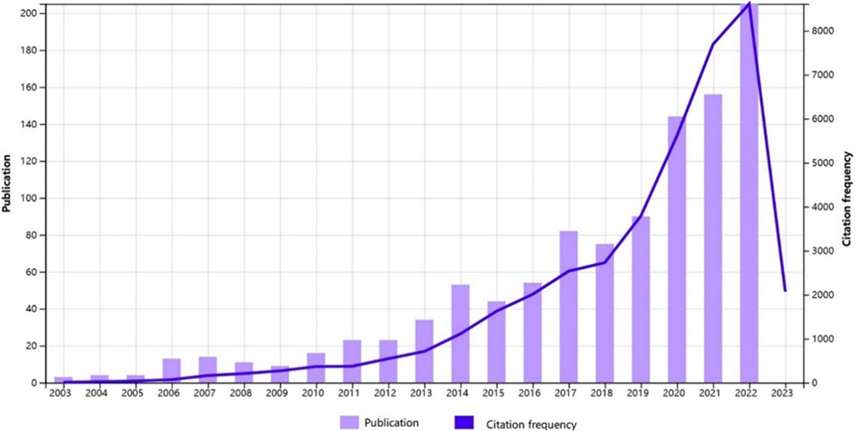
Fig 1: Number of publications indexed on PubMed per year using search terms “targeted” and “cholangiocarcinoma” from [15].
Cholangiocarcinoma (CCA) represents diverse tumors originating from cholangiocytes in the bile ducts. Depending on their anatomical location, CCA is classified as intrahepatic (iCCA) or extrahepatic (eCCA), and eCCA is further classified as perihilar or distal eCCA [16] (Fig2). Consideration of an iCCA diagnosis in patients with CUP could improve timely diagnosis, molecular characterisation and treatment [17].
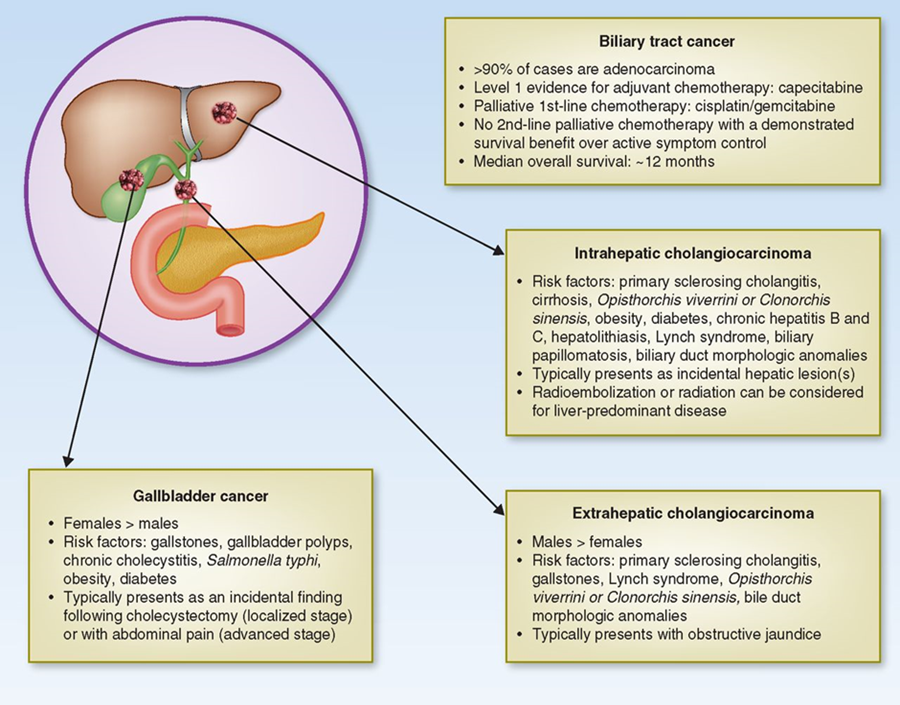
Fig 2: BTCs are a group of different diseases which includes ICC, ECC, and gallbladder cancer. They differ in many aspects, such as anatomical location, demographics, clinical presentations, and treatment options (from[16, 18]).
CCA is a relatively rare cancer with an incidence rate in the United States of 1.20 per 100 000 person-years from 2000 to 2015, based on data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program.
Estimated incidence rates in the United States for iCCA and eCCA during this period were 0.77 and 0.43 per 100 000 person-years, respectively.
Recent retrospective data analyses suggest that the incidence of CCA has increased in past decades in both the United States and most European countries[19, 20](see Fig.3):
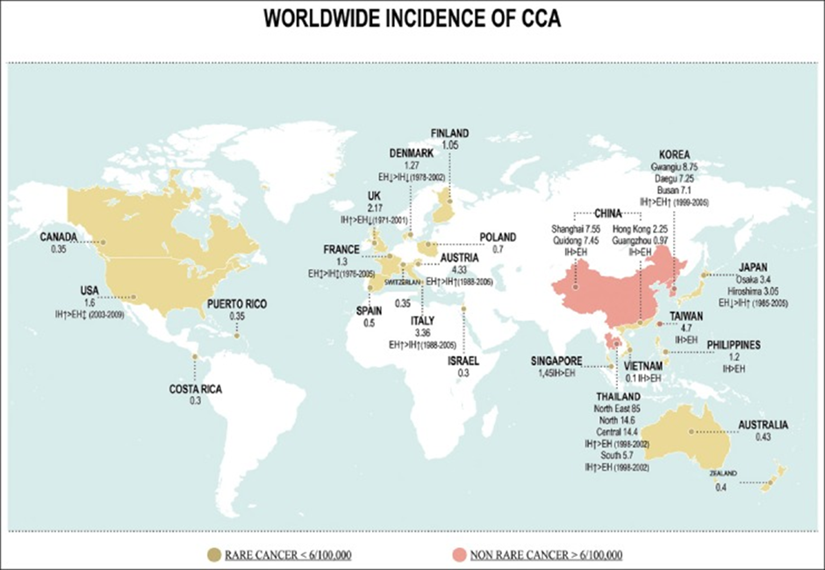
Fig. 3: Worldwide incidence (cases/100,000) of cholangiocarcinoma (CCA) [20] from particularly that of iCCA. In the United States, the annual percentage increases from 2003 to 2015 in iCCA and eCCA were 7.0 and 2.1, respectively.
In Western and Central Europe, age-adjusted incidence rates (per 100 000 person-years) from 2008 to 2012 for iCCA were highest in the UK (1.15), France (1.13), and Germany (1.05), and those for eCCA were highest in Germany (0.74), the Netherlands (0.69), and Ireland (0.68) (see Fig 3):
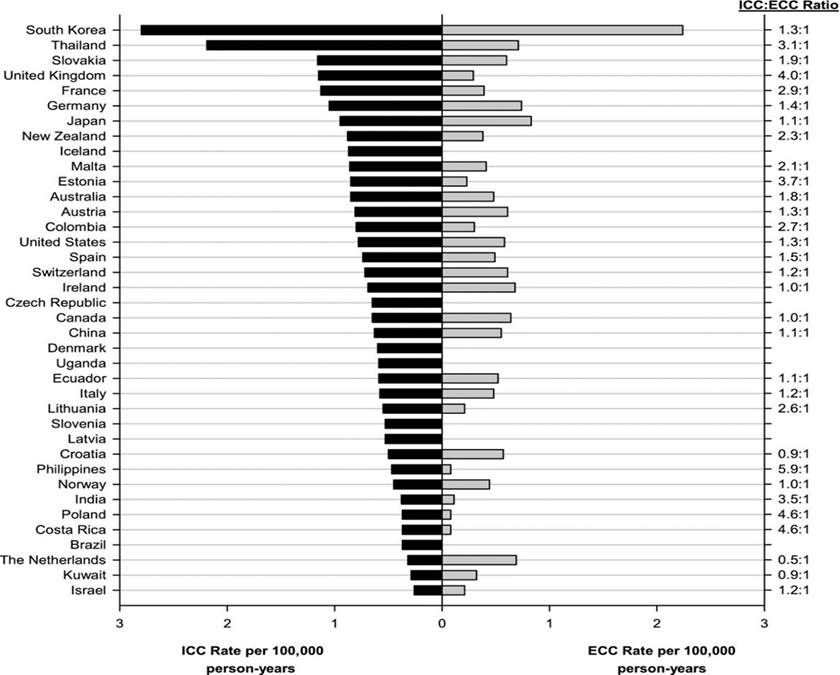
Fig 4: Age-standardized ICC and ECC incidence rates by country, 2008-2012. ECC indicates (extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; from[19]).
