Current Issue : Article / Volume 3, Issue 1
- Case Report | DOI:
- https://doi.org/10.58489/2836-2187/020
Pet-Related Pasteurella Multocida-Induced Peritonitis in Peritoneal Dialysis: A Case Report
- Charles Nicolle Hospital, Laboratory of Microbiology, National Reference Lab on AMR surveillance, 1006, Tunis, Tunisia
- University of Tunis El Manar, Faculty of Medicine of Tunis, LR99ES09, Research Laboratory, «Antimicrobial Resistance », 1007, Tunis, Tunisia
- Charles Nicolle Hospital, Department of Internal Medicine A, 1006, Tunis, Tunisia
Ahmed Fakhfakh
Ahmed Fakhfakh, et.al., (2024). Pet-Related Pasteurella Multocida-Induced Peritonitis in Peritoneal Dialysis: A Case Report. Journal of Microbes and Research. 3(1); DOI: 10.58489/2836-2187/020
© 2024 Ahmed Fakhfakh, this is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Received Date: 16-04-2024
- Accepted Date: 23-04-2024
- Published Date: 26-04-2024
Abstract
Pasteurella multocida is a small, Gram-negative cocco-bacillus usually found in the oral cavities of most healthy cats and dogs as part of their natural oral flora. This zoonotic pathogen can cause a variety of infections in humans. The number of reported cases of peritoneal dialysis-associated peritonitis caused by P. multocida has been limited worldwide.
We report here the case of a 38-year-old woman on continuous peritoneal dialysis (PD) that presented to the emergency department with abdominal pain and vomiting lasting for two days. A sample of peritoneal dialysis fluid was explored in the microbiology laboratory where grown microorganisms was identified by the VITEK2 system as P. canis with 90% accuracy.
Then whole genome sequencing concluded that the species is rather P. multocida and that the strain was sensitive to all antibiotics tested and harbored various virulence genes. Patient was treated by intraperitoneal antibiotic course with cefotaxime for 3 weeks.
Introduction
Peritoneal dialysis (PD) is a renal replacement therapy (RRT) modality that is part of the integrated care of end-stage renal disease (ESRD). Currently, automated PD (APD) experiences a large extent due to its convenience and lower cost. Additionally, it offers effective social and occupational integration since it can be performed at home and does not require travel to hospitals or outpatient dialysis units [1, 2]. The success of PD is limited by the occurrence of peritonitis, which is the primary cause of hospitalizations in PD patients. The main pathogens associated with peritoneal dialysis-associated peritonitis (PD peritonitis) are Gram-positive cocci, Staphylococcus aureus, and S. epidermidis, which account for 42% of cases [3]. Gram-negative organisms are less frequently detected, representing 19% of cases [1, 3]. Other microorganisms were described as causing PD peritonitis, such as Pasteurella multocida, with an increasing incidence during the last few years, especially in patients with indoor pets [4]. We describe herein the case of a 38-year-old woman receiving APD who developed peritonitis related to P. multocida, initially diagnosed as Pasteurella canis by the VITEK2 system but later confirmed as Pasteurella multicidia using whole genome sequencing.
Case report
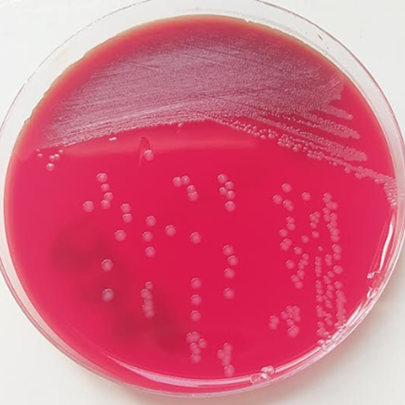
A 38-year-old woman, undergoing APD for 3 years because of ESRD related to familial nephropathy, presented to the emergency department with abdominal pain and vomiting lasting for two days. Physical examination revealed a temperature of 38.3°C, a blood pressure of 140/100 mmHg, a heart rate of 72 beats/min, a soiled PD catheter dressing and cloudy PD effluent. Serum chemistry analysis showed the following findings: sodium 134 mmol/L, potassium 4.1 mmol/L, chloride 96 mmol/L, creatinine 988 µmol/L, and C-reactive protein 287.2 mg/L. A complete blood count revealed a white blood cell (WBC) of 4.5 × 103/mm3 with 80% neutrophils, hemoglobin of 10.1 g/dL, hematocrit of 30.6%, and platelet count of 201 × 103/μL. An abdominal CT scan showed low-abundance peritoneal effusion. Patient was diagnosed with peritonitis, and empiric intraperitoneal vancomycin treatment was initiated after taking a sample of peritoneal fluid for bacteriological examination. Initial microbiological examination showed purulent peritoneal fluid with 80% PNN, and Gram staining was negative. The sample was inoculated on thyoglycolate broth, ordinary agar, blood agar (COH), chocolate + polyvitex (PVX) agar, Chapman medium, and deoxycholate lactose agar (GDL). These media were incubated at 37°C in an aerobic atmosphere with 5% CO2 for the blood media (COH and PVX). Grown colonies were non-hemolytic, 1-1.5 mm in diameter, smooth, slightly raised, and grayish-yellow after 24 hours of incubation (Figure 1).
