Archive : Article / Volume 2, Issue 2
Case Report | DOI: https://doi.org/10.58489/2836-2187/014
Dietary Probiotic Supplementation; Effects on Fecundity and Relationship with Biometric Parameters of Clarias gariepinus
1Department of Fisheries and Aquaculture, Faculty of Environmental Management, Nigeria Maritime University, P.M.B. 1005, Okerenkoko, Delta State, Nigeria
2Department of Fisheries and Aquaculture, Faculty of Oceanography, University of Calabar, P.M.B. 1115, Calabar, Cross River State, Nigeria
Correspondng Author: Victor Oscar Eyo
Citation: Victor Oscar Eyo, Albert Philip Ekanem, (2023). Dietary Probiotic Supplementation; Effects on Fecundity and Relationship with Biometric Parameters of Clarias gariepinus. Journal of Microbes and Research. 2(2). DOI: 10.58489/2836-2187/014
Copyright: © 2023 Victor Oscar Eyo; this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received Date: 2023-07-22, Received Date: 2023-07-22, Published Date: 2023-08-05
Abstract Keywords: Fecundity; Dietary probiotics supplementation; Fecundity; Relationship; Ovary weight
Abstract
The implications of probiotics in aquaculture including host health, reproductive development, welfare and nutrition has driven the demand for commercial probiotics for use in aquaculture operations either as feed supplements or through direct application. This study was conducted to evaluate the long-term effects of varying dietary amounts of probiotic supplements on C. gariepinus. Five isonitrogenous diets (42% crude protein) were supplemented with probiotics as follows: Feed A (0 g/kg TPS), Feed B (0.5 g/kg TPS), Feed C (1.0 g/kg TPS), Feed D (1.5 g/kg TPS) and Feed E (2 g/kg TPS) and fed to the experimental fishes in triplicate groups for 38 weeks. Results obtained showed that there was no significant difference in mean fecundity between fish fed the control and probiotic-enriched diets (P > 0.05), with diet B containing 0.5 g/kg Taslyprobiotics having the highest fecundity (61,714.00 ± 4,469.43 eggs) and diet C (1.0 g) having the lowest fecundity (49,568.14 ± 3,920.68 eggs). There was a linear relationship between fecundity of Clarias gariepinus with all body parameters such as body weight, ovary weight, and total length and fecundity of female fish.In addition, a positive and significant correlation was obtained between fecundity and all body parameters. Additionally, there was no uniform increase in fecundity with increasing dietary probiotic inclusion level. In conclusion, probiotics supplementation in diets may boost fecundity but increase in probiotic dietary inclusion level will not result in a corresponding increase in fecundity of C. gariepinus.
Introduction
From a global perspective, biotechnologies to enhance fish growth, reproduction and health are increasingly gaining popularity. Recent highlights clearly indicate that the intestinal microbiota plays a crucial role in the growth, well-being, and health of aquatic animals such as fish [1]. Metabolic activities of microbiota enhance energy uptake and nutrients absorption, gastric development promotion, stimulation of epithelial cell differentiation and proliferation, with maintenance of mucosal tolerance and provision of protective functions to combat pathogens [2]. Biotechnology is important in aquaculture because it allows aquaculturists to identify and combine characteristics of fishes to improve yield and quality. Probiotics which are live microorganisms with several beneficial impacts are increasingly utilized to enhance general welfare, growth, treatment of pathogenic diseases and disease prevention in aquaculture [1]. According to the World Health Organization, probiotics are defined as “live microorganisms that confer health benefits on the host when administered in adequate amounts” [3]. In aquaculture, probiotics can be applied as an additive to the fish culture water to improve and enhance quality or by feed supplementation to boost fish growth, optimize health and control diseases [4] and [5]. Probiotics exhibits four modes of action when applied in aquaculture including competitive exclusion of disease causing or pathogenic bacteria, enhancement of physicochemical parameters; enhancement of immunostimulatory response, and host nutrition enhancement [6]. Since fecundity involves the average reproductive attributes of fish, it is an important aspect of aquaculture [7]. Fecundity or the number of eggs in an ovary of a gravid female fish before spawning, is one of the various reproductive traits of fish species. As a result, estimation of fecundity is of great significance in fisheries science as it can be used as an index in elaborating life histories of numerous aquatic species [8]. In addition, fecundity is used to assess fish stocks, egg and larval survival, estimate stock size, predict stock exploitation, and predict fish recruitment [9] and [10]. Fecundity information can be used to estimate the number of fry and fingerlings produced and is an important criterion for aquaculture professionals to consider when selecting new species for aquaculture [11] [12] [13] [14]. Clarias gariepinus is a popular and most preferred farmed fish species in Nigeria [14]. C. gariepinus has proven to be very hardy, disease-resistant, fast-growing, attractive market size, high stocking density, low environmental tolerance, acceptance of artificial diets, and high market value [15], [16], [17]. Furthermore, Eyo et al., [14] have shown that C. gariepinus also exhibits excellent reproductive traits, such as high fecundity, ease of induction or artificial propagation, and rapid gonad maturation in captivity. Currently, Nigeria's aquaculture production costs are increasing due to several factors such as increasing feed costs [18] (Eriegha et al., 2022). Recently, the removal of fuel subsidies by the government of President Bola Tinubu on May 29, 2023 has had a negative impact on all aquaculture products, including feed, as the price of fuel at pumps directly affects the cost of transporting goods and services, which in turn affects the market price of feed. This challenge has led farmers to look for cheaper and viable alternative feeds. Some farmers now formulate farm-made feeds from locally available raw materials to feed their fish. This local and farm-made feed can slow fish growth and degrade water quality. This may be due to the fact that this local or farm-made diet lacks some of the key minerals and nutritional additives that fish need for optimal growth [19]. However, many of these farmers use this farm-made diet to raise fish from the juvenile or juvenile stage to broodstock without considering the impact on fecundity, an important factor to consider when choosing broodstock. Several studies have been documented on the effects of probiotics on fish fertility in several fish species [20], [21] but in some of these studies fish were exposed to probiotics for only a short duration. Therefore, this study was conducted to evaluate the long-term effects of varying dietary amounts of probiotic supplements on C. gariepinus.
Materials and Methods
Experimental feed composition
The composition of the experimental feeds included Tasly probiotics supplement, wheat bran, bonga fish meal (BFM), soybean meal, groundnut cake, sodium chloride, corn flour, calcium, lysine, methionine, vitamin premix, and fish oil.
Probiotics Supplement
Tasly probiotics supplement was purchased from a licensed distributors in Calabar, Cross River State and introduced into the experimental diets as a dietary supplement. Tasly probiotics is composed of five microorganism namely: Lactobacilluscasei, Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis and Streptococus thermophiles.
Experimental Design
This research work was carried out using fifteen (15) tarpaulin ponds of size (100×80×100 cm3). Three hundred and seventy-five (375) juvenile C. gariepinus of average (13.11 ± 2.07 g) were collected from the Hatchery facility of the University of Calabar and used for the study. The 375 juvenile C. gariepinus were randomly stocked in the tarpaulin ponds prior to the start of the feeding trial. The fishes were acclimated for 14 days duration, and managed under optimum environmental conditions including optimal water quality and sufficient feeding to satiation. In this study, five isonitrogenous diets containing 42 % crude protein (Table 1) were formulated with different levels of Tasly probiotics supplement (TPS) using Pearson square method. Feed A contained 0 g/kg TPS, Feed B (0.5 g/kg TPS), Feed C (1.0 g/kg TPS), Feed D (1.5 g/kg TPS) and Feed E (2 g/kg TPS) (Table 1). The experimental feeds were stored in sealed bags at a temperature of 4°C. The method of AOAC [22] was adopted to determine the proximate composition of the feeds (Table 1). Indices analyzed were crude protein (using micro-Kjeldahl N×6.25), crude fibre, lipid, moisture content, ash and nitrogen-free extract which was determined by differential. The juvenile C. gariepinus in the tarpaulin tanks were starved for 24 hours to empty their gastrointestinal tract before starting the feeding experiment. The experimental fishes were fed twice daily at 8:30 am and 5:30 pm at 3% of their body weight. Biometric parameters, including fish weight (g) and length were measured fortnightly using a sensitive scale (Metlar MT-5000D) for weight and measuring board for length according to the protocol of [23] and [17].
Measurement of Physicochemical parameters
The physicochemical parameters measured were ammonia (Nutrifan kit), dissolved oxygen (HI9142 DO meter), pH (multifunction pen type Hanna pH meter), and temperature (mercury in a glass thermometer). These parameters were measured on daily basis and were maintained at optimal levels.