Mortality rates for 2018 for iCCA and eCCA in males and females are shown in in Fig.4, Fig.5 respectively[21],
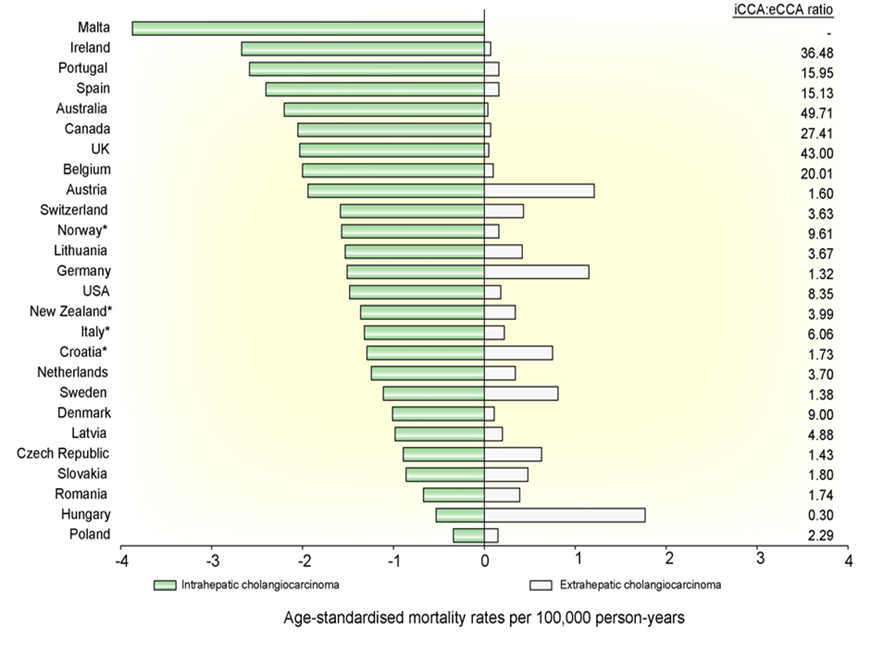
Fig 5: Age-standardised mortality rates for intrahepatic and extrahepatic cholangiocarcinoma per 100,000 person-years for males from Western countries for 2018.(from [21]).
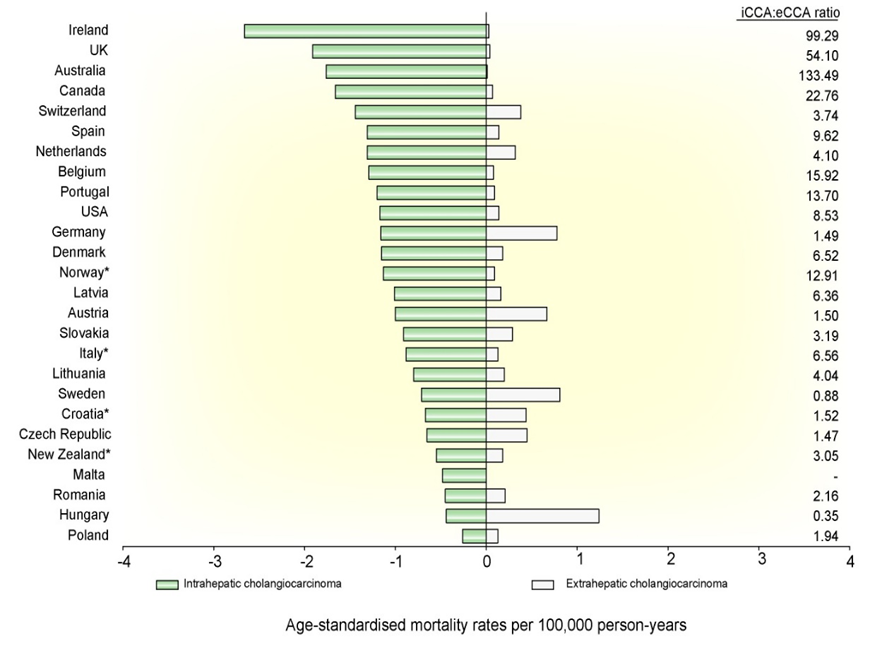
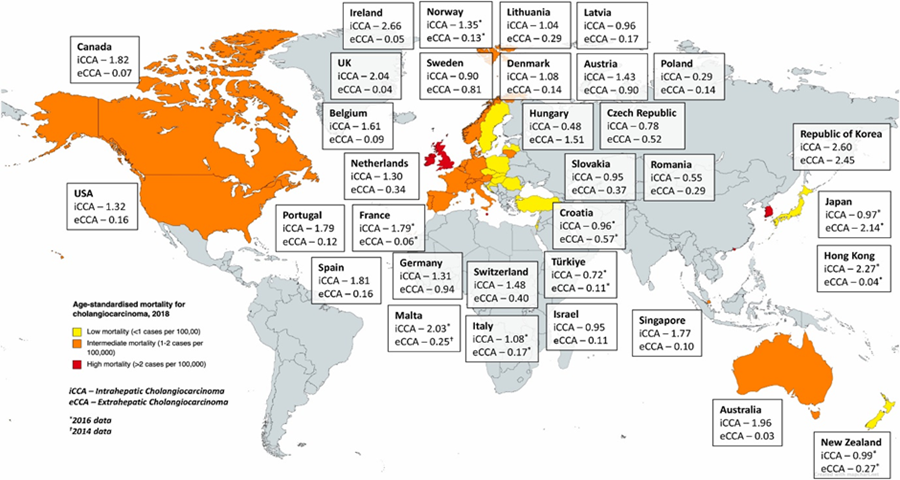
Fig 6: Age-standardised mortality for cholangiocarcinoma per 100,000 person-years – adapted from [22]
Although CCA is essentially a sporadic disease, diverse factors have been associated with increased risk of CCA, including bile duct cyst, Caroli's disease, primary sclerosing cholangitis, cholelithiasis or choledocholithiasis, parasitic liver infections, liver cirrhosis, hepatitis B or C virus infection, and hepatolithiasis (iCCA only).
The high incidence of CCA in some East Asian countries, such as South Korea and Thailand, is due to the endemic presence of OPISTHORCHIS, VIVERRINI and Clonorchis sinensis liver flukes (see Fig.7) and vertical hepatitis B virus transmission.
The CCA incidence is 40 times higher in endemic areas of Thailand and China. Liver fluke-induced chronic inflammation plays a crucial role in cholangiocarcinogenesis, as aflatoxin exposure in Chile, or other chemical exposures.
Fig 7: Clonorchis sinensis Liver Flukes( from[23])
Several high-throughput genomic sequencing analyses have elucidated the distinct mutational landscapes of iCCA, pCCA and dCCA, thus revealing differences in biology between these anatomical subtypes of the disease.
The genomic heterogeneity within biliary tract carcinoma is illustrated in the following Figure 8 and Figure 9:
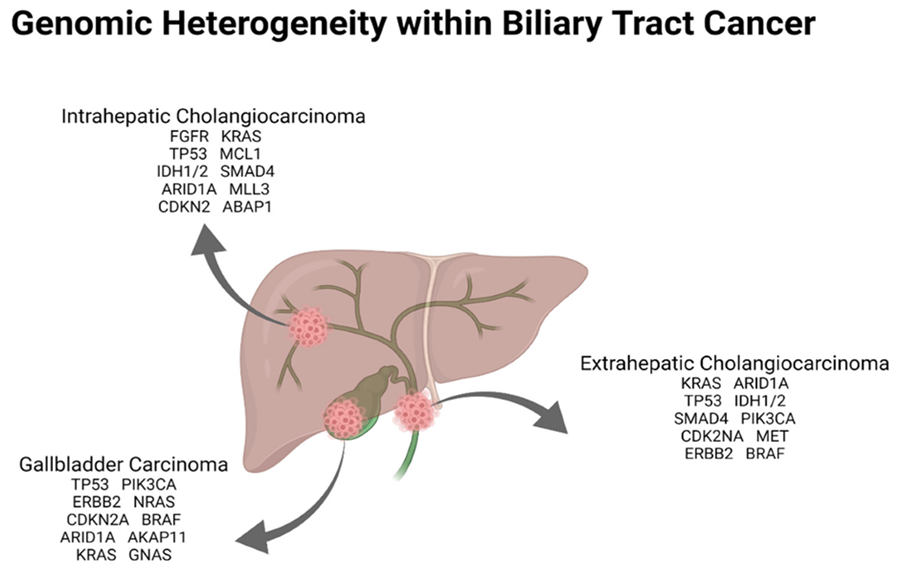
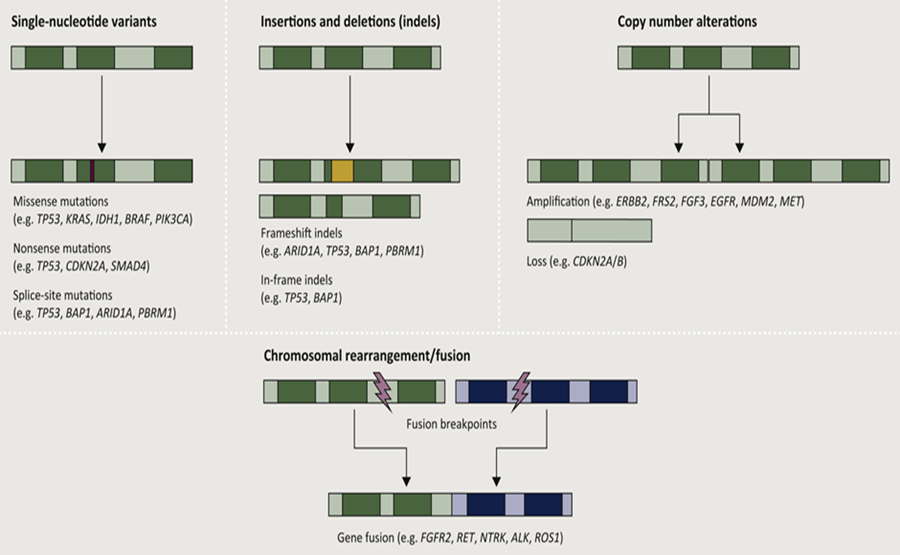
Cytotoxic chemotherapy has been the mainstay of systemic treatment and is well established as a means to prolong life and to palliate cancer-related symptoms, though an explosion of drug development in recent years has led to the adoption of several new forms of therapy[24] (see Fig.9). It is certainly not surprising that metabolic inhibitors were targeted decades ago, such as the development of aminopterin[26]. In this context, the successes now achieved are the cornerstone for precision medicine[18, 27-32] or personalized medicine[33], which is based on molecular genetic data and is specifically directed against driver mutations, for example. The extension or complementation of the modern therapeutic armamentarium includes monoclonal antibodies, tyrosine kinase inhibitors, immune checkpoint inhibitors, CRISP based therapeutics and as well as cellular therapies such as CAR-Tcell therapy (see Fig. 10, Fig. 11).