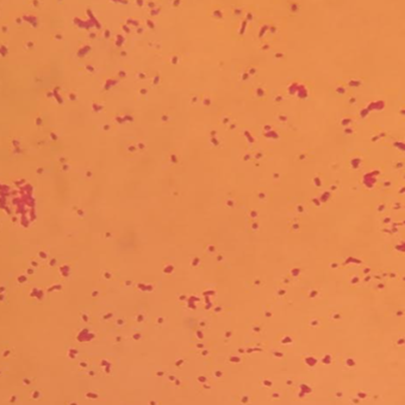
After COH Gram staining, microscopic examination found Gram-negative capsulated and not sporulated coccobacilli (Figure 2).
In view of the VITEK2 system (BioMérieux, Marcy-l'Étoile, France) allowed us to identify grown microorganisms as P. canis with an accuracy of 90%. Isolated bacteria were tested sensitive to Amoxicillin + Clavulanic Acid, Tetracycline, Ciprofloxacin, Nalidixic Acid, Cefotaxime, and Levofloxacin.
Laboratory findings, further patient anamnesis revealed a close and affectionate relationship with her cat, adopted one month ago. The diagnosis of pasteurellosis was made, and an intraperitoneal antibiotic course with cefotaxime for 3 weeks resulted in a good outcome. There was a rapid resolution of abdominal pain with a negative DP effluent culture.
We continued further investigations with a molecular diagnosis after the isolation of the genomic DNA according to the protocol of the manufacturer of the QIAamp DNA Mini Kit (Qiagen, Hilden - Germany). DNA preparation kit (Illumina, San Diego, CA, USA) was used for library preparation. Whole genome sequencing was performed on an iSeq100™ instrument (Illumina, San Diego, CA, USA) with matching reagent kit v2 chemistry (300 cycles). Raw sequencing reads were assembled using the Spades component (SPAdes version 3). Molecular analysis concluded that the species is P. multocida and that the bacterial genome does not contain a plasmid or resistance genes, serotype D: 3, belonged to clone ST283. however it harbored various virulence genes fur, iscA, pasT, pe, pilQ, sodA, sodC, tdeA, tdhA, crp and ompA. GenBank accession number: JAWZSE000000000
Discussion
P. multocida, described for the first time in 1880 by Louis Pasteur [3], as a species of the Pasteurella genus that consists of Gram-negative, non-motile coccobacilli. It is a component of cats' and dogs' commensal flora [5, 6]. Pasteurella genus contains nine species; only four of these species (P. multocida, P. dagmatis, P. canis, and P. stomatis) are implicated in human pathology [7, 8].
In humans, these zoonotic pathogens can cause a variety of infections through bites, scratches, or licking. Infections range from less severe cases, such as infected animal bites, to more severe cases, sepsis, and meningitis [1, 9]. In addition to these common presentations, P. multocida has been reported to cause peritonitis in patients undergoing PD, especially those with close contact with indoor pets. Microorganisms could be transmitted through contaminated machines when the animal is playing or resting on them, contaminated lines, or patient hands that could be touched or licked before or during PD preparation or processing [3]. The count of peripheral white blood cells may be normal or high with a dominance of polymorphonuclear leukocytes, and the peritoneal dialysate WBC count is usually very high [10], as it was observed in our patient. In all cases, presentation includes fever, severe abdominal pain, occasional nausea and vomiting, in addition to cloudy dialysate effluent [1]. Therefore, it is crucial that the patient's anamnesis specify any potential contact with pets in order to educate them regarding the risk of close intimate contact with domestic animals. Such preventive approaches should be introduced into our daily practice with universal hygiene measures to ensure avoiding peritonitis. PD peritonitis could lead to peritoneal membrane failure which needs to stop PD and switching to hemodialysis [2].
The VITEK 2 GN is validated as an automated method for the rapid identification of Gram-negative bacteria at the genus level but remains inaccurate at the species level [11]. The sensitivity of VITEK 2 GN was evaluated at 97.4% in previously published studies [11, 12]. Despite the good sensitivity of VTEK2 GN, the right diagnosis of causal microorganism specie was identified using whole genome sequencing, which, despite being very expensive and not accessible in all laboratories, remains the reference diagnosis method.
Empirical antibiotic therapy should be started as early as possible using intraperitoneal and oral or intravenous routes [13]. Penicillin, ampicillin, ampicillin-sulbactam, amoxicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam, and carbapenem are generally effective against Pasteurella infections, according to findings from clinical and in vitro studies [9, 14, 15].