Table 1: Experimental feeds formulations (g/kg) and proximate composition (%) of the diets
| Ingredients | 0.0 g/kg TPS | 0.5 g/kg TPS | 1.0 g/kg TPS | 1.5 g/kg TPS | 2.0 g/kg TPS |
| Tasly probiotics Supplement | 0.00 | 0.50 | 1.00 | 1.50 | 2.00 |
| Wheat bran | 207.10 | 206.60 | 206.10 | 205.60 | 205.10 |
| Fish meal (FM) | 244.30 | 244.30 | 244.30 | 244.30 | 244.30 |
| Soybean meal (SBM) | 244.30 | 244.30 | 244.30 | 244.30 | 244.30 |
| Groundnut meal (GNM) | 244.30 | 244.30 | 244.30 | 244.30 | 244.30 |
| Sodium chloride | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Corn flour | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Calcium | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Lysine | 10.00 | 10.00 | 10.00 | 10.00 | 1.00 |
| Methionine | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Vitamin premix | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Fish oil | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Proximate Composition (%) | |||||
| Crude protein | 41.37± 0.17a | 41.80 ± 015 a | 41.67± 0.18 a | 41.90 ± 0.21 a | 41.77 ± 0.18 a |
| Crude fibre | 7.40 ± 0.05 a | 7.33 ± 0.30 a | 7.53 ± 0.20 a | 7.57 ± 0.09 a | 7.43 ± 0.23 a |
| Lipid | 2.47 ± 0.03 a | 2.60 ± 0.06 a | 2.61 ± 0.04 a | 2.57 ± 0.03 a | 2.60 ± 0.06 a |
| Moisture | 1.89 ± 0.10 a | 1.63 ± 0.09 a | 1.90 ± 0.10 a | 1.65 ± 0.09 a | 1.83 ± 0.09 a |
| Ash | 5.00 ± 0.00 a | 5.67 ± 0.33 a | 5.00 ± 0.00 a | 5.33 ± 0.33 a | 5.33 ± 0.33 a |
| Nitrogen Free Extract | 41.87 ± 0.19 a | 40.97 ± 0.15 a | 41.30 ± 0.17 a | 41.00 ± 0.11 a | 41.03 ± 0.26 a |
*Values represents the mean ± standard error for the triplicate experimental units. Means with the same superscript indicates that values are not significantly different (P > 0.05). TBS = Tasly Probiotics Supplement
Feed conversion parameters of the test feed
Feed indices including feed intake, food conversion ratio, and feed conversion efficiency were calculated according to the methods of De Silva & Anderson [24] as follows:
Feed intake (g) = 3percentage × fish bulk bodyweight × Number of days used in the study
Feed conversion ratio (FCR) =
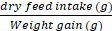
Feed conversion efficiency (percentage FCE) =
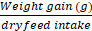
x 100
Fecundity Estimation
The methods of Viveen et al., [25] was used in estimating the fecundity of fish fed the experimental diets. The weight of the egg mass was multiplied by the number of eggs per gram of egg mass. All the females in each treatment were collected and used for fecundity studies. Biometric parameters including total length (TL - cm), total body weight (TW - g) and ovary weight (OW - g) were measured using a Metlar 5000-D electronic weighing balance for weight and measuring board for length. Ovarian sub-samples (1 g) were dissected from different parts of the ovary (posterior, middle and anterior) and preserved in Gilson’s fluid to harden the tissues and facilitation of oocytes isolation and counting. Gilson’s fluid is composed of the following:
i. Nitric acid 17 ml
ii. Glacial acetic acid 4 ml
iii. Mercuric chloride 20g
iv. Ethanol (95 percentage) 70 ml
v. Distilled water 900 ml
Statistical Analysis
Data obtained in this study were analyzed using Predictive Analytical Software (PASW, V19). First, the descriptive statistics of the various parameters were determined, followed by testing the normality and homogeneity of the dataset were tested. A one-way analysis of variance (ANOVA) was performed when the assumption of normality was upheld. Significant differences between treatments were assessed at the probability level of P < 0>
Results
Food utilization indices of experimental fish
Results obtained showed that food conversion ratio (Table 2) was 2.26 ± 0.01 in Feed A (0 g/kg TPS), 2.27 ± 0.003 in Feed B (0.5 g/kg TPS), 2.28 ± 0.003 in Feed C (1.0 g/kg TPS), 2.25 ± 0.05 in Feed D (1.5 g/kg TPS) and 2.37 ± 0.10 in Feed E (2.0 g/kg TPS). Food conversion efficiency was 44.31±0.23 % in Feed A (0 g/kg TPS), 44.13 ± 0.03% in Feed B (0.5 g/kg TPS), 43.75 ± 0.08 % in Feed C (1.0 g/kg TPS), 44.55 ± 0.99 % in Feed D (1.5 g/kg TPS), and 42.41 ±1.89 % in Feed E (2.0 g/kg TPS).