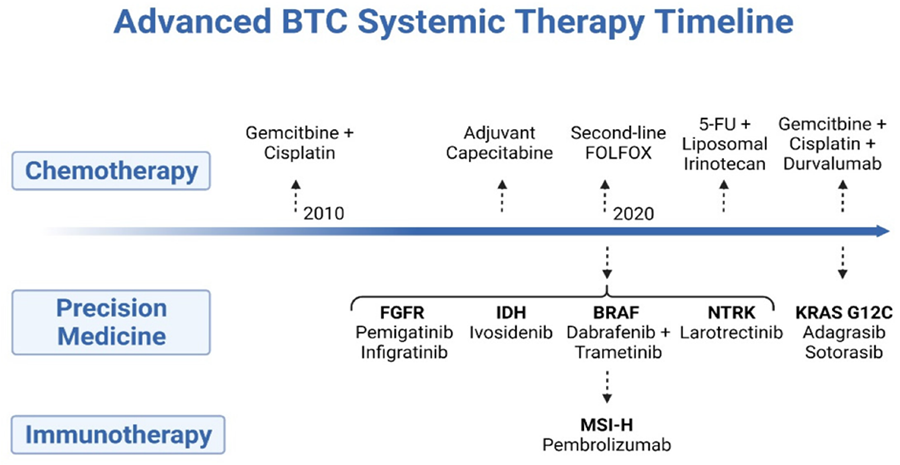
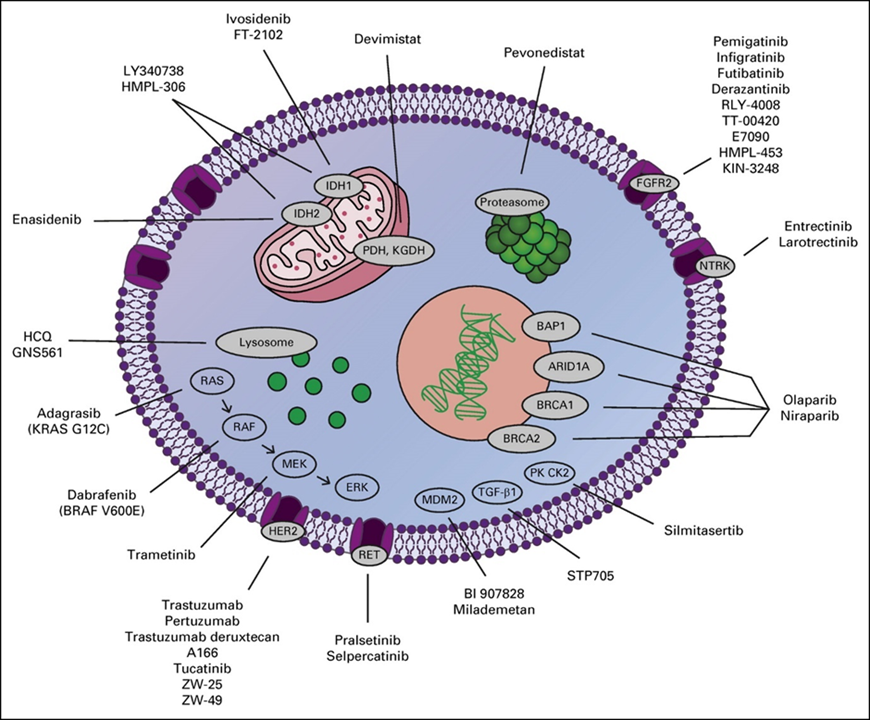
Fig. 11: New and emerging targets in cholangiocarcinoma. ARID1A, AT-rich interaction domain 1A; BAP1, BRCA1-associated protein 1; BRAF, v-raf murine sarcoma viral oncogene homolog B1; BRCA, breast cancer gene; ERK, extracellular signal-regulated kinase; FGFR2, fibroblast growth factor receptor 2; HCQ, hydroxychloroquine; HER2, human epidermal growth factor receptor 2; IDH, isocitrate dehydrogenase; KGDH, alpha ketoglutarate dehydrogenase; KRAS, Kirsten rat sarcoma virus; MDM2, mouse double minute 2; MEK, mitogen-activated protein kinase; NTRK, neurotrophic tropomyosin receptor kinase; PDH, pyruvate dehydrogenase; PK CK2, protein kinase CK2; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma virus; RET, rearranged during transfection; TGF-β1, transforming growth factor beta 1. (from[30])
From 2000 to 2022, R. Weinberg and D. Hanahan summarized and developed the hallmarks of cancer every ten years to explain the mechanisms of the occurrence, development, and treatment response characteristics of malignant tumors[34-36]. Characteristics of cancer summarized in Fig.12 will provide a reasonable explanation for the multi-level process of human tumor pathology.
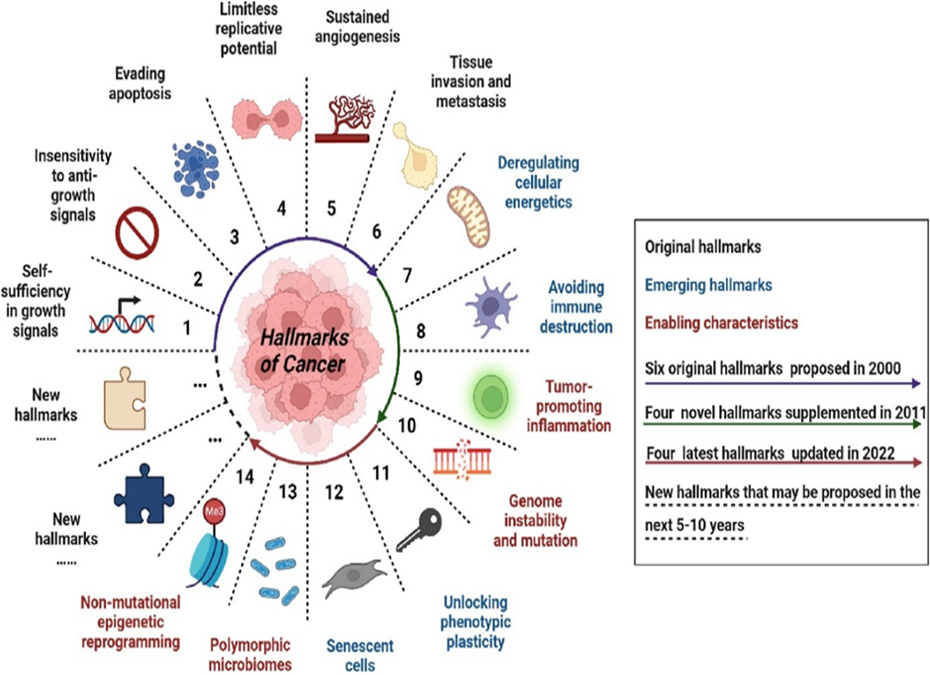
Original hallmarks are the initially identified cancer feature; emerging hallmarks are the features that have not been determined in the corresponding period and need further research and confirmation; enabling characteristics are the features that have been proposed in the corresponding period; colored circular arrows represent the time when the cancer hallmarks were presented; “…” represents the time when new cancer characteristics may be proposed in the future. ([37] from [34, 35]
Metabolic reprogramming is a hallmark of cancer and is one of the basic characteristics of cancer and has been proved to be an important cancer treatment strategy[38-40].