However, because of their lack of efficaciousness, antistaphylococcal penicillins such dicloxacillin and oxacillin should not be used. While second- and third-generation cephalosporins show good in vitro activity and are thought to be effective penicillin alternatives, especially in patients allergic to penicillin, first-generation cephalosporins should also be avoided [9, 14, 15].
It is currently advised to administer intraperitoneal antibiotics for duration of three weeks [10]. In our patient, intraperitoneal vancomycin was initially started as per our empirical protocol to manage PD peritonitis. It was subsequently switched to cefotaxime, given intravenously, on the basis of the antibiogram findings. This therapeutic regimen resulted in a good outcome with complete resolution of clinical symptoms and biological abnormalities.
Conclusion
The present case highlights the risk of zoonotic infections contracted by pets in patients undergoing PD. Where appropriate, emphasis should be placed on educating these patients on the proper handling of home PD equipment.
References
- Mirzai, S., Rifai, A. O., Tidrick, A., Huang, Q., & Hale, J. (2019). A case report on Pasteurella multocida peritoneal dialysis-associated peritonitis: when cats think medical equipment are toys. Case Reports in Nephrology, 2019.
- Nishina, M., Yanagi, H., Koizumi, M., Kimura, M., Kakuta, T., Endoh, M., ... & Takagi, A. (2012). Pasteurella multocida peritonitis associated with a cat in a peritoneal dialysis patient using an automated cycler device. CEN case reports, 1, 73-76.
- Dresselaars, H. F., Zwart, B., Pettersson, A. M., Rijnsburger, M. C., & Ho-dac-Pannekeet, M. M. (2014). Peritoneal dialysis-associated peritonitis of zoonotic origin, when minor gets major. Neth J Med, 72(10), 551-3.
- Mu, H., Yang, M., Zhang, Y., Zhang, Y., Wang, J., Yuan, W., & Rong, S. (2020). Pet-related Pasteurella multocida induced peritonitis in peritoneal dialysis: a case report and review of the literatures. BMC nephrology, 21, 1-8.
- Fernández-Vecilla, D., Aspichueta-Vivanco, C., & de Tuesta-del, J. L. D. (2022). Cellulitis due to Pasteurella stomatis and Actinomyces canis following dog bite. Revista Española de Quimioterapia, 35(6), 584.
- Sinnott-Stutzman V (2023), Chapter Title, In Sykes E (Ed.), Greene's Infectious Diseases of the Dog and Cat (5th Ed., pp:), Saunders, USA,
- Christensen H, Bisgaard M. (2006). The Genus Pasteurella. In: Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, KH., Stackebrandt, E. (Eds.), The Prokaryotes. Springer, New York, NY.
- https://www.sfm-microbiologie.org/wp content/uploads/2019/07/BACTERIE_Pasteurella.pdf
- Giacona, J. M., Weiner, M., Hanna, J., Jodlowski, T., Bedimo, R., Giacona, J., ... & Hanna, J. J. (2022). Pasteurella multocida Bacteremia Secondary to Peritoneal Dialysis Associated Peritonitis: A Case Report and Literature Review. Cureus, 14(4).
- Rondon-Berrios, H., & Trevejo-Nunez, G. J. (2010). Pets or pest: peritoneal dialysis-related peritonitis due to Pasteurella multocida. Journal of Microbiology, Immunology and Infection, 43(2), 155-158.
- Crowley, E., Bird, P., Fisher, K., Goetz, K., Boyle, M., Benzinger, Jr, M. J., ... & Johnson, R. (2012). Evaluation of the VITEK 2 Gram-negative (GN) microbial identification test card: collaborative study. Journal of AOAC International, 95(3), 778-785.
- Renaud, F. N. R., Bergeron, E., Tigaud, S., Fuhrmann, C., Gravagna, B., & Freney, J. (2005). Evaluation of the new Vitek 2 GN card for the identification of gram-negative bacilli frequently encountered in clinical laboratories. European Journal of Clinical Microbiology and Infectious Diseases, 24, 671-676.
- Liakopoulos, V., Nikitidou, O., Kalathas, T., Roumeliotis, S., Salmas, M., & Eleftheriadis, T. (2017). Peritoneal dialysis-related infections recommendations: 2016 update. What is new?. International urology and nephrology, 49, 2177-2184.
- Chiang AD, Zurlo JJ (2020). Pasteurella species. In Bennett JE, Dolin R, Blaser MJ (Eds.), Mandell Douglas and Bennett's principles and practice of infectious diseases (pp.:2774-8). Elsevier, Philadelphia.
- Lion, C., Conroy, M. C., Carpentier, A. M., & Lozniewski, A. (2006). Antimicrobial susceptibilities of Pasteurella strains isolated from humans. International journal of antimicrobial agents, 27(4), 290-293.