Table 2: Food utilization indices of fish fed the experiment diets
| Indices | Feed A (0 g/kg TPS) | Feed B (0.5 g/kg TPS) | Feed C (1.0 g/kg TPS) | Feed D (1.5 g/kg TPS) | Feed E (2.0 g/kg TPS) |
| Initial weight (g) | 327.33±3.71 | 329.33±4.37 | 326.67±2.73 | 327.00±2.08 | 328.00±3.61 |
| Final weight (g) | 14295.38±20.68 | 14545.00±38.81 | 14776.00±49.91 | 15271.67±318.56 | 15005.00±574.48 |
| Weight gain (g) | 13968.00 ± 21.20a | 14215.67±34.84 a | 14449.33±44.41 a | 14944.67±320.14 a | 14677.00±573.60 a |
| Food consumed (g) | 31527.42±156.70 a | 32217.50±88.20 b | 33031.48±150.24 c | 33543.65±64.46d | 34624.22±185.78e |
| FCR | 2.26± 0.01 a | 2.27± 0.003 a | 2.28±0.003 a | 2.25±0.05 a | 2.37±0.10 a |
| FCE (%) | 44.31±0.23 a | 44.13±0.03 a | 43.75±0.08 a | 44.55±0.99 a | 42.41±1.89 a |
| Protein intake (g) | 13241.52±65.81 a | 13531.35± 37.05 b | 13873.22± 63.10 c | 14088.33 ± 27.08 d | 14542.17± 78.03 e |
| PER | 1.06± 0.01 a | 1.05 ± 0.00 a | 1.04 ± 0.00 a | 1.06 ± 0. 02 a |
|
*Values represent the mean ± standard error for triplicate unit of each treatment. Mean values having the same superscript are not significantly different (P > 0.05)
Mean fecundity of C. gariepinus fed the experimental diets
Results obtained in this study showed that mean fecundity (Table 3) was highest (61,714.00 ± 4469.43 eggs) in fish fed feed B (0.5 g/kg TPS), followed by 54,807.06 ± 4766.92 eggs obtained for fish fed feed E (2.0 g/kg TPS), followed by 51779.82 ± 4225.01 eggs recorded forfish fed feed A (control 0.0 g/kg TPS), followed by 50014.71 ±3564.69 eggs obtained forfish fed feed D (1.5 g/kg TPS) and least (49568.14 ± 3920.68 eggs) in fish fed feed C (1.0 g/kg TPS).
Table 3: Mean fecundity of fish fed the experimental diets
| Number of fish | Range | Mean fecundity |
| Feed A (0 g/kg TPS) | 28 | 24480 – 106568 | 51779.82 ± 4225.01a |
| Feed B (0.5 g/kg TPS) | 35 | 22144 – 113774 | 61714.00 ± 4469.43 a |
| Feed C (1.0 g/kg TPS) | 41 | 19432 – 97995 | 49568.14 ± 3920.68 a |
| Feed D (1.5 g/kg TPS) | 29 | 21952 – 95215 | 50014.71 ±3564.69 a |
| Feed E (2.0 g/kg TPS) | 32 | 21576 – 111252 | 54807.06 ± 4766.92 a |
*Values represent the mean ± standard error for triplicate unit of each treatment. Mean values having the same superscript are not significantly different (P > 0.05)
Relationship between biometric indices and fecundity of fish fed the experimental diets
Results obtained in this study showed a linear relationship between fecundity body parameters (total length, body weight, ovary weight, and mean egg diameter) of fish fed the experimental feeds. Correlation co-efficient (r) obtained in this study revealed that there was a positive significant (P < 0>
Physicochemical Parameters
The mean physicochemical parameters of the tarpaulin ponds (Table 5), including dissolved oxygen, pH, temperature, and ammonia were all within the recommended level for optimal growth and good health of C. gariepinus.