The hallmark of “Deregulating cellular metabolism" involves the mitochondrial metabolism which is reprogrammed in cancer[38, 41-46]. This year, in 2024, the discovery of the Warburg effect[38, 41-43, 47-53] will celebrate its 100th anniversary. Although it has been foretold that “no one can doubt that we understand the origin of cancer cells if …. we know how the damaged respiration and the excessive fermentation of the cancer cells originate”. Here, Warburg showed that tumor tissue slices continued to produce lactate even when oxygen was abundant, a phenomenon of aerobic glycolysis now dubbed [54].
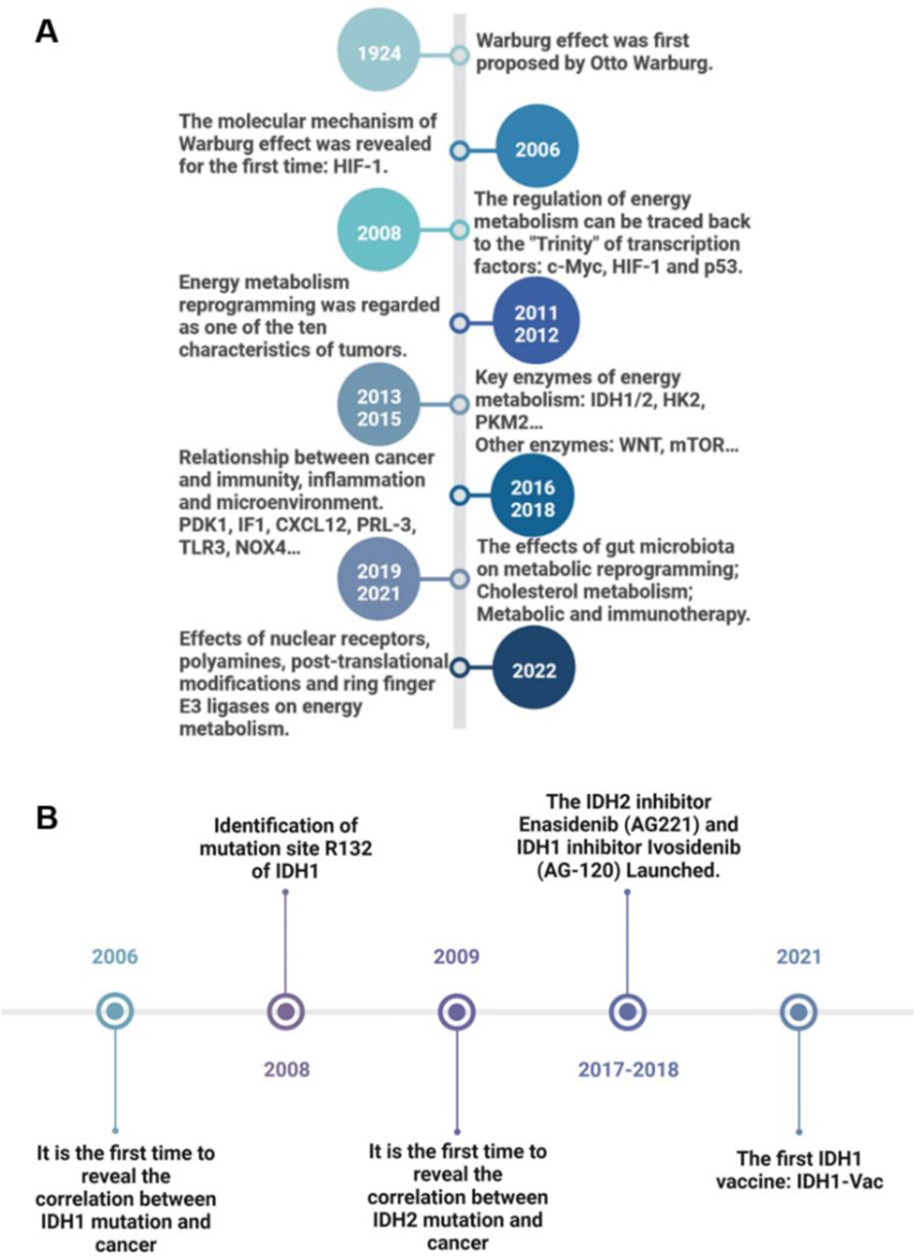
Fig 13: Research timeline of cancer energy metabolism reprogramming and IDH1/2. (A) Milestone events of cancer energy metabolism reprogramming from 1924 to 2022. (B) Milestone events of IDH1/2 research from 2006 to 2021. The description of key events is on the opposite side of the time. (from [37]).
When the activity of metabolic enzymes is disrupted due to mutations or changes in expression levels, it can result in various metabolic disorders that have also been linked to cancer development. Recently The Cancer Genome Atlas (TCGA) study in ICC was published, and this integrated analysis of somatic mutations, RNA expression, copy number, and DNA methylation also led to a molecular classification scheme and identified an IDH mutant–enriched subtype with distinct molecular features, including low expression of chromatin modifiers, elevated expression of mitochondrial genes, and increased mitochondrial DNA copy number [55]. By means of this integrative genomic analysis four distinct subtypes were identified. One of it fulfilled one of the hallmarks of cancer “Deregulating cellular metabolism” as shown in the following Fig. 14:

With the rise of genetic and molecular sequencing, several driver mutations have been identified and targeted as novel therapeutic approaches. The most common genomic alterations include changes in FGFR2, IDH1, IDH2, KRAS, BRAF, HER2, and the tumor suppressor p53. In addition, increased understanding of the cellular and molecular constituents of the tumor microenvironment (TME) has created opportunities for further novel therapeutic approaches. Molecular profiling using next-generation sequencing (NGS) has revealed a complex genomic landscape of CCA that likely reflects its heterogeneous etiology.
Notably, using NGS, the most commonly altered genes have been identified for iCCA.
Certainly, to the surprise of any hematologist and oncologist, identical mutations of metabolic enzymes have been found in both solid and hematologic malignancies. An important aspect is that mitochondrial metabolism is reprogrammed in cancer. In this year, in 2024, the discovery of the Warburg effect will celebrate its 100th anniversary.
When the activity of metabolic enzymes is disrupted due to mutations or changes in expression levels, it can result in various metabolic disorders that have also been linked to cancer development.
Cancer-associated mutations in IDH1 and IDH2 I represent one of the most comprehensively studied mechanisms of IDH pathogenic effect.
Ultimately, mitochondrial dysfunction and aerobic glycolysis have become widely accepted as hallmarks of cancer.
The mitochondrial pathways in cancer as the Tricarboxylic Acid cycle (TCA), the electron transport chain (ETC), the oxidative phosphorylation (OXPHOS) and the mitochondrial One-Carbon metabolism are mainly affected (see Fig.15):

Especially one mutated enzyme of the TCA came into the focus: Isocitrate dehydrogenase 1-3 (IDH 1, IDH 2, IDH3).