Table 4:Intercept (a), regression coefficient (b) and correlation coefficient (r) in relationship between fecundity of fish fed experimental diets and total length (F/TL)
Relationship | ||||||||
| Feed | Ordinate | Abscissa | N | Intercept (a) | Slope (b) | R | r2 | Inference on rat P = 0.05 |
| Feed A | Fecundity (F) | TL (cm) | 28 | 0.0544 | 3.5903 | 0.7921 | 0.6274 | Significant |
| Feed B | Fecundity (F) | TL (cm) | 35 | 0.0033 | 4.3629 | 0.9044 | 0.8180 | Significant |
| Feed C | Fecundity (F) | TL (cm) | 41 | 0.0658 | 3.5817 | 0.9276 | 0.8604 | Significant |
| Feed D | Fecundity (F) | TL (cm) | 29 | 0.0227 | 3.845 | 0.9008 | 0.8114 | Significant |
| Feed E | Fecundity (F) | TL (cm) | 32 | 0.0340 | 3.7391 | 0.9474 | 0.8975 | Significant |
| Feed A | Fecundity (F) | TW (g) | 28 | 67.982 | 1.0064 | 0.7877 | 0.6205 | Significant |
| Feed B | Fecundity (F) | TW (g) | 35 | 61.378 | 1.3971 | 0.9566 | 0.9151 | Significant |
| Feed C | Fecundity (F) | TW (g) | 41 | 61.741 | 1.0475 | 0.9110 | 0.8300 | Significant |
| Feed D | Fecundity (F) | TW (g) | 29 | 32.380 | 1.1386 | 0.9519 | 0.9062 | Significant |
| Feed E | Fecundity (F) | TW (g) | 32 | 56.243 | 1.0563 | 0.9663 | 0.9338 | Significant |
| Feed A | Fecundity (F) | OW (g) | 28 | 669.62 | 1.0056 | 0.9993 | 0.9988 | Significant |
| Feed B | Fecundity (F) | OW (g) | 35 | 659.84 | 1.0090 | 0.9997 | 0.9995 | Significant |
| Feed C | Fecundity (F) | OW (g) | 41 | 674.94 | 1.0063 | 0.9999 | 0.9999 | Significant |
| Feed D | Fecundity (F) | OW (g) | 29 | 661.61 | 1.0110 | 0.9998 | 0.9997 | Significant |
| Feed E | Fecundity (F) | OW (g) | 32 | 674.23 | 1.0058 | 0.9999 | 0.9998 | Significant |
TW = Total weight (g), TL = Total length (cm), MED = Mean egg diameter (mm), OW = Ovary weight (g). Value for Pearson’s product moment correlation for df = 27 is 0.367, 34 (0.325), 40 (0.304), 28 (0.361) and 31 (0.349), P = 0.05.
Table 5:Mean physicochemical parameters of the tarpaulin ponds
| Parameters | Tarpaulin A (0.0 g/kgTPS) | Tarpaulin B (0.5 g/kgTPS) | Tarpaulin C (1.0 g/kgTPS) | Tarpaulin D (1.5 g/kgTPS) | Tarpaulin A (2.0 g/kgTPS) |
| Mean Ammonia (mg/L) | 0.13 ± 0.01a | 0.13 ± 001 a | 0.14 ± 0.01 a | 0.14 ± 0.02 a | 0.14 ± 0.01 a |
| Mean DO (mg/L) | 4.87 ± 0.15a | 4.76 ± 0.02 a | 4.72 ± 0.02 a | 4.75 ± 0.03 a | 4.74 ± 0.19 a |
| Mean Ph | 6.94 ± 0.05a | 7.00 ± 0.04 a | 6.95 ± 0.12 a | 6.93 ± 010 a | 6.93 ± 0.19 a |
| Mean Tempt (°C) | 29.98 ± 0.33a | 29.98 ± 0.44 a | 29.98 ± 0.04 a | 30.00 ± 0.03 a | 30.00 ± 0.04 a |
*Values represents the mean ± standard error for the triplicate experimental units. The same superscript indicates that mean values are not significantly different (P > 0.05)
Discussion
Fecundity which is the number of eggs in the ovary of a gravid female fish is a very crucial area of fish culture system due to its relationship to fish reproductive characteristics [13]. The results of this study suggest that egg size and fecundity may be affected by feed quality in C. gariepinus. There was no significant difference in mean fecundity between fish fed the control and probiotic-enriched diets (P > 0.05), with diet B containing 0.5 g/kg Tasly probiotics having the highest fecundity (61,714.00 ± 4,469.43 eggs) and diet C (1.0 g) having the lowest fecundity (49,568.14 ± 3,920.68 eggs). More recently, Eyo et al., [13] documented that fecundity of C. gariepinus could be greatly influenced by feed quality. Positive effects of probiotics (Lactobacillus rhamnosus) on fecundity have been reported in zebra fish [20] and [26]. Similarly, studies of Qin et al., [27] found that a probiotic strain (Lactobacillus rhamnosu) enhanced fecundity and oocyte maturation. According to Qin et al., [27] , continuous feeding of probiotic-enriched diets may maintain higher reproductive performance of female parent fish. Qin et al., [27] observed that if probiotics supplemented feed was withdrawn in zebra fish, the beneficial effects of probiotics quickly disappeared, when probiotic-rich diets were discontinued in zebrafish, regardless of whether the probiotic strain used was an adherent or non-adherent strain. However, an insignificant variation (P > 0.05) in the fecundity of fish fed all the diets was obtained and the reason could be ascribed to the nutritional qualities of all the five diets. To Shim et al., [28] , the composition and quantity of dietary protein utilized by fish are known to influence fecundity. Fecundity results obtained in this research work could be ascribed to the proximate characteristics of the experimental feeds which showed that crude protein, moisture, crude fibre, crude fat, ash and Nitrogen free extract of the five experimental diets were within the recommended range by NRC [29] for optimal growth and health of the African Catfish (C. gariepinus). Findings of this study is similar to results of Ekanem et al., [12] who demonstrated the effects of Unical aqua feed and Coppens feed on C. gariepinus reared in ponds. Findings of this study are consistent with those of Ekanem et al., [12] and Eyo et al., [13], wherein utilization of feed properly resulted in gonad growth and higher fecundity of C. gariepinus. However, non-significant difference (P > 0.05) was observed in fecundity of fish fed all diets, likely due to the nutritional value of all five diets. According to Shim et al., [28], it is known that the composition and amount of dietary protein ingested by fish affects fecundity. The fecundity results obtained in this study were traceable to the direct characterization of the experimental diets, showing that the crude protein, moisture, crude fiber, crude fat, ash and nitrogen free extracts of the five experimental diets were within the ranges recommended by [29] for optimal growth and health of African catfish (C. gariepinus). The results of this study are similar to those of Ekanem et al., [12] who demonstrated the effects of unical aqua and coppen feeds on C. gariepinus grown in ponds. The results of this study are consistent with those of Ekanem et al., [12] and Eyo et al., [12] who agreed that appropriate use of food resulted in improved gonadal growth and fertility in C. gariepinus. However, the significant difference (P>0.05) obtained for feed conversion rate (FCR) and feed conversion efficiency (FCE) of experimental fish in this study may explain the non-significant variation in fecundity (P>0.05). The present study found that fish of similar body size differed in ovary size, weight, and fecundity. These results are similar to those of Ekanem et al., [12] who recorded the same results with fish reared in ponds on Unical and Coppens diets. Musa & Bhuiyan [30] also studied Mystus bleekeri collected from the Padma River near the town of Rajshahi. Similarly, differences in fish ovary weight and fecundity according to fish size have been reported for the endangered fish species of Tripua (Ompok pabo) [31], Carassius Carassius from Kashmirdal Lake [32], and Puntius sophore from Upper Assam [33]. In this study, fecundity and ovary weight of all fed fish were affected by fish body size relative to body length and body weight. Females with the highest ovarian weight and fecundity were not found to be the biggest females in each treatment. For example, in fish diet A (control diet), the largest fish with a total weight of 1108 g and a total length of 53.6 cm had an ovary weight of 150 g and an egg count of 102,750, whereas the fish with the largest ovary weight (154) had an ovary weight of 150 g. g) and fecundity (106,568 eggs) had a total weight of 1068 and a total length of 51.7 cm. Similar observations were made in other fed fish [12] and [13], which agrees to the results of other authors. The fecundity of fish species has been reported to increase with the square of their length [32]. The results of this study show that there is a linear relationship between all body parameters such as body weight, ovary weight, average egg diameter and total length and fecundity of female fish. These results are consistent with findings of Shaheena [32] who reported a linear relationship between fish length and fecundity. Similar results have been reported for various fish species by other authors [33] and [34]. The correlation coefficients (r) determined in this study showed that fishes fed different experimental diets had positive significance values (P < 0>C. gariepinus on other diets [12] and [13]. External factors such as food availability and environmental parameters such as pH, ammonia levels, dissolved oxygen levels and temperature levels. They have been observed to affect fish fertility [31]. The range of physicochemical parameters (water temperature, dissolved oxygen level, pH, and ammonia level) measured in the tarpaulin ponds in this study did not vary significantly (P > 0.05) and were within recommended tolerances for optimal growth and good health of freshwater fish [35]. This implies that physicochemical parameters did not impose any negative influence on the fecundity of fish in the different experimental groups.