Mutations in isocitrate dehydrogenase (IDH) have been identified in a spectrum of human malignancies. Mutations in
IDH1
were first identified in an exome resequencing analysis of patients with colorectal cancer [57]. Isocitrate dehydrogenase (IDH) is an important metabolic enzyme in the tricarboxylic acid cycle (TCA), whose mutated genes are associated with a variety of tumors, including acute myeloid leukemia (AML), myelodysplastic Syndrome (MDS), glioma, cholangiocarcinoma, colon cancer, prostate cancer and chondrosarcoma[58-66] (see Fig. 16 see Fig.17):
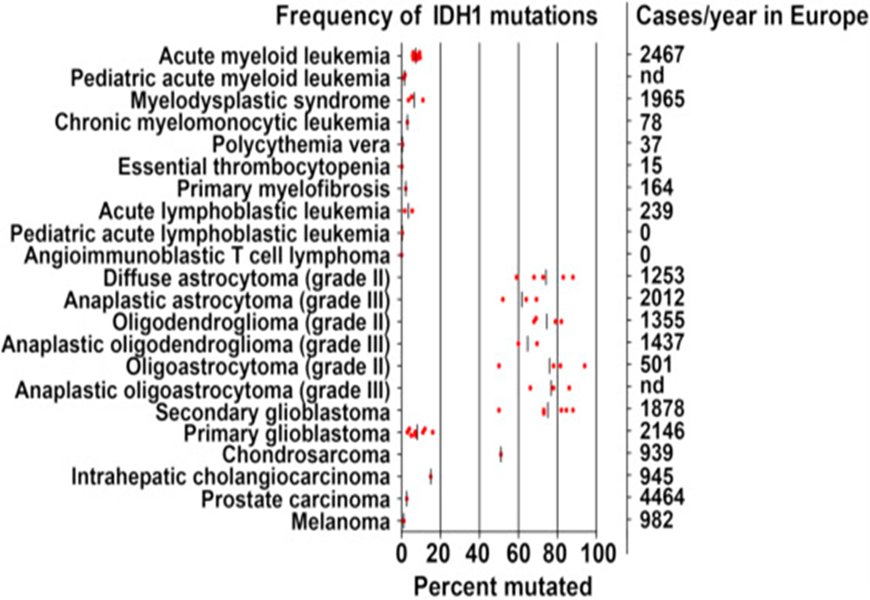

IDH mutation detection and 2-Hydroxyglutarate (D2HG) measurement have already been incorporated into clinical practice in a number of settings and are under evaluation for their use as tools in the management of several diseases. The rapid incorporation of IDH in the sense from “bench to the bedside” is illustrated in the following Figure 18:
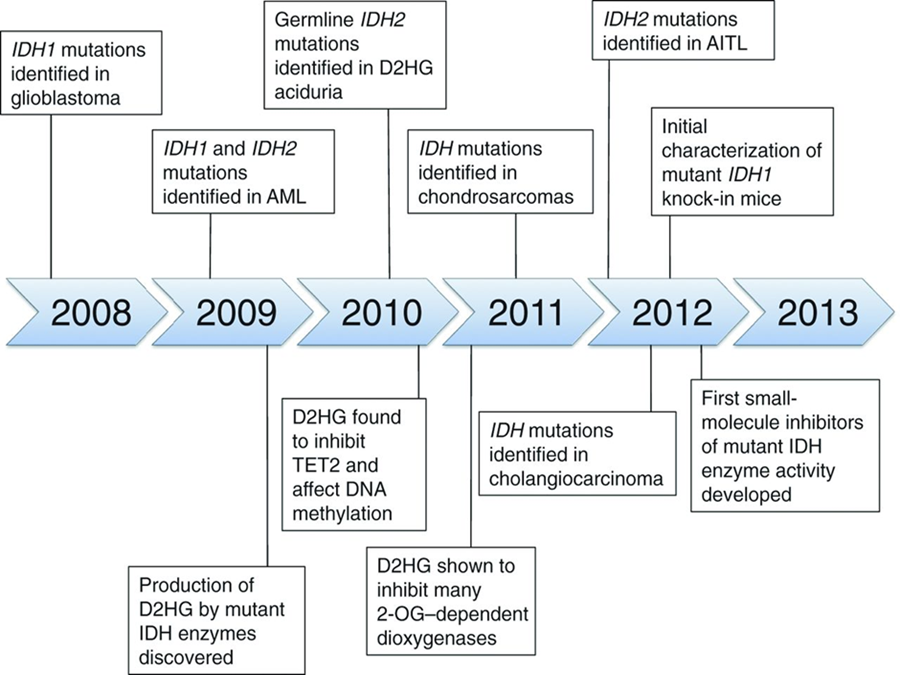
Fig 18: Time line illustrating many of the key clinical and mechanistic discoveries made during the investigation of cancer-associated IDH mutations. (from[66]).
Cancer-associated mutations in IDH1 and IDH2 represent one of the most comprehensively studied mechanisms of IDH pathogenic effect (see Fig19). I
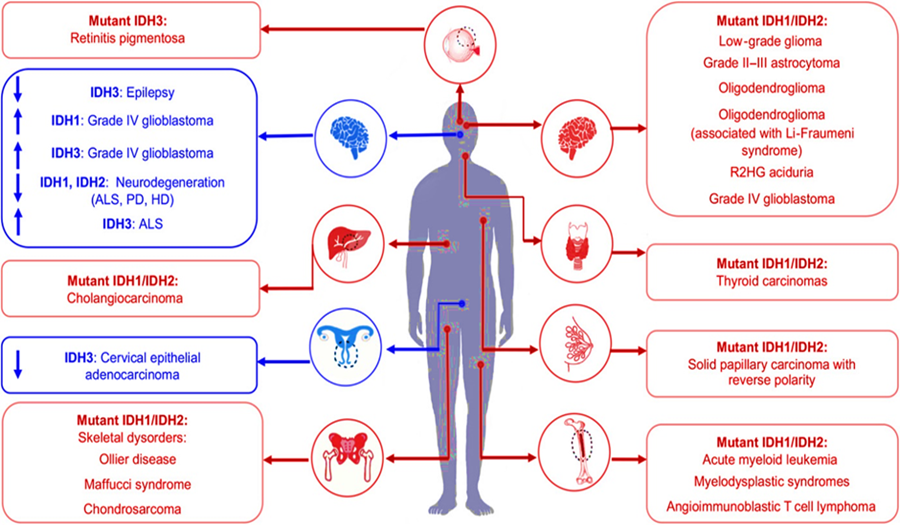
Fig 19: Deregulation of IDH enzymatic activity is associated with human disease. (from[59]) Upward or downward pointing arrows indicate overexpression or downregulation of wild-type IDHs (shown in blue), respectively.
IDH1 and IDH2 are enzymes located in the cytoplasm and mitochondria, respectively. They are involved in cell metabolism and transform decarboxylation of isocitrate to α-KG, resulting in the reduction of NADP+ to NADPH (see Fig.20)
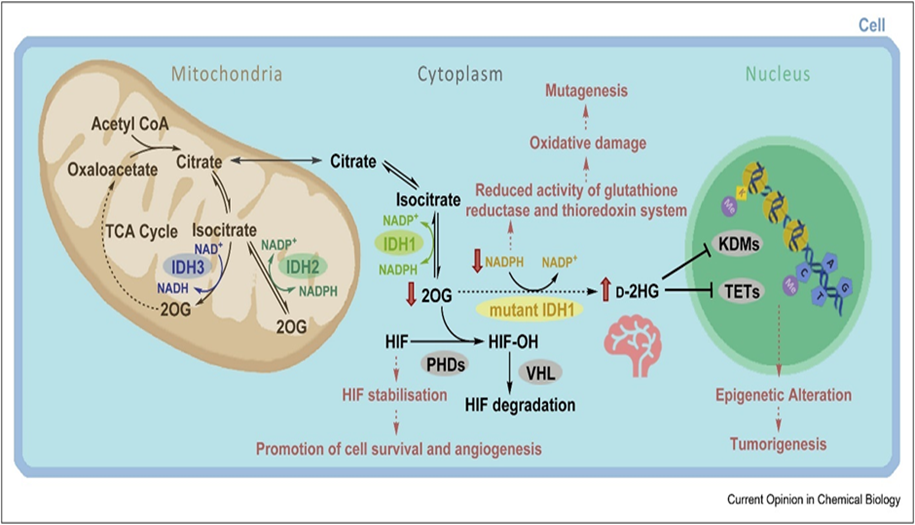
Different subcellular localisations (IDH1: cytoplasm; IDH2/3: mitochondria) and cosubstrate usage (IDH1/2: NADP+; IDH3: NAD+) distinguish the 3 human IDHs. The effects of IDH1 variants, including promotion of tumorigenesis, are proposed to manifest because of metabolic changes including d-2HG accumulation, depletion of NADPH and/or reduced 2OG. Changes in d-2HG/2OG levels are proposed to inhibit 2OG oxygenases involved in regulation of expression, for example, PHD, JmjC KDM, or TET enzymes. 2OG, 2-oxoglutarate; d-2HG, d-2-hydroxyglutarate; IDH, isocitrate dehydrogenase; HIF, hypoxia-inducible factor; HIF–OH, hydroxylated HIF; PHD, HIF prolyl hydroxylase domain enzyme; KDM, histone lysine demethylase; NADP+, oxidised nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; TCA, tricarboxylic acid; TET, ten-eleven translocation oxygenase; VHL, Von Hippel-Lindau; wt, wild-type.( from [67] .
In the presence of mutant IDH1 or IDH2, there is a pathognomic accumulation of the oncometabolite 2-HG[37, 68-70] which can activate cancer-related processes. The product of the novel reaction, 2-HG, is a poorly understood metabolite that does not participate in any known productive metabolic pathway. This oncometabolite is a very reliable biomarker for CCA, because in other gastrointestinal cancers it cannot be detected(from[67] .
R-2-Hydroxyglutarate is found in all tumors with canonical IDH1 and IDH2 mutations. Elevated d-2HG levels serve as a robust biomarker for IDH1/2 mutations in gliomas [26]. d-2HG levels in plasma/serum can be analysed by liquid chromatography-mass spectrometry (LC-MS).[71, 72].