Summary and Conclusion
In summary, dietary probiotic supplementation in the diets did not show any negative impact on the fecundity of C. gariepinus. Mean fecundity of fish fed the control diet did not differ significantly from fecundity of fish fed probiotics supplemented diets. There was a linear relationship and positive significant correlation between fecundity of C. gariepinus fed the five experimental diets with body parameters such as ovary weight, total length, body weight, and mean egg diameter. In addition, there was no uniform increase in fecundity with increasing dietary probiotic inclusion level. In conclusion, probiotics supplementation in diets may boost fecundity but increase in probiotic dietary inclusion level will not result in a corresponding increase in fecundity of C. gariepinus.
References
- Lauzon, H. L., Dimitroglou, A., Merrifield, D. L., Ringø, E., & Davies, S. J. (2014). Probiotics and prebiotics: concepts, definitions and history. Aquaculture nutrition: Gut health, probiotics and prebiotics, 169-184.
- Guarner, F., & Malagelada, J. R. (2003). Gut flora in health and disease. The lancet, 361(9356), 512-519.
- FAO/WHO. (2001). Food and Agriculture Organization of the United Nations, World Health Organization. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food.
- Lara-Flores, M., Olvera-Novoa, M. A., Guzmán-Méndez, B. E., & López-Madrid, W. (2003). Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture, 216(1-4), 193-201.
- Moriarty, D. J. (1999). Disease control in shrimp aquaculture with probiotic bacteria. In Proceedings of the 8th international symposium on microbial ecology (pp. 237-243). Atlantic Canada Society for Microbial Ecology, Halifax, Canada.
- Verschuere, L., Rombaut, G., Sorgeloos, P., & Verstraete, W. (2000). Probiotic bacteria as biological control agents in aquaculture. Microbiology and molecular biology reviews, 64(4), 655-671.
- Eyo, V. O., Ekanem, A. P., & Jimmy, U. I. U. (2014). A comparative study of the gonado-somatic index (GSI) and gonad gross morphology of African catfish (Clarias gariepinus) fed unical aqua feed and coppens commercial feed. Croatian Journal of Fisheries, 72(2), 63-69.
- Hunter, J. R., Goldberg, P. (1980). Spawning incidence and batch fecundity in northern anchorvy, Engraulis mordax. Fish Bulletin, 77, 641–652.
- Mayer, I., Shackley, S. E., & Witthames, P. R. (1990). Aspects of the reproductive biology of the bass, Dicentrarchus labrax L. II. Fecundity and pattern of oocyte development. Journal of Fish Biology, 36(2), 141-148.
- Shalloof, K. A. S., & Salama, H. M. (2008). Investigations on some aspects of reproductive biology in Oreochromis niloticus (Linnaeus, 1757) inhabited Abu-zabal Lake, Egypt. Global Veterinaria, 2(6), 351-359.
- Akanse, N.N., Eyo, V. O. (2018). Length-weight Relationship, Condition Factor and Length Frequency Distribution of the Tongue Sole Cynoglossus senegalensis from AkpaYafe River, Bakassi, Cross River State, Nigeria. Asian Journal of Advances in Agricultural Research, 6, 1–8.
- Ekanem, A. P., Eyo, V. O., James, P. U., & Udo, N. E. (2013). Effects of unical feed on fecundity and gonad development of Clarias gariepinus; A comparative study with Coppens commercial feed in earthen pond. International Journal of Science and research, 2(10), 8-14.
- Eyo, V. O., Ekanem, A. P., & Ajom, V. A. (2016). Fecundity studies of the African catfish Clarias gariepinus (Burchell, 1822) fed Coppens feed and Unical aqua feed in circular concrete tanks. Journal of Coastal Life Medicine, 4(7), 531-535.
- Eyo, V. O., Eze, F., & Eriegha, O. J. (2021). Reproductive performance of hatchery-bred, wild-caught broodstock, and their outbreed of the African Catfish Clarias gariepinus (Burchell, 1822). Indonesian Aquaculture Journal, 15(2), 59-65.
- Arong, G., & Eyo, V. (2017). Evaluation of house fly (Musca domestica) maggot meal and termite (Macrotermes subhyalinus) meal as supplementary feed for African catfish Clarias gariepinus (Burchell, 1822). International Journal of Entomology and Nematology, 3(1), 42–50.