Added to this is the advantage of this marker versus the widespread use of CA19 – 9 in the serum, as its concentration does not depend on the Lewis and secretor genotypes of the blood groups[68, 73].
The spectrum of genetic events associated with cholangiocarcinoma is not completely characterized, but two studies examining mutations in the tumors of several hundred patients with cholangiocarcinoma have been conducted. IDH mutations were identified in 10% to 23% of intrahepatic cholangiocarcinomas, but not in extrahepatic cholangiocarcinomas.
Last year T. B. Karasic et al. gave a very informative and readable overview on “Precision Medicine and Immunotherapy Have Arrived for Cholangiocarcinoma”[13]. In Fig. 21 are all new and emerging targets depicted without taking into account anatomical classification and heterogeneity. This is so important because, for example, the "genetic make-up between iCCA and eCCA is too distinctly different as shown in Fig.21.

BRAF proto-oncogene B-Raf, BTC biliary tract cancer, CDKN2A/B cyclin-dependent kinase inhibitor 2A/B, dMMR/MSI-H deficient mismatch repair/microsatellite instability-high, eCCA extrahepatic cholangiocarcinoma, ERBB erb-b2 receptor tyrosine kinase, FGFR fibroblast growth factor receptor, GBC gallbladder cancer, iCCA intrahepatic cholangiocarcinoma, IDH isocitrate dehydrogenase, KRAS Kirsten rat sarcoma viral oncogene homolog, MET MET proto-oncogene receptor tyrosine kinase, MGMT methylguanine-DNA methyltransferase, mTOR mammalian target of rapamycin, NTRK neurotrophic tyrosine receptor kinase, PI3K phosphoinositide 3-kinase, PRKACA/B protein kinase A/B catalytic subunit, PTEN phosphatase and tensin homolog, TP53 tumor protein P53.
Patients with advanced ECC have low rates of molecular profiling, possibly in part because of insufficient tissue.[4, 74-76] They also have low rates of targeted therapy use and clinical trial enrollment. While these rates are higher in advanced ICC, the prognosis for both subtypes of cholangiocarcinoma remains poor, and a pressing need exists for new effective targeted therapies and broader access to clinical trials[75, 77-80].
The characterization of a targetable mutation must be followed by the development of a drug, preferably orally. For the IDH1 and IDH 2, it was successful until the market approval[81, 82]
Remarkably, non-small cell lung cancer (NSCLC) has become the poster child for a lethal malignancy in which numerous molecular aberrations have become druggable[83, 84]. Similar to NSCLC, there are limited responses in cholangiocarcinoma (CCA) to conventional chemotherapy. Next-generation sequencing has identified novel genomic alterations in CCA that vary between patients and iCC versus eCC( see Fig.15).
The development of specific inhibitors of IDH1 and IDH2 very quickly led to FDA approval on August 25, 2021 for oral treatment with Ivosidenib (Tibvoso) for AML and CCA[81, 82, 85, 86]. On August 25, 2021, the FDA approved ivosidenib for the treatment of adult patients with unresectable locally advanced or metastatic hepatocellular isocitrate dehydrogenase 1 (IDH1) mutated cholangiocarcinoma (CCA) as detected by an FDA-approved test with disease progression after 1 to 2 prior lines of systemic therapy for advanced disease[81]. Ivosidenib was approved for locally advanced or metastatic cholangiocarcinoma with IDH1 mutations based on the results from the ClarIDHy study (JAMA Oncol 2021; doi: 10.1001/jamaoncol.2021.3836). This was an international, randomized, double-blind, Phase III study comparing ivosidenib 500 mg by mouth once daily (n=126) to matched placebo (n=61).[81, 87]. The efficacy analyses were conducted in the intention-to-treat population (ITT), defined as all patients who were randomly assigned to treatment. Kaplan–Meier (K-M) methodology was applied to summarize PFS and OS (see Fig.22 and Fig. 23):
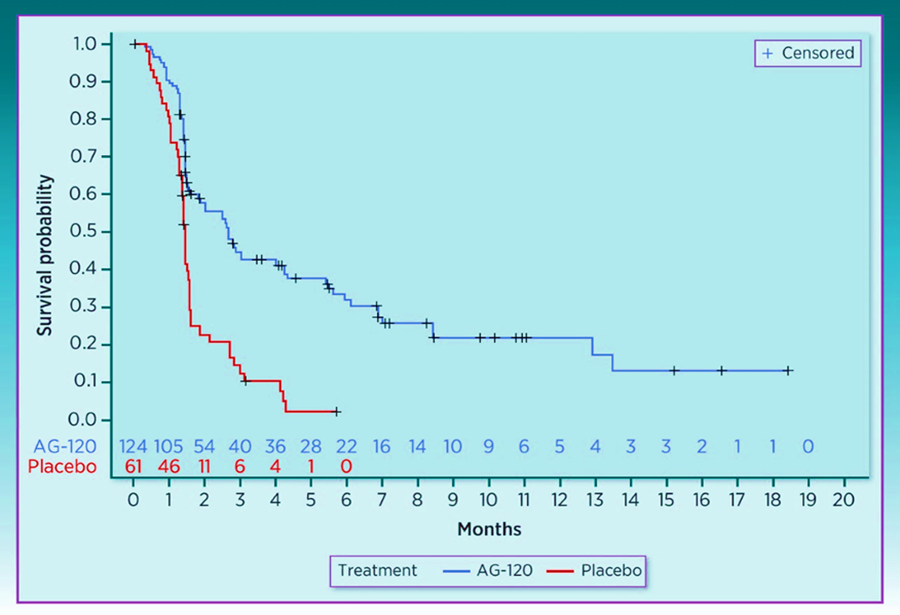

The discovery of mutations in IDH genes (IDH1 and IDH2) has revolutionized the therapeutic approaches and opened a new research way focused on possible targeted therapies capable of inhibiting the aberrant activity of the mutated isoforms. Isocitrate dehydrogenase 2 (IDH2) is a mitochondrial protein which also promotes tumorigenesis via 2-HG through activating mutations in codons 140 and 172. Mutations in IDH2 occur less frequently in ICC (2%-5%) than mutations in IDH1, and the only reported study targeting IDH2 in patients with cholangiocarcinoma enrolled four patients and observed no objective responses with enasidenib [88].
The complexity of cholangiocarcinomas' molecular genomics has opened avenues for improving the outcome for this therapeutically challenging rare disease. The European Society for Medical Oncology (ESMO) Precision Medicine Working Group recommends routine next-generation sequencing in all cholangiocarcinoma patients,[88] given the prevalence of multiple driver alterations that can be matched to standard-of-care therapies or clinical trial recruitment. The ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) can be used to guide patient selection for targeted therapy. Integration of ESCAT into treatment management, notably for intrahepatic cholangiocarcinoma, offers clinicians a valuable tool to expand therapeutic opportunities in a chemotherapy-refractory setting[89].
In the era of precision oncology, molecular tumour boards (MTBs) have an increasingly important role in optimizing treatment selection to improve outcomes by reviewing and interpreting molecular-profiling data and matching patients with appropriate available molecularly targeted therapies, which can include investigational drugs[90].
Nowadays, the identification of targetable mutations is a hot topic in oncologic field, since patients carrying one or more actionable lesions could have broad opportunities for treatment. The identifications of differences in terms of targetable mutations’ incidence between IDH1 m and IDH1 wt patients could suggest novel therapeutic strategies which could be investigated in concomitating and/or in sequencing to the recently studied IDH inhibitors.
The emerging therapies outlined in this review are suitable to reshape the treatment landscape for cholangiocarcinoma in the coming years.
Declarations
Competing interests: I have nothing to declare.
Funding: No Funding.
References
- Alvaro, D., Gores, G. J., Walicki, J., Hassan, C., Sapisochin, G., Komuta, M., ... & Sangro, B. (2023). EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. Journal of hepatology, 79(1), 181-208.
- Rushbrook, S. M., Kendall, T. J., Zen, Y., Albazaz, R., Manoharan, P., Pereira, S. P., ... & Khan, S. A. (2023). British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma. Gut.
- Vogel, A., Bridgewater, J., Edeline, J., Kelley, R. K., Klümpen, H. J., Malka, D., ... & Ducreux, M. (2023). Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Annals of Oncology, 34(2), 127-140.
- Banales, J. M., Marin, J. J., Lamarca, A., Rodrigues, P. M., Khan, S. A., Roberts, L. R., ... & Gores, G. J. (2020). Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nature reviews Gastroenterology & hepatology, 17(9), 557-588.
- Brindley, P. J., Bachini, M., Ilyas, S. I., Khan, S. A., Loukas, A., Sirica, A. E., ... & Gores, G. J. (2021). Cholangiocarcinoma. Nature reviews Disease primers, 7(1), 65.
- Ilyas, S. I., Affo, S., Goyal, L., Lamarca, A., Sapisochin, G., Yang, J. D., & Gores, G. J. (2023). Cholangiocarcinoma—novel biological insights and therapeutic strategies. Nature Reviews Clinical Oncology, 1-17.
- Izquierdo-Sanchez, L., et al., Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. Journal of Hepatology, 2022. 76(5): p. 1109-1121.
- Lamarca, A., Edeline, J., & Goyal, L. (2022). How I treat biliary tract cancer. ESMO open, 7(1), 100378.
- Moris, D., Palta, M., Kim, C., Allen, P. J., Morse, M. A., & Lidsky, M. E. (2023). Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA: a cancer journal for clinicians, 73(2), 198-222.
- Wheless, M., Agarwal, R., Goff, L., Lockney, N., Padmanabhan, C., & Heumann, T. (2024). Current Standards, Multidisciplinary Approaches, and Future Directions in the Management of Extrahepatic Cholangiocarcinoma. Current Treatment Options in Oncology, 1-34.
- Scott, A. J., Sharman, R., & Shroff, R. T. (2022). Precision medicine in biliary tract cancer. Journal of Clinical Oncology, 40(24), 2716-2734.
- Adeva, J., Sangro, B., Salati, M., Edeline, J., La Casta, A., Bittoni, A., ... & Valle, J. W. (2019). Medical treatment for cholangiocarcinoma. Liver International, 39, 123-142.
- Karasic, T. B., Eads, J. R., & Goyal, L. (2023). Precision Medicine and Immunotherapy Have Arrived for Cholangiocarcinoma: An Overview of Recent Approvals and Ongoing Clinical Trials. JCO Precision Oncology, 7, e2200573.
- LaPelusa, M., Heumann, T., Goff, L., & Agarwal, R. (2023). Targeted therapies in advanced biliary tract cancers—a narrative review. Chinese clinical oncology, 12(2), 14.
- Huang, P., Wen, F., Wu, Q., Zhang, P., & Li, Q. (2023). Research trends of targeted therapy for cholangiocarcinoma from 2003 to 2022: a bibliometric and visual analysis. Clinical and Experimental Medicine, 1-14.
- Zabron, A., Edwards, R. J., & Khan, S. A. (2013). The challenge of cholangiocarcinoma: dissecting the molecular mechanisms of an insidious cancer. Disease models & mechanisms, 6(2), 281-292.
- Conway, A. M., Morris, G. C., Smith, S., Vekeria, M., Manoharan, P., Mitchell, C., ... & Cook, N. (2022). Intrahepatic cholangiocarcinoma hidden within cancer of unknown primary. British Journal of Cancer, 127(3), 531-540.
- Valle, J. W., Lamarca, A., Goyal, L., Barriuso, J., & Zhu, A. X. (2017). New horizons for precision medicine in biliary tract cancers. Cancer discovery, 7(9), 943-962.
- Florio, A. A., Ferlay, J., Znaor, A., Ruggieri, D., Alvarez, C. S., Laversanne, M., ... & Petrick, J. L. (2020). Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer, 126(11), 2666-2678.
- Bragazzi, M. C., Ridola, L., Safarikia, S., Di Matteo, S., Costantini, D., Nevi, L., & Cardinale, V. (2018). New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Annals of Gastroenterology, 31(1), 42.
- Vithayathil, M. and S.A. Khan, Current epidemiology of cholangiocarcinoma in Western countries. Journal of Hepatology, 2022. 77(6): p. 1690-1698.
- Qurashi, M., Vithayathil, M., & Khan, S. A. (2023). Epidemiology of cholangiocarcinoma. European Journal of Surgical Oncology, 107064.
- Cai, S. and M. Kuang, Clonorchis sinensis Liver Flukes. New England Journal of Medicine, 2023. 389(26): p. e57.
- Cowzer, D., & Harding, J. J. (2022). Advanced bile duct cancers: a focused review on current and emerging systemic treatments. Cancers, 14(7), 1800.
- Bekaii-Saab, T. S., Bridgewater, J., & Normanno, N. (2021). Practical considerations in screening for genetic alterations in cholangiocarcinoma. Annals of Oncology, 32(9), 1111-1126.
- Farber, S., Diamond, L. K., Mercer, R. D., Sylvester Jr, R. F., & Wolff, J. A. (1948). Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). New England Journal of Medicine, 238(23), 787-793.
- Jones, S., Anagnostou, V., Lytle, K., Parpart-Li, S., Nesselbush, M., Riley, D. R., ... & Velculescu, V. E. (2015). Personalized genomic analyses for cancer mutation discovery and interpretation. Science translational medicine, 7(283), 283ra53-283ra53.
- Schilsky, R. L. (2010). Personalized medicine in oncology: the future is now. Nature reviews Drug discovery, 9(5), 363-366.
- Gambardella, V., Tarazona, N., Cejalvo, J. M., Lombardi, P., Huerta, M., Roselló, S., ... & Cervantes, A. (2020). Personalized medicine: recent progress in cancer therapy. Cancers, 12(4), 1009.
- Karasic, T. B., Eads, J. R., & Goyal, L. (2023). Precision Medicine and Immunotherapy Have Arrived for Cholangiocarcinoma: An Overview of Recent Approvals and Ongoing Clinical Trials. JCO Precision Oncology, 7, e2200573.
- Wang, R. C., & Wang, Z. (2023). Precision medicine: Disease subtyping and tailored treatment. Cancers, 15(15), 3837.
- Tsimberidou, A. M., Fountzilas, E., Nikanjam, M., & Kurzrock, R. (2020). Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer treatment reviews, 86, 102019.
- Muharremi, G., Meçani, R., & Muka, T. (2023). The Buzz Surrounding Precision Medicine: The Imperative of Incorporating It into Evidence-Based Medical Practice. Journal of Personalized Medicine, 14(1), 53.
- Hanahan, D. (2022). Hallmarks of cancer: new dimensions. Cancer discovery, 12(1), 31-46.
- Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. cell, 144(5), 646-674.
- Heuser, M., Cruz, M. M. A., Goparaju, R., & Chaturvedi, A. (2015). Enigmas of IDH mutations in hematology/oncology. Experimental hematology, 43(8), 685-697.
- Liu, Y., Xu, W., Li, M., Yang, Y., Sun, D., Chen, L., ... & Chen, L. (2023). The regulatory mechanisms and inhibitors of isocitrate dehydrogenase 1 in cancer. Acta Pharmaceutica Sinica B.
- Raggi, C., Taddei, M. L., Rae, C., Braconi, C., & Marra, F. (2022). Metabolic reprogramming in cholangiocarcinoma. Journal of hepatology, 77(3), 849-864.
- Ward, P. S., & Thompson, C. B. (2012). Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer cell, 21(3), 297-308.
- Schmidt, D. R., Patel, R., Kirsch, D. G., Lewis, C. A., Vander Heiden, M. G., & Locasale, J. W. (2021). Metabolomics in cancer research and emerging applications in clinical oncology. CA: a cancer journal for clinicians, 71(4), 333-358.
- DeBerardinis, R. J., & Chandel, N. S. (2016). Fundamentals of cancer metabolism. Science advances, 2(5), e1600200.
- Tang, Z., Xu, Z., Zhu, X., & Zhang, J. (2021). New insights into molecules and pathways of cancer metabolism and therapeutic implications. Cancer Communications, 41(1), 16-36.
- Wang, S. F., Tseng, L. M., & Lee, H. C. (2023). Role of mitochondrial alterations in human cancer progression and cancer immunity. Journal of Biomedical Science, 30(1), 61.
- Missiroli, S., Perrone, M., Genovese, I., Pinton, P., & Giorgi, C. (2020). Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. EBioMedicine, 59.
- Pavlova, N. N., & Thompson, C. B. (2016). The emerging hallmarks of cancer metabolism. Cell metabolism, 23(1), 27-47.
- Finley, L.W.S., What is cancer metabolism? Cell, 2023. 186(8): p. 1670-1688.
- DeBerardinis, R. J., & Chandel, N. S. (2020). We need to talk about the Warburg effect. Nature metabolism, 2(2), 127-129.
- Wang, Y. and G.J. Patti, The Warburg effect: a signature of mitochondrial overload. Trends in Cell Biology, 2023. 33(12): p. 1014-1020.
- Vaupel, P., & Multhoff, G. (2021). Revisiting the Warburg effect: Historical dogma versus current understanding. The Journal of physiology, 599(6), 1745-1757.
- Wallace, D. C. (2005, January). Mitochondria and cancer: Warburg addressed. In Cold Spring Harbor symposia on quantitative biology (Vol. 70, pp. 363-374). Cold Spring Harbor Laboratory Press.
- Warburg, O. (1956). On the origin of cancer cells. Science, 123(3191), 309-314.