- Emeka, A. I., & Oscar, E. V. (2016). Comparative study of growth performance, food utilization and survival of the African Catfish Clarias gariepinus (Burchell, 1822) fingerlings fed live maggot (Musca domestica) and coppens commercial feed. International Journal of Scientific Research in Science, Engineering and Technology, 2(2), 379-386.
- Oscar, E. V., Mfon, A. M., & Solomon, U. I. (2015). Some aspects of the biology of the female blue crab Callinectes amnicola (De Rocheburne) from the Cross River estuary, Nigeria. Journal of Coastal Life Medicine, 3(4), 259-264.
- Eriegha, O. J., Ekelemu, J. K., Eyo, V. O. (2022). The drivers and impacts of inflation on fish production in Nigeria. In R. A. Sundaray, J. K., Bhat (Ed.), Research Trends in Fisheries and Aquatic Sciences (pp. 103 – 136). AkiNik Publications.
- Eyo, V. O. (2017). Effects of Probiotics on the growth performance, fecundity and gonadal development of the African catfish Clarias gariepinus (Burchell 1822) under aquaculture conditions., Ph.D Thesis, University of Calabar, Nigeria.
- Gioacchini, G., Giorgini, E., Ferraris, P., Tosi, G., Bizzaro, D., & Silvi, S. (2010). Could probiotics improve fecundity? Danio rerio as case of study. Journal of Biotechnology, (150), 59-60.
- Gioacchini, G., Maradonna, F., Lombardo, F., Bizzaro, D., Olivotto, I., & Carnevali, O. (2010). Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction (Cambridge, England), 140(6), 953-959.
- AOAC (2005). Official method of analysis of the AOAC (Horwitz E (ed.); 18th ed.).
- Eyo, V. O., & Ekanem, A. P. (2011). Effect of feeding frequency on the growth, food utilization and survival of African catfish (Clarias gariepinus) using locally formulated diet. African journal of environmental pollution and health, 9(2), 11-17.
- De Silva, S. S., & Anderson, T. A. (1995). Fish nutrition in aquaculture. Chapman and Hall.
- Viveen, W. A. R., Richter, C. J. J., Van Oordt, P. G. W. J., Janssen, J. A. L., & Huisman, E. A. (1985). Practical manual for the culture of the African catfish (Clarias gariepinus).
- Gioacchini, G., Giorgini, E., Merrifield, D. L., Hardiman, G., Borini, A., Vaccari, L., & Carnevali, O. (2012). Probiotics can induce follicle maturational competence: the Danio rerio case. Biology of reproduction, 86(3), 65-1.
- Qin, C., Xu, L., Yang, Y., He, S., Dai, Y., Zhao, H., & Zhou, Z. (2014). Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction, 147(1), 53-64.
- Shim, K. F., Landesman, L., & Lam, T. J. (1989). Effect of dietary protein on growth, ovarian development and fecundity in the dwarf gourami Colisa lalia (Hamilton). Journal of Tropical Aquaculture, 4, 111–123.
- NRC. (1983). Nutrients requirements of warm water fishes and shell-fishes. (1st ed.). National Academy Press.
- Musa, A. S. M., & Bhuiyan, A. S. (2007). Fecundity on Mystus bleekeri (Day, 1877) from the River Padma near Rajshahi city. Turkish Journal of Fisheries and Aquatic Sciences, 7(2).
- Bhattacharya, P., Banik, S. (2015). Study of fecundity of Ompok pabo (Hamilton, 1822) an endangered fish species of Tripua. Indian Journal of Fisheries and Livestock Production, 3, 153–158.
- Shafi, S. (2012). Study on fecundity and GSI of Carassius carassius (Linneaus, 1758-introduced) from Dal Lake Kashmir. Journal of Biology, Agriculture and Healthcare, 2(3), 68-75.
- Phukon, H. K., Biswas, S. P. (2012). Observation on the maturity index and fecundity of Puntius sophore (Ham-Buch) from upper Assam. Asian Journal of Biology and Experimental Biological Science, 3(1), 247–250.
- Arup, B., Goswami, M. M. (2014). Relationship of Fecundity and different body parameters of Clarias magur (Hamilton, 1822) in captive condition in the Agro climatic condition of Assam, India. IOSR Journal of Agriculture and Veterinary Science, 3(1), 44 – 50.
- Boyd, C. E. (1979). Water quality in warmwater fish ponds. Auburn University Agriculture Experiment Station, Auburn, Alabama.