- Warburg, O., Wind, F., & Negelein, E. (1926). Ueber den stoffwechsel von tumoren im körper. Klinische Wochenschrift, 5(19), 829-832.
- Cassim, S., Vučetić, M., Ždralević, M., & Pouyssegur, J. (2020). Warburg and beyond: the power of mitochondrial metabolism to collaborate or replace fermentative glycolysis in cancer. Cancers, 12(5), 1119.
- Warburg, O. (1925). The metabolism of carcinoma cells. The Journal of Cancer Research, 9(1), 148-163.
- Farshidfar, F., Zheng, S., Gingras, M. C., Newton, Y., Shih, J., Robertson, A. G., ... & Rathmell, W. K. (2017). Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell reports, 18(11), 2780-2794.
- Farshidfar, F., Zheng, S., Gingras, M. C., Newton, Y., Shih, J., Robertson, A. G., ... & Rathmell, W. K. (2017). Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell reports, 18(11), 2780-2794.
- Sjoblom, T., Jones, S., Wood, L. D., Parsons, D. W., Lin, J., Barber, T. D., ... & Velculescu, V. E. (2006). The consensus coding sequences of human breast and colorectal cancers. science, 314(5797), 268-274.
- McKenney, A. S., & Levine, R. L. (2013). Isocitrate dehydrogenase mutations in leukemia. The Journal of Clinical Investigation, 123(9), 3672-3677.
- Tommasini-Ghelfi, S., Murnan, K., Kouri, F. M., Mahajan, A. S., May, J. L., & Stegh, A. H. (2019). Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Science advances, 5(5), eaaw4543.
- Yan, H., Parsons, D. W., Jin, G., McLendon, R., Rasheed, B. A., Yuan, W., ... & Bigner, D. D. (2009). IDH1 and IDH2 mutations in gliomas. New England journal of medicine, 360(8), 765-773.
- Franceschi, E., De Biase, D., Di Nunno, V., Pession, A., Tosoni, A., Gatto, L., ... & Brandes, A. A. (2021). IDH1 non-canonical mutations and survival in patients with glioma. Diagnostics, 11(2), 342.
- Lugowska, I., Teterycz, P., Mikula, M., Kulecka, M., Kluska, A., Balabas, A., ... & Ostrowski, J. (2018). IDH1/2 mutations predict shorter survival in chondrosarcoma. Journal of Cancer, 9(6), 998.
- Testa, U., Castelli, G., & Pelosi, E. (2020). Isocitrate dehydrogenase mutations in myelodysplastic syndromes and in acute myeloid leukemias. Cancers, 12(9), 2427.
- Chaturvedi, A., Araujo Cruz, M. M., Jyotsana, N., Sharma, A., Yun, H., Görlich, K., ... & Heuser, M. (2013). Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood, The Journal of the American Society of Hematology, 122(16), 2877-2887.
- Ghiam, A. F., Cairns, R. A., Thoms, J., Dal Pra, A., Ahmed, O., Meng, A., ... & Bristow, R. G. (2012). IDH mutation status in prostate cancer. Oncogene, 31(33), 3826-3826.
- Cairns, R. A., & Mak, T. W. (2013). Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer discovery, 3(7), 730-741.
- Liu, S., Cadoux-Hudson, T., & Schofield, C. J. (2020). Isocitrate dehydrogenase variants in cancer—Cellular consequences and therapeutic opportunities. Current opinion in chemical biology, 57, 122-134.
- Borger, D. R., Goyal, L., Yau, T., Poon, R. T., Ancukiewicz, M., Deshpande, V., ... & Zhu, A. X. (2014). Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clinical Cancer Research, 20(7), 1884-1890.
- Collins, R. R., Patel, K., Putnam, W. C., Kapur, P., & Rakheja, D. (2017). Oncometabolites: a new paradigm for oncology, metabolism, and the clinical laboratory. Clinical chemistry, 63(12), 1812-1820.
- Yang, M., Soga, T., & Pollard, P. J. (2013). Oncometabolites: linking altered metabolism with cancer. The Journal of clinical investigation, 123(9), 3652-3658.
- Kalinina, J., Carroll, A., Wang, L., Yu, Q., Mancheno, D. E., Wu, S., ... & Van Meir, E. G. (2012). Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. Journal of molecular medicine, 90, 1161-1171.
- Radoul, M., Hong, D., Gillespie, A. M., Najac, C., Viswanath, P., Pieper, R. O., ... & Ronen, S. M. (2021). Early noninvasive metabolic biomarkers of mutant IDH inhibition in glioma. Metabolites, 11(2), 109.
- Vestergaard, E.M., et al., Reference Values and Biological Variation for Tumor Marker CA 19-9 in Serum for Different Lewis and Secretor Genotypes and Evaluation of Secretor and Lewis Genotyping in a Caucasian Population. Clinical Chemistry, 1999. 45(1): p. 54-61.
- Nimish, T., Gonzalez, T., Nano, O., Shin, S. H., Samuels, S., & Hussein, A. M. (2022). Extrahepatic cholangiocarcinoma versus intrahepatic cholangiocarcinoma: A comparison of survival outcomes and use of palliative care in diagnosed patients.
- Ahn, D. H., & Bekaii-Saab, T. (2017). Biliary cancer: intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: classification and therapeutic implications. Journal of gastrointestinal oncology, 8(2), 293.
- Clements, O., Eliahoo, J., Kim, J. U., Taylor-Robinson, S. D., & Khan, S. A. (2020). Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Journal of hepatology, 72(1), 95-103.
- Spencer, K., Pappas, L., Baiev, I., Maurer, J., Bocobo, A. G., Zhang, K., ... & Goyal, L. (2023). Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma. JNCI: Journal of the National Cancer Institute, djad046.
- Esnaola, N. F., Meyer, J. E., Karachristos, A., Maranki, J. L., Camp, E. R., & Denlinger, C. S. (2016). Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer, 122(9), 1349-1369.
- O'Rourke, C. J., Salati, M., Rae, C., Carpino, G., Leslie, H., Pea, A., ... & Braconi, C. (2023). Molecular portraits of patients with intrahepatic cholangiocarcinoma who diverge as rapid progressors or long survivors on chemotherapy. Gut.
- Israel, M. A., Danziger, N., McGregor, K. A., Murugesan, K., Gjoerup, O., Sokol, E. S., ... & Ross, J. S. (2021). Comparative genomic analysis of intrahepatic cholangiocarcinoma: biopsy type, ancestry, and testing patterns. The Oncologist, 26(9), 787-796.
- Casak, S. J., Pradhan, S., Fashoyin-Aje, L. A., Ren, Y., Shen, Y. L., Xu, Y., ... & Lemery, S. J. (2022). FDA Approval Summary: Ivosidenib for the treatment of patients with advanced unresectable or metastatic, chemotherapy refractory cholangiocarcinoma with an IDH1 mutation. Clinical Cancer Research, 28(13), 2733-2737.
- Sproat, M. (2023). Ivosidenib (Tibsovo®). Oncology Times, 45(22), 11.
- Janku, F., Stewart, D. J., & Kurzrock, R. (2010). Targeted therapy in non-small-cell lung cancer—is it becoming a reality?. Nature reviews Clinical oncology, 7(7), 401-414.
- Majeed, U., Manochakian, R., Zhao, Y., & Lou, Y. (2021). Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. Journal of hematology & oncology, 14(1), 1-20.
- Adeva, J. (2022). Current development and future perspective of IDH1 inhibitors in cholangiocarcinoma. Liver Cancer International, 3(1), 17-31.
- Frampton, J. E. (2023). Ivosidenib: A Review in Advanced Cholangiocarcinoma. Targeted Oncology, 18(6), 973-980.
- Zhu, A. X., Macarulla, T., Javle, M. M., Kelley, R. K., Lubner, S. J., Adeva, J., ... & Abou-Alfa, G. K. (2021). Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA oncology, 7(11), 1669-1677.
- Cellgene, Study of Orally Administered Enasidenib (AG-221) in Adults With Advanced Solid Tumors, Including Glioma, or Angioimmunoblastic T-Cell Lymphoma, With an IDH2 Mutation, 2021.
- Stenzinger, A., et al., Molecular profiling in cholangiocarcinoma: A practical guide to next-generation sequencing. Cancer Treatment Reviews, 2024. 122: p. 102649.
- Verdaguer, H., Saurí, T., Acosta, D. A., Guardiola, M., Sierra, A., Hernando, J., ... & Macarulla, T. (2022). ESMO scale for clinical actionability of molecular targets driving targeted treatment in patients with cholangiocarcinoma. Clinical Cancer Research, 28(8), 1662-1671.
- Tsimberidou, A. M., Kahle, M., Vo, H. H., Baysal, M. A., Johnson, A., & Meric-Bernstam, F. (2023). Molecular tumour boards—current and future considerations for precision oncology. Nature Reviews Clinical Oncology, 20(12), 843-863.


